Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.


electrical and thermal conductivities are required, and is copper which has
been refined electrolytically.
Fire-refined grades of copper can be either tough pitch or deoxidised
according to their subsequent application. The former contains small
amounts of oxygen (present as copper (I) oxide, O12O) absorbed during
the manufacturing process. It is usually present in amounts of the order
of 0.04-0.05% oxygen (equivalent to 0.45-0.55% copper(I)oxide). This
copper(I) oxide is present as tiny sky-blue globules which were originally
part of a CU/O12O eutectic. Hot-working of the copper ingots breaks down
the Cu
2
O layers of this eutectic into globules. These globules have a neglig-
ible effect as far as electrical conductivity, and most other properties, are
concerned. The presence of copper(I) oxide is, in fact often advantageous,
since harmful impurities, like bismuth, appear to collect as oxides associ-
ated with the copper (I) oxide globules, instead of occurring as brittle inter-
crystalline films, as they would otherwise do.
For processes such as welding and tube-making, however, the existence
of these globules is extremely deleterious, since reducing atmospheres con-
taining hydrogen cause gassing of the metal. Hydrogen is interstitially
soluble in solid copper so that it comes into contact with subcutaneous
globules of copper(I) oxide, reducing them thus:
CU
2
O H- H
2
^ 2Cu + H
2
O
Equilibrium proceeds to the right according to the Law of Mass Action
because the concentration of dissolved hydrogen is high relative to that of
copper (I) oxide. The water formed is present as steam at the temperature
of the reaction. Since steam is virtually insoluble in solid copper it is
precipitated at the crystal boundaries, thus, in effect, pushing the crystals
apart and reducing the ductility by as much as 85% and the tensile strength
by 30-40%. Under the microscope gassed tough-pitch copper is recognised
by the thick grain boundaries, which are really minute fissures, and by the
absence of copper(I) oxide globules.
For such purposes as welding, therefore, copper is deoxidised before
being cast by the addition of phosphorus, which acts in the same way as
the manganese used in deoxidising steels. A small excess of phosphorus,
of the order of 0.04%, dissolves in the copper after deoxidation, and this
small amount is sufficient to reduce the electrical conductivity by as much
as 25%. So, whilst copper which is destined for welding or thermal treat-
ment in hydrogen-rich atmospheres should be of this type, copper deoxi-
dised by phosphorus would be unsuitable for electrical purposes, where
either electrolytic copper or good-quality tough-pitch copper must be used.
16.23 Mention was made (16.10) of the use of arsenic in hardening
copper by Balkans metal workers some 6000 years ago. In more recent
times up to 0.5% arsenic was added to much of the copper used in the
construction of locomotives. This addition considerably increased the
strength at elevated temperatures by raising the softening temperature
from about 190
0
C for the pure metal to 550
0
C for arsenical copper. This
made arsenical copper useful in the manufacture of steam locomotive fire-

boxes,
boiler tubes
and
rivets, since
the
alloy, whilst being stronger
at
high
temperatures still
had a
high thermal conductivity.
The addition
of
0.5%
lead
or
tellurium imparts free-cutting properties
to copper (6.64)
and
provides
a
material which
can be
machined
to
close
tolerances whilst still retaining
95%
of the
conductivity
of
pure copper.
16.24 Although copper
has
only
a
moderate tensile strength (16.21)
it
is
a
metal with very high malleability
and
ductility.
It is
very suitable
for
both
hot- and
cold-working
by the
main processes. Mention
has
been made
of 'deformation twins' (4.18)
but
another type
of
twinning occurs when
certain
of the
FCC
metals—particularly copper
and its
alloys
and
austenitic
steels—are annealed following cold-work.
The
presence
of
these annealing
twins
is
indicated
by
what appear
to be
pairs
of
parallel straight lines
crossing individual crystals (Plates
16.1c and
16.3c). Like deformation
twins,
these regions represent
a
part
of the
crystal
in
which
a
change
in
direction
of the
crystal lattice occurs
and in
this instance
are
formed during
re-crystallisation.
The
growth
of
annealing twins
is
related
to the
amount
of internal energy associated with
the
formation
of
dislocation faults within
the crystals during previous Cold-work.
In
aluminium
and its
alloys this
form
of
internal energy
is
high
so
that
new
crystal boundaries tend
to
form
whereas
in
copper alloys this energy
is low and
twinning occurs instead.
Thus copper
and its
alloys show twinned crystals
in the
cold-worked/
annealed state whilst aluminium alloys
do not.
16.25
The
Effects
of
Impurities
on the
electrical properties
of
copper
CONDUCTIVITY
PER CENT.
ADDED ELEMENT PER CENT
•+-
TO THE
LIMIT
OF
SOLID SOLUBILITY.
Fig.
16.1 The
effect
of
impurities
on the
electrical conductivity
of
copper.
SILVER
CADMIUM
ZINC
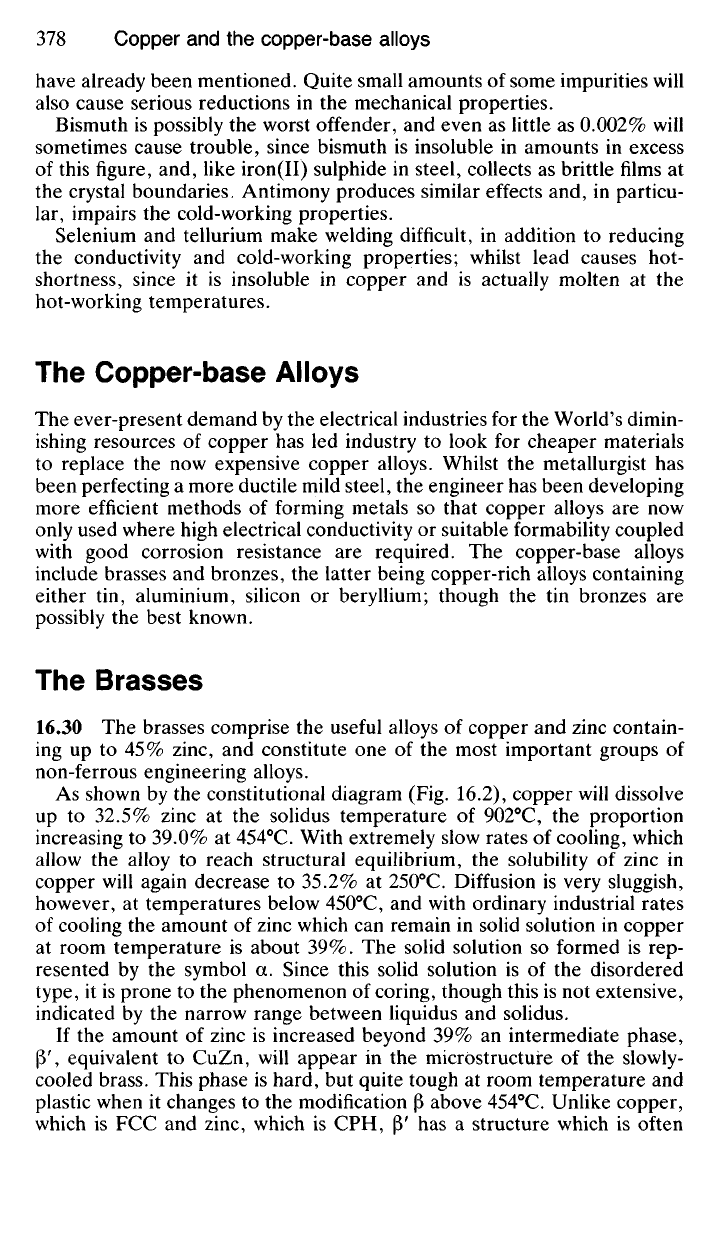
have already been mentioned. Quite small amounts of some impurities will
also cause serious reductions in the mechanical properties.
Bismuth is possibly the worst offender, and even as little as 0.002% will
sometimes cause trouble, since bismuth is insoluble in amounts in excess
of this figure, and, like iron(II) sulphide in steel, collects as brittle films at
the crystal boundaries, Antimony produces similar effects and, in particu-
lar, impairs the cold-working properties.
Selenium and tellurium make welding difficult, in addition to reducing
the conductivity and cold-working properties; whilst lead causes hot-
shortness, since it is insoluble in copper and is actually molten at the
hot-working temperatures.
The Copper-base Alloys
The ever-present demand by the electrical industries for the World's dimin-
ishing resources of copper has led industry to look for cheaper materials
to replace the now expensive copper alloys. Whilst the metallurgist has
been perfecting a more ductile mild steel, the engineer has been developing
more efficient methods of forming metals so that copper alloys are now
only used where high electrical conductivity or suitable formability coupled
with good corrosion resistance are required. The copper-base alloys
include brasses and bronzes, the latter being copper-rich alloys containing
either tin, aluminium, silicon or beryllium; though the tin bronzes are
possibly the best known.
The Brasses
16.30 The brasses comprise the useful alloys of copper and zinc contain-
ing up to 45% zinc, and constitute one of the most important groups of
non-ferrous engineering alloys.
As shown by the constitutional diagram (Fig. 16.2), copper will dissolve
up to 32.5% zinc at the solidus temperature of 902
0
C, the proportion
increasing to 39.0% at 454°C. With extremely slow rates of cooling, which
allow the alloy to reach structural equilibrium, the solubility of zinc in
copper will again decrease to 35.2% at 250
0
C. Diffusion is very sluggish,
however, at temperatures below 450
0
C, and with ordinary industrial rates
of cooling the amount of zinc which can remain in solid solution in copper
at room temperature is about 39%. The solid solution so formed is rep-
resented by the symbol a. Since this solid solution is of the disordered
type,
it is prone to the phenomenon of coring, though this is not extensive,
indicated by the narrow range between liquidus and solidus.
If the amount of zinc is increased beyond 39% an intermediate phase,
P',
equivalent to CuZn, will appear in the microstructure of the slowly-
cooled brass. This phase is hard, but quite tough at room temperature and
plastic when it changes to the modification (3 above 454°C. Unlike copper,
which is FCC and zinc, which is CPH, (3' has a structure which is often
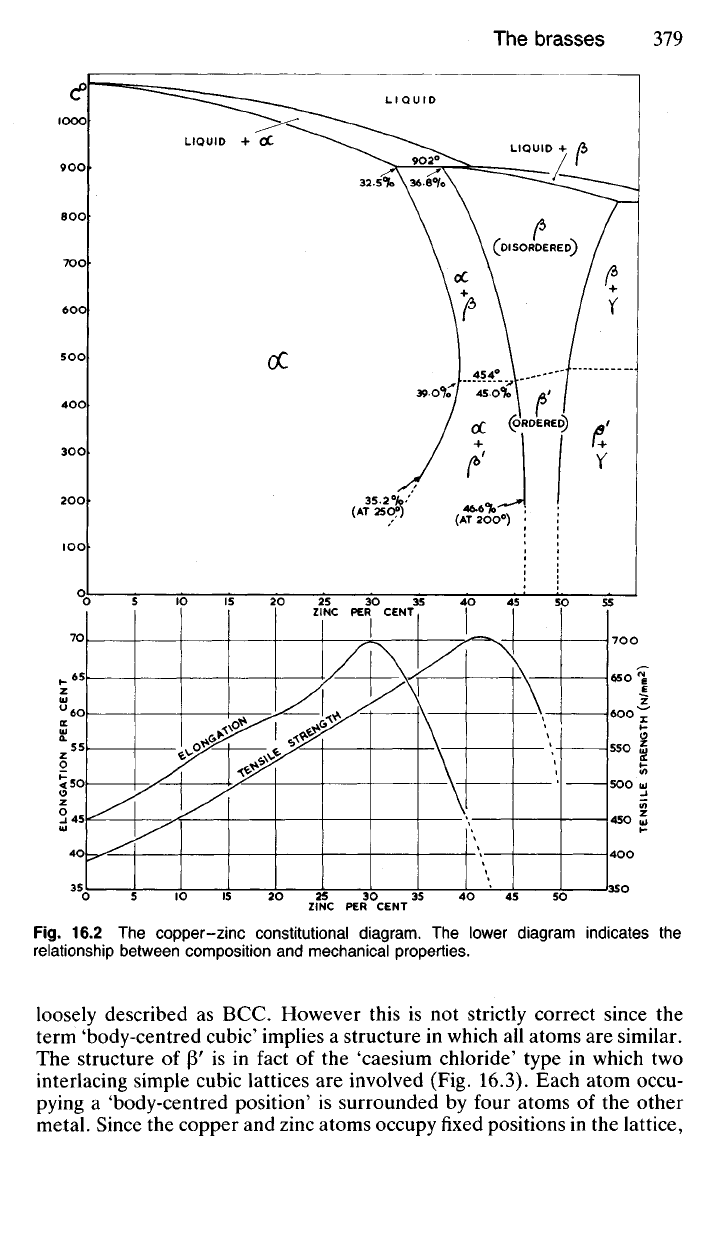
Fig.
16.2 The copper-zinc constitutional diagram. The lower diagram indicates the
relationship between composition and mechanical properties.
loosely described as BCC. However this is not strictly correct since the
term 'body-centred cubic' implies a structure in which all atoms are similar.
The structure of |3' is in fact of the 'caesium chloride' type in which two
interlacing simple cubic lattices are involved (Fig. 16.3). Each atom occu-
pying a 'body-centred position' is surrounded by four atoms of the other
metal. Since the copper and zinc atoms occupy fixed positions in the lattice,
ELONGATION
PER CENT
TENSILE
STRENGTH
(N/mm2)
<?
LIQUID
LIQUID + CC
LIQUID
(DISORDERED)
ORDERED
ZINC
PER
CENT
ZINC
PER
CENT
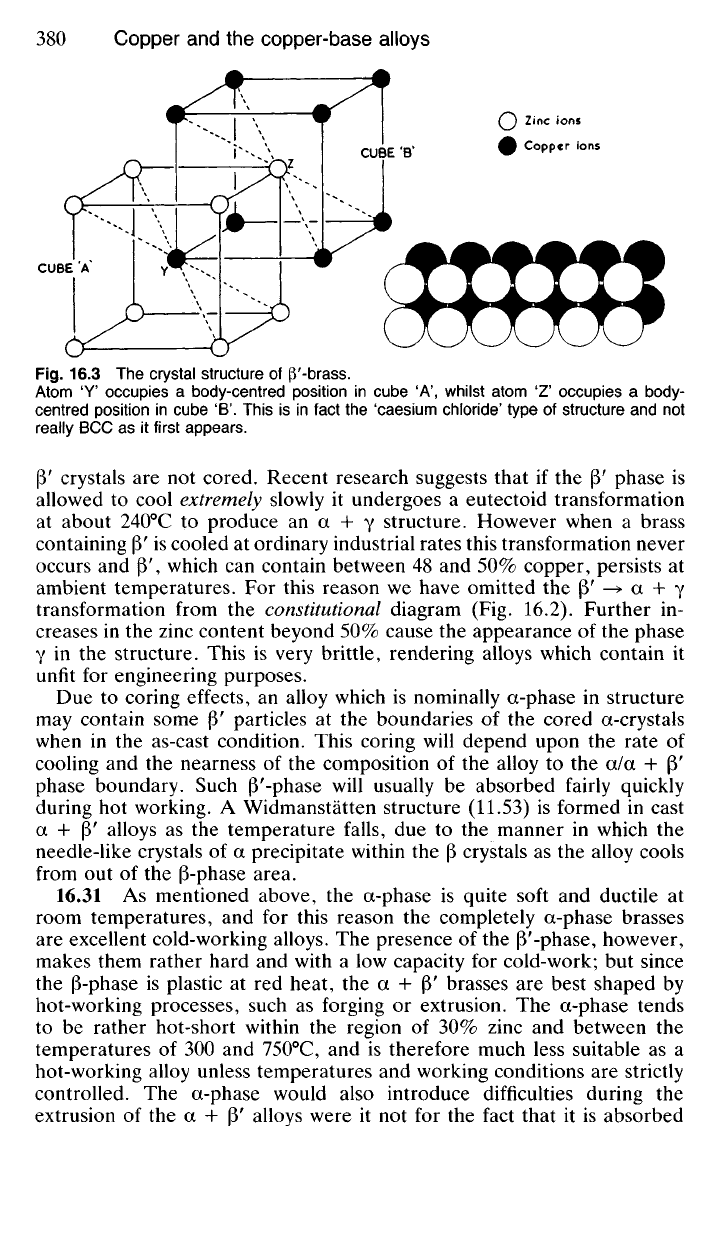
Fig.
16.3 The crystal structure of P'-brass.
Atom 'Y' occupies a body-centred position in cube 'A', whilst atom
1
Z occupies a body-
centred position in cube 'B'. This is in fact the 'caesium chloride' type of structure and not
really BCC as it first appears.
P'
crystals are not cored. Recent research suggests that if the P' phase is
allowed to cool extremely slowly it undergoes a eutectoid transformation
at about 240
0
C to produce an a + y structure. However when a brass
containing P' is cooled at ordinary industrial rates this transformation never
occurs and P', which can contain between 48 and 50% copper, persists at
ambient temperatures. For this reason we have omitted the (3' —» a + y
transformation from the constitutional diagram (Fig. 16.2). Further in-
creases in the zinc content beyond 50% cause the appearance of the phase
Y in the structure. This is very brittle, rendering alloys which contain it
unfit for engineering purposes.
Due to coring effects, an alloy which is nominally a-phase in structure
may contain some (3' particles at the boundaries of the cored a-crystals
when in the as-cast condition. This coring will depend upon the rate of
cooling and the nearness of the composition of the alloy to the a/a + (3'
phase boundary. Such P'-phase will usually be absorbed fairly quickly
during hot working. A Widmanstatten structure (11.53) is formed in cast
a + p' alloys as the temperature falls, due to the manner in which the
needle-like crystals of a precipitate within the P crystals as the alloy cools
from out of the P-phase area.
16.31 As mentioned above, the a-phase is quite soft and ductile at
room temperatures, and for this reason the completely a-phase brasses
are excellent cold-working alloys. The presence of the P'-phase, however,
makes them rather hard and with a low capacity for cold-work; but since
the p-phase is plastic at red heat, the a 4- P' brasses are best shaped by
hot-working processes, such as forging or extrusion. The a-phase tends
to be rather hot-short within the region of 30% zinc and between the
temperatures of 300 and 750
0
C, and is therefore much less suitable as a
hot-working alloy unless temperatures and working conditions are strictly
controlled. The a-phase would also introduce difficulties during the
extrusion of the a + P' alloys were it not for the fact that it is absorbed
Zinc ions
Copper ions
CUBE 'A
CUBE 'B

into the (3-phase when the 60-40 composition (one of the most popular
alloys of this group) is heated to a point above the a + (3/(3 phase boundary
in the region of 750
0
C, thus producing a uniform plastic structure of
(3-phase only. The a-phase is usually in process of being precipitated whilst
hot-working is taking place, so that, instead of the Widmanstatten structure
being formed again as the temperature falls, it is replaced by a refined
granular a + |3' structure which possesses superior mechanical properties
to those of the directional Widmanstatten structure. The needle-shaped
crystals of the a-phase are prevented from forming by the mechanical
disturbances which accompany the working process.
Thus the brasses can conveniently be classified according to whether
they are hot-working or cold-working alloys.
16.32 The Cold-working (/-brasses These are generally completely
a-phase in structure, though a limited amount of cold-work may also be
applied to those a + |3' alloys which contain only small amounts of the
P'-phase. The a + (3' alloys proper are, however, shaped by hot-working
processes in the initial stages, and such cold-work as is applied is merely
for finishing to size or to produce the correct degree of work-hardening
for subsequent use.
The a-brasses are useful mainly because of their high ductility, which
reaches a maximum at 30% zinc, as shown in Fig. 16.2. Such alloys need
to be of very high purity, since the inclusion of even small amounts of
impurity will lead to a big loss in ductility. The need to use high-purity
copper and zinc in the manufacture of 70-30 brass makes it a very ex-
pensive alloy. For this reason it has been replaced in many instances in
engineering design by BOP mild steel which has a high ductility because
of its low nitrogen content. The a-brasses are also rather sensitive to
annealing temperatures and, since grain growth is rapid at elevated tem-
peratures, it is easy to burn the alloy. (This trade term should not be
confused with oxidation of the metal. It is widely used industrially to signify
overheating.) a-Brasses should be annealed at about 600
0
C. If overheated
to 750
0
C, grain growth is so rapid that on subsequent pressing an orange
peel effect is apparent on the surface. This is due to coarse grain being
large enough to be visible on the surface.
16.33 Cold-worked a-brasses are subject to 'season cracking'. Dislo-
cations become piled-up at crystal boundaries as a result of cold-work,
making these regions zones of high energy. Therefore, any corrosion which
takes place tends to be intercrystalline since the high-energy zones are
'anodic'
(21.40) to their surroundings. As a result of corrosion the grain
boundaries become weakened and fracture occurs there because of the
locked-up stresses which are present. The term 'season-cracking' was origi-
nally used to describe the spontaneous cracking found in stored cartridge
cases in India during the monsoon season. It was particularly prevalent
when the damp atmosphere contained ammonia emanating from nearby
cavalry stables. Season cracking can be avoided by giving the components
a low-temperature, stress relief anneal at about 250
0
C after fabrication.
16.34 Other elements may be added in small amounts to the a-brasses
in order to improve either corrosion-resistance or mechanical properties.
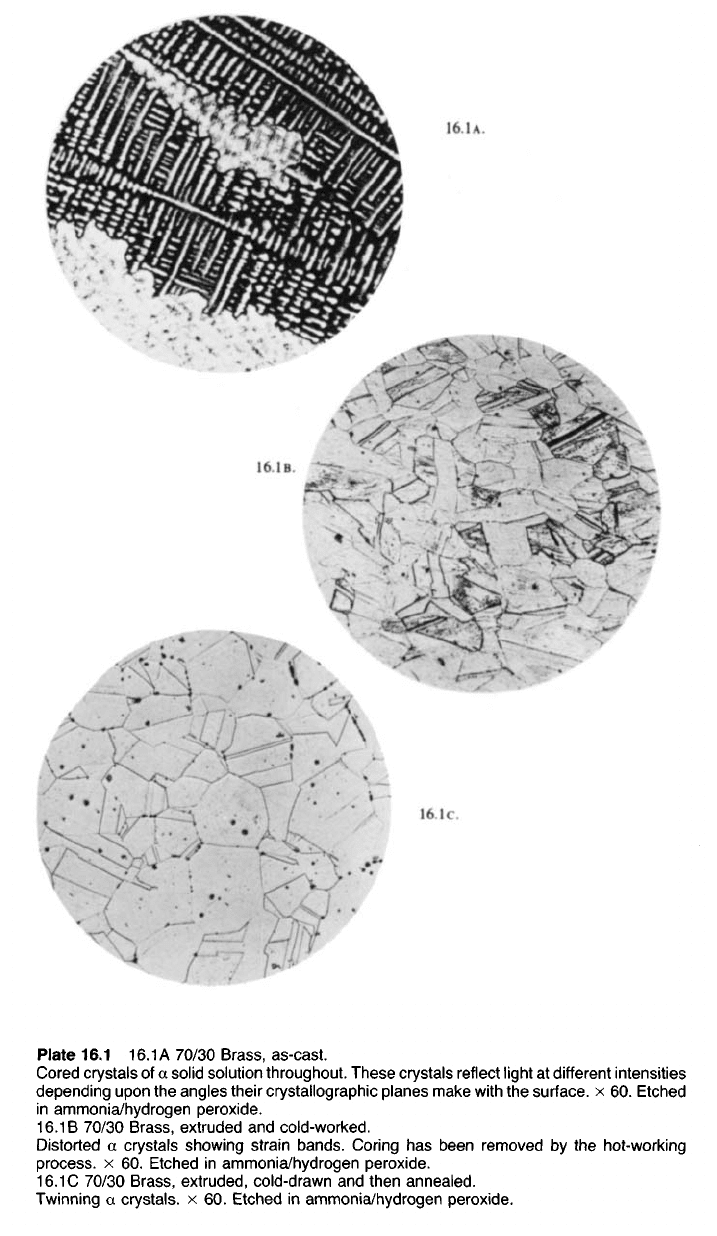
Plate 16.1 16.1A 70/30 Brass, as-cast.
Cored crystals of a solid solution throughout. These crystals reflect light at different intensities
depending upon the angles their crystallographic planes make with the surface, x 60. Etched
in ammonia/hydrogen peroxide.
16.1 B 70/30 Brass, extruded and cold-worked.
Distorted a crystals showing strain bands. Coring has been removed by the hot-working
process, x 60. Etched in ammonia/hydrogen peroxide.
16.1 C 70/30 Brass, extruded, cold-drawn and then annealed.
Twinning a crystals, x 60. Etched in ammonia/hydrogen peroxide.
16.1c.
16.1B.
16.1A.

Plate 16.2 16.2A 60/40 Brass, as-cast.
Widmanstatten structure of a (light) and P' (dark), x 150. Etched in acid iron(lll) chloride.
16.2B 60/40 Brass, extruded.
Extrusion has stimulated recrystailisation of the coarse as-cast structure and produced a
fine granular structure of a (light) in a matrix of P' (dark), x 150. Etched in acid iron(lll)
chloride.
16.2C 50/50 Brass, as-cast.
Crystals of P' only. These are not cored since p' is an intermediate phase of fixed composition
(CuZn). The individual crystals reflect light at different intensities because their crystallo-
graphic planes cut the surface at different angles, x 15. Etched in acid iron(lll) chloride.
16.2c.
16.2B.
16.2A.
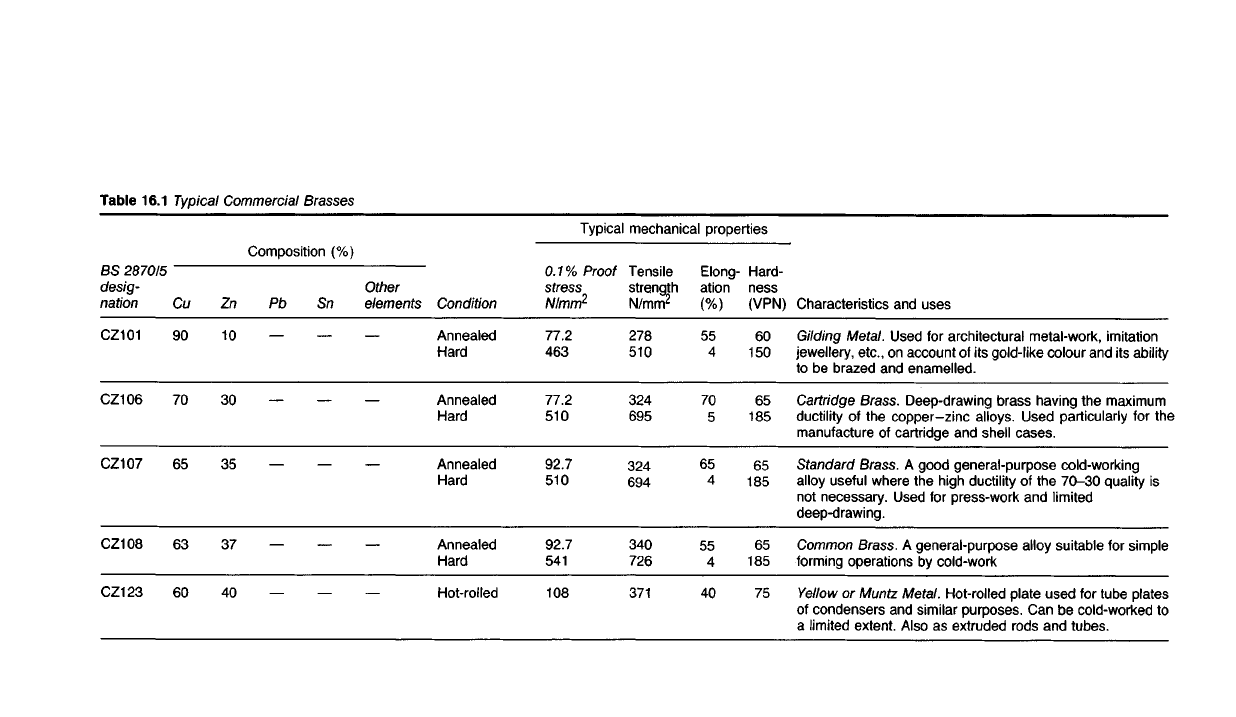
Table 16.1
Typical
Commercial
Brasses
Characteristics and uses
Gilding
Metal. Used for architectural metal-work, imitation
jewellery, etc., on account of its
gold-like
colour and its
ability
to be brazed and enamelled.
Cartridge
Brass.
Deep-drawing brass having the maximum
ductility of the
copper-zinc
alloys. Used particularly for the
manufacture of cartridge and shell cases.
Standard
Brass.
A good general-purpose cold-working
alloy useful where the high
ductility
of the
70-30
quality is
not
necessary. Used for press-work and
limited
deep-drawing.
Common
Brass.
A general-purpose alloy suitable for simple
forming operations by cold-work
Yellow
or
Muntz
Metal. Hot-rolled plate used for tube plates
of
condensers and similar purposes. Can be cold-worked to
a
limited
extent. Also as extruded rods and tubes.
Typical mechanical properties
Hard-
ness
(VPN)
60
150
65
185
65
185
65
185
75
Elong-
ation
(%)
55
4
70
5
65
4
55
4
40
Tensile
strength
N/mm
2
278
510
324
695
324
694
340
726
371
0.1%
Proof
stress
N/mm
2
77.2
463
77.2
510
92.7
510
92.7
541
108
Condition
Annealed
Hard
Annealed
Hard
Annealed
Hard
Annealed
Hard
Hot-rolled
Composition (%)
Other
elements
Sn
Pb
Zn
10
30
35
37
40
Cu
90
70
65
63
60
BS
2870/5
desig-
nation
CZ101
CZ106
CZ107
CZ108
CZ123
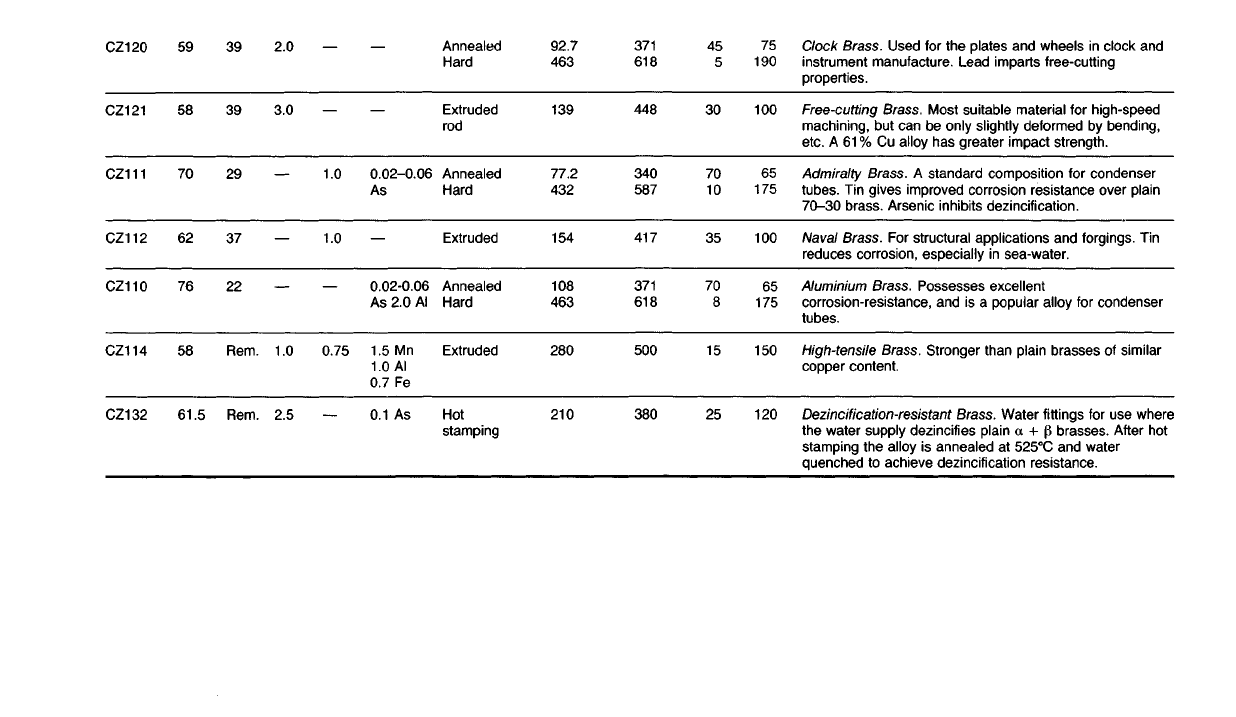
Clock
Brass. Used for the plates and wheels in clock and
instrument manufacture. Lead imparts free-cutting
properties.
Free-cutting
Brass.
Most suitable material for high-speed
machining,
but can be only
slightly
deformed by
bending,
etc. A 61 % Cu alloy has greater impact strength.
Admiralty
Brass.
A standard composition for condenser
tubes. Tin gives improved corrosion resistance over
plain
70-30
brass. Arsenic
inhibits
dezincification.
Naval
Brass.
For structural
applications
and forgings. Tin
reduces corrosion, especially in sea-water.
Aluminium
Brass.
Possesses excellent
corrosion-resistance, and is a popular alloy for condenser
tubes.
High-tensile
Brass.
Stronger than
plain
brasses of similar
copper content.
Dezincification-resistant
Brass. Water fittings for use where
the water supply dezincifies plain a + p brasses.
After
hot
stamping the alloy is annealed at 525°C and water
quenched to achieve dezincification resistance.
75
190
100
65
175
100
65
175
150
120
45
30
70
10
35
70
8
15
25
371
618
448
340
587
417
371
618
500
380
92.7
463
139
77.2
432
154
108
463
280
210
Annealed
Hard
Extruded
rod
Annealed
Hard
Extruded
Annealed
Hard
Extruded
Hot
stamping
0.02-0.06
As
0.02-0.06
As
2.0 Al
1.5Mn
1.0 Al
0.7Fe
0.1 As
1.0
1.0
0.75
2.0
3.0
. 1.0
. 2.5
39
39
29
37
22
Rem
Rem
59
58
70
62
76
58
61.5
CZ120
CZ121
CZ111
CZ112
CZ110
CZ114
CZ132
