Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.

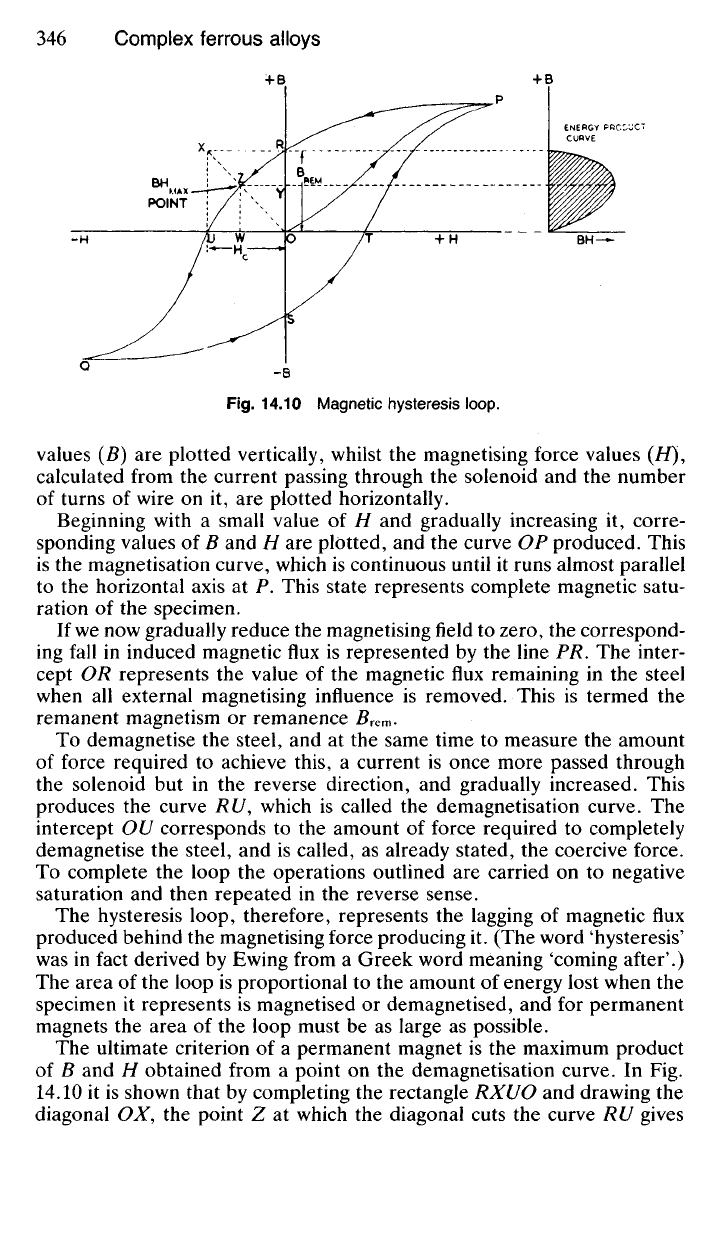
Fig.
14.10 Magnetic hysteresis loop.
values (B) are plotted vertically, whilst the magnetising force values (//),
calculated from the current passing through the solenoid and the number
of turns of wire on it, are plotted horizontally.
Beginning with a small value of H and gradually increasing it, corre-
sponding values of B and H are plotted, and the curve OP produced. This
is the magnetisation curve, which is continuous until it runs almost parallel
to the horizontal axis at P. This state represents complete magnetic satu-
ration of the specimen.
If we now gradually reduce the magnetising field to zero, the correspond-
ing fall in induced magnetic flux is represented by the line PR. The inter-
cept OR represents the value of the magnetic flux remaining in the steel
when all external magnetising influence is removed. This is termed the
remanent magnetism or remanence
B
rGm
.
To demagnetise the steel, and at the same time to measure the amount
of force required to achieve this, a current is once more passed through
the solenoid but in the reverse direction, and gradually increased. This
produces the curve RU, which is called the demagnetisation curve. The
intercept OU corresponds to the amount of force required to completely
demagnetise the steel, and is called, as already stated, the coercive force.
To complete the loop the operations outlined are carried on to negative
saturation and then repeated in the reverse sense.
The hysteresis loop, therefore, represents the lagging of magnetic flux
produced behind the magnetising force producing it. (The word 'hysteresis'
was in fact derived by Ewing from a Greek word meaning 'coming after'.)
The area of the loop is proportional to the amount of energy lost when the
specimen it represents is magnetised or demagnetised, and for permanent
magnets the area of the loop must be as large as possible.
The ultimate criterion of a permanent magnet is the maximum product
of B and H obtained from a point on the demagnetisation curve. In Fig.
14.10 it is shown that by completing the rectangle RXUO and drawing the
diagonal OX, the point Z at which the diagonal cuts the curve RU gives
BH
MAX
POINT
ENERGY PRODUCT
CURVE
an approximation to the point at which the product of YZ and WZ is a
maximum for the curve. This is called
BH
mdX
,
and corresponds to the
maximum energy the magnet can provide in a circuit external to
itself.
14.36 Fully hardened carbon steels containing about 1.0 % carbon
were originally used for permanent magnets. These were later replaced by
alloy steels containing chromium and tungsten, and later cobalt. The cobalt
steels are the only ones of any practical value today and these have been
superseded by alloys of the Alni-Alnico-Alcomax series. These were
developed from magnetic aluminium-nickel alloys discovered by Japan-
ese workers in 1930.
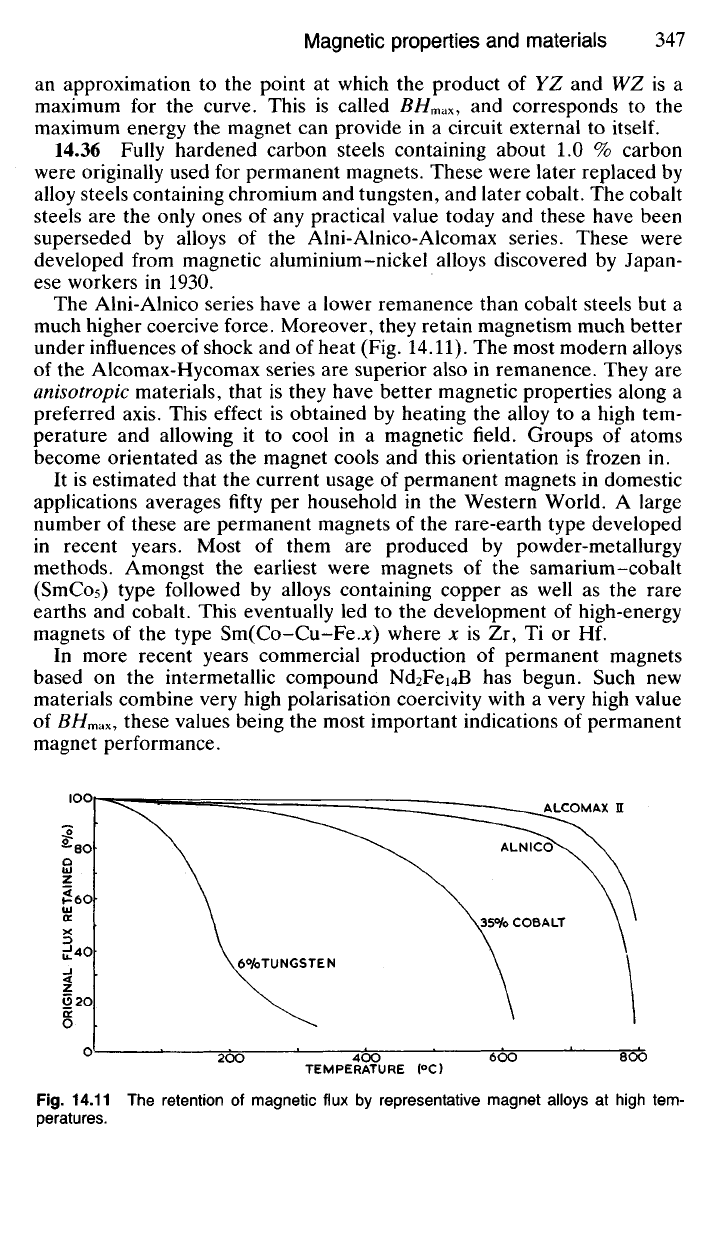
The Alni-Alnico series have a lower remanence than cobalt steels but a
much higher coercive force. Moreover, they retain magnetism much better
under influences of shock and of heat (Fig. 14.11). The most modern alloys
of the Alcomax-Hycomax series are superior also in remanence. They are
anisotropic materials, that is they have better magnetic properties along a
preferred axis. This effect is obtained by heating the alloy to a high tem-
perature and allowing it to cool in a magnetic field. Groups of atoms
become orientated as the magnet cools and this orientation is frozen in.
It is estimated that the current usage of permanent magnets in domestic
applications averages fifty per household in the Western World. A large
number of these are permanent magnets of the rare-earth type developed
in recent years. Most of them are produced by powder-metallurgy
methods. Amongst the earliest were magnets of the samarium-cobalt
(SmCo
5
) type followed by alloys containing copper as well as the rare
earths and cobalt. This eventually led to the development of high-energy
magnets of the type Sm(Co-Cu-Fe.x) where x is Zr, Ti or Hf.
In more recent years commercial production of permanent magnets
based on the intermetallic compound Nd
2
Fe^B has begun. Such new
materials combine very high polarisation coercivity with a very high value
of Z?//max, these values being the most important indications of permanent
magnet performance.
ORIGINAL
FLUX
RETAINED
(°/o)
TEMPERATURE (
0
C)
Fig.
14.11 The retention of magnetic flux by representative magnet alloys at high tem-
peratures.
6°/oTUNGSTEN
35<Vo COBALT
ALNICO
ALCOMAX H
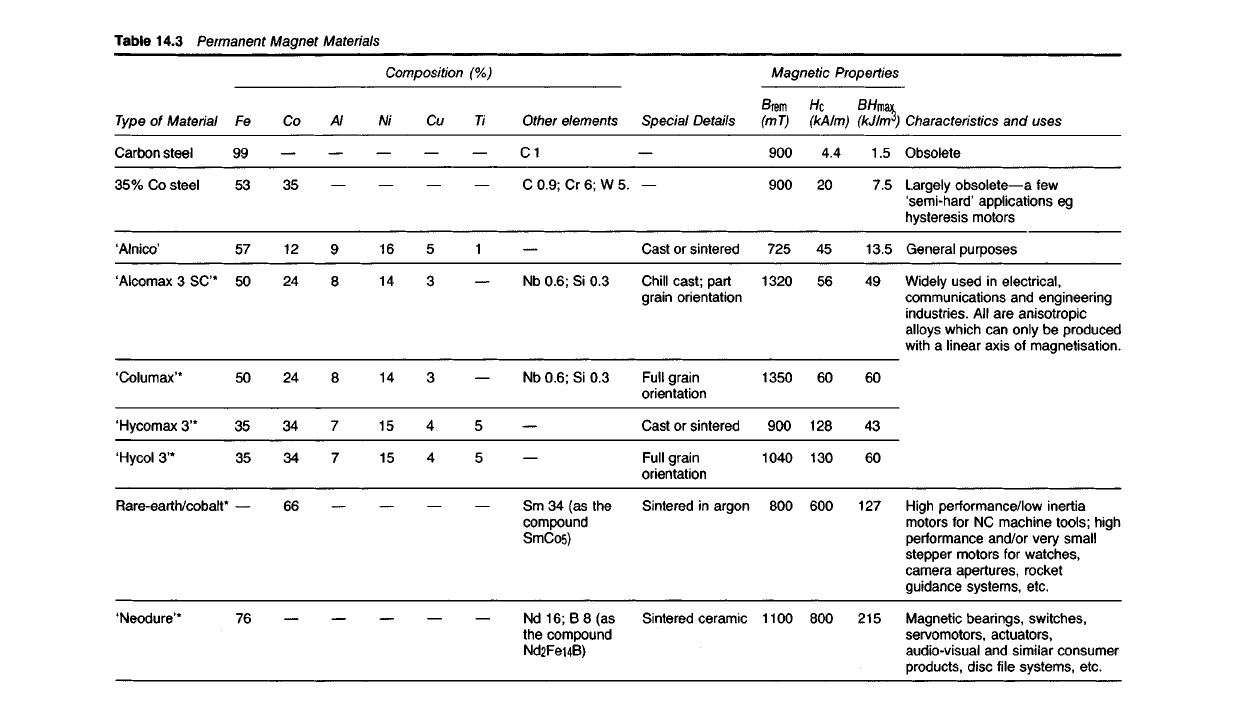
Table
14.3 Permanent
Magnet
Materials
Characteristics and uses
Obsolete
Largely obsolete—a few
'semi-hard' applications eg
hysteresis
motors
General purposes
Widely used in electrical,
communications and engineering
industries. All are anisotropic
alloys which can only be produced
with
a linear axis of magnetisation.
High performance/low inertia
motors
for NC machine tools; high
performance and/or very small
stepper motors for watches,
camera apertures, rocket
guidance systems, etc.
Magnetic bearings, switches,
servomotors, actuators,
audio-visual and similar consumer
products, disc file systems, etc.
Magnetic
Properties
(kJ/m
3
)
1.5
7.5
13.5
49
60
43
60
127
215
(kA/m)
4.4
20
45
56
60
128
130
600
800
(mT)
900
900
725
1320
1350
900
1040
800
1100
Special
Details
Cast or sintered
Chill
cast; part
grain
orientation
Full grain
orientation
Cast or sintered
Full grain
orientation
Sintered in argon
Sintered ceramic
Composition
(%)
Other elements
C1
C 0.9; Cr 6; W 5.
Nb 0.6; Si 0.3
Nb 0.6; Si 0.3
Sm 34 (as the
compound
SmCo5)
Nd 16; B 8 (as
the compound
Nd2FeuB)
Ti
1
5
5
Cu
5
3
3
4
4
Ni
16
14
14
15
15
Al
9
8
8
7
7
Co
35
12
24
24
34
34
66
Fe
99
53
57
50
50
35
35
76
Type
of Material
Carbon steel
35% Co steel
'Alnico'
'Alcomax 3 SC*
'Columax'*
'Hycomax 3'*
'Hycol 3'*
Rare-earth/cobalt
'Neodure*
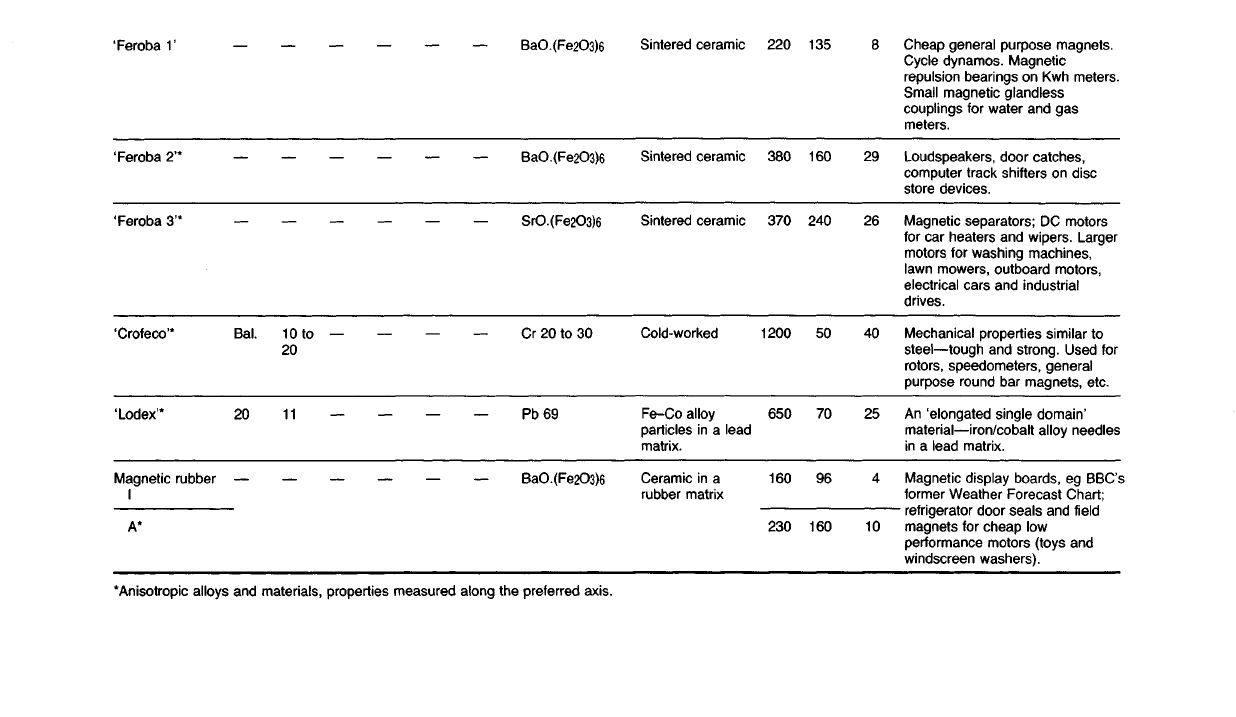
Cheap
general
purpose magnets.
Cycle dynamos.
Magnetic
repulsion bearings on Kwh meters.
Small magnetic glandless
couplings for water and gas
meters.
Loudspeakers, door catches,
computer track
shifters
on disc
store devices.
Magnetic
separators; DC motors
for
car heaters and wipers. Larger
motors for washing machines,
lawn mowers, outboard motors,
electrical cars and industrial
drives.
Mechanical properties similar to
steel—tough
and strong.
Used
for
rotors,
speedometers,
general
purpose round bar magnets, etc.
An
'elongated single domain'
material—iron/cobalt alloy needles
in
a
lead
matrix.
Magnetic display boards, eg BBC's
former
Weather Forecast Chart;
refrigerator
door seals and
field
magnets for cheap low
performance motors (toys and
windscreen washers).
29
26
40
25
4
10
135
160
240
50
70
96
160
220
380
370
1200
650
160
230
Sintered ceramic
Sintered ceramic
Sintered ceramic
Cold-worked
Fe-Co
alloy
particles in a
lead
matrix.
Ceramic in a
rubber matrix
BaO.(Fe2O3)6
BaO.(Fe2O3)6
SrO.(Fe2O3)6
Cr 20 to 30
Pb 69
BaO.(Fe2O3)6
10to
20
11
BaI.
20
'Feroba
1'
'Feroba
2'*
'Feroba
3'*
'Crofeco'*
'Lodex'*
Magnetic
rubber
A*
*Anisotropic alloys and
materials,
properties measured along the preferred axis.
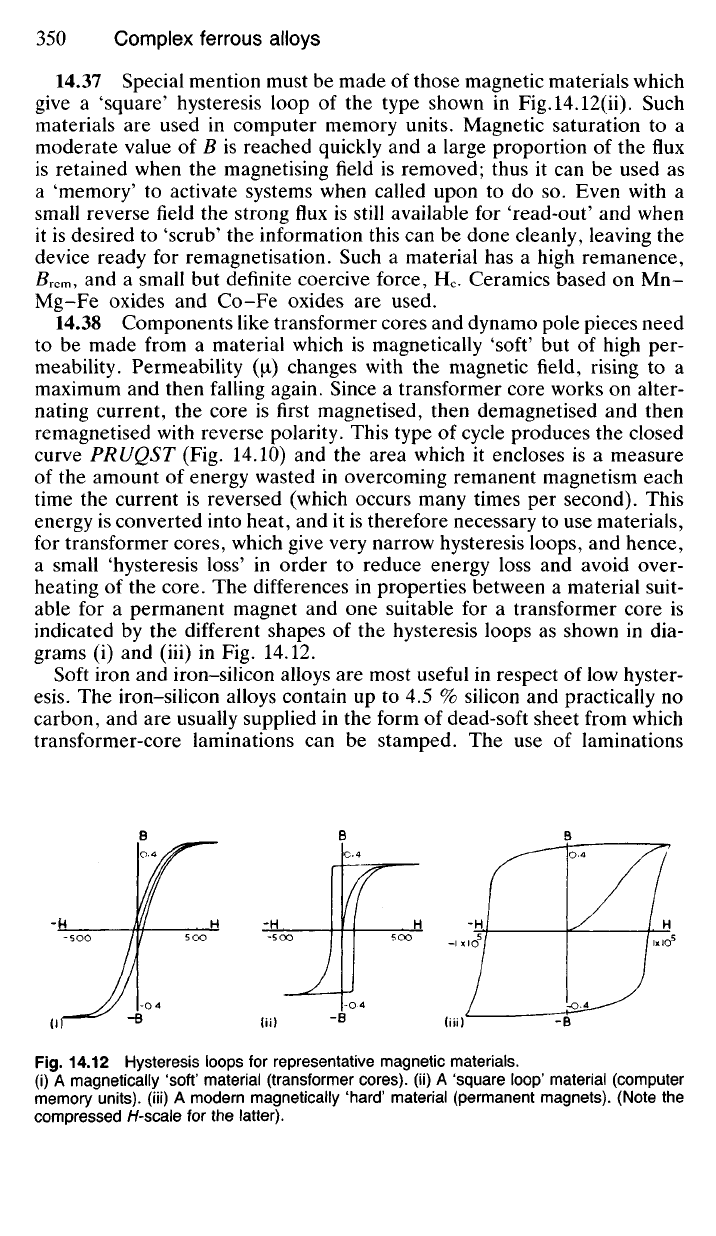
14.37 Special mention must be made of those magnetic materials which
give a 'square' hysteresis loop of the type shown in Fig.l4.12(ii). Such
materials are used in computer memory units. Magnetic saturation to a
moderate value of B is reached quickly and a large proportion of the flux
is retained when the magnetising field is removed; thus it can be used as
a 'memory' to activate systems when called upon to do so. Even with a
small reverse field the strong flux is still available for 'read-out' and when
it is desired to 'scrub' the information this can be done cleanly, leaving the
device ready for remagnetisation. Such a material has a high remanence,
/?rem, and a small but definite coercive force, H
c
. Ceramics based on Mn-
Mg-Fe oxides and Co-Fe oxides are used.
14.38 Components like transformer cores and dynamo pole pieces need
to be made from a material which is magnetically 'soft' but of high per-
meability. Permeability (\i) changes with the magnetic field, rising to a
maximum and then falling again. Since a transformer core works on alter-
nating current, the core is first magnetised, then demagnetised and then
remagnetised with reverse polarity. This type of cycle produces the closed
curve PRUQST (Fig. 14.10) and the area which it encloses is a measure
of the amount of energy wasted in overcoming remanent magnetism each
time the current is reversed (which occurs many times per second). This
energy is converted into heat, and it is therefore necessary to use materials,
for transformer cores, which give very narrow hysteresis loops, and hence,
a small 'hysteresis loss' in order to reduce energy loss and avoid over-
heating of the core. The differences in properties between a material suit-
able for a permanent magnet and one suitable for a transformer core is
indicated by the different shapes of the hysteresis loops as shown in dia-
grams (i) and (iii) in Fig. 14.12.
Soft iron and iron-silicon alloys are most useful in respect of low hyster-
esis.
The iron-silicon alloys contain up to 4.5 % silicon and practically no
carbon, and are usually supplied in the form of dead-soft sheet from which
transformer-core laminations can be stamped. The use of laminations
Fig.
14.12 Hysteresis loops for representative magnetic materials,
(i) A magnetically 'soft' material (transformer cores), (ii) A 'square loop' material (computer
memory units), (iii) A modern magnetically 'hard' material (permanent magnets). (Note the
compressed H-scale for the latter).

reduces
losses
from eddy currents, which would be more prevalent in a
solid core.
Nickel-iron
alloys are also of importance as low-hysteresis, high-
permeability alloys. 'Permalloy' contains №78 and Fe22 with carbon and
silicon kept as low as possible.
'MumetaF
contains №74; Cu5; Mn up to
1; Fe-bal. Both alloys are used in communications engineering, where the
high permeability
will
utilise to the full the small currents involved. In
addition
to being used for transformer cores, these alloys are used as
shields for submarine cables. Like the iron-silicon alloys, they are used in
the
dead-soft condition, the best treatment consisting of heating to 900
0
C,
followed by
slow
cooling, and
then
heating to 600
0
C, followed by cooling
in
air. This treatment avoids the ordered structure which would otherwise
form in these alloys. Since the magnetic properties are
very
sensitive to
work-hardening these alloys must
always
be used in the annealed state.
In
all magnetically soft materials properties depend upon the ease of
domain
movement and so work-hardening and the presence of impurities
must be avoided. In iron-silicon alloys the crystal orientation of the sheet
is also controlled. Since magnetisation is easiest in the <100> directions,
the
sheet is prepared with the (100) planes parallel to the plane of the
sheet. This leads to a lower power
loss
in transformer cores.
A number of ceramic-type magnetically-soft materials are now used in
electronics and radio-communications equipment. Most of these materials
are based on the formula
Fe
3
O
4
('magnetite' which also occurs naturally
as a magnetic mineral—the iodestone' of the ancient mariners). In this
formula the
Fe
++
ions are replaced by various mixtures of
Mn
++
,
Zn
++
,
Ni
++
and Cu
++
ions and are generally known as 'ferrites'. This term has
no
direct connection with 'ferrite' which
signifies
a-iron but is a reference
to
the general formula M-Fe
2
O
4
. They are powder metallurgy products.
Exercises
1.
Discuss
reasons
for and
against
the use of
molybdenum
as a
replacement
for
tungsten
in
high-speed
steels.
(14.10;
Table
14.1)
2.
By
reference
to Fig. 14.1
estimate
the %
carbon
in
solution
in
austenite
at (i)
900
0
C;
(ii)
1300
0
C,
for a
typical
high-speed
steel.
Show
how
this
affects
the
quenching
temperature
for
such
a
steel.
(14.13;
14.14)
3.
Discuss
the
significance
of Fig. 14.2 on the
heat-treatment
programme
of a
high-speed
steel.
(14.15)
4.
(a)
What
do you
understand
by the
term
'tool
steel'?
(b)
Give
typical
approximate
chemical
compositions
for
tool
steels
suitable
for
thread
rolls
or
other
components
that
need
great
resistance
to
wear
and
abrasion.
(c)
Describe
how you
would
heat-treat
such
a
tool,
mentioning
the
precautions
that
would
be
necessary
to
produce
a
satisfactory
article.
(Table
14.1;
14.14;
14.15)
5.
What
are the
basic
requirements
of a
tool
steel?
Describe
the
heat-treatment
of
a
high-speed
tool
steel,
giving
reasons
for
each
step.
Explain
how the

microstructure is related to the service requirements of the tool.
(14.11;
14.14;
14.15)
6. Distinguish between
ferromagnetism,
paramagnetism and
diamagnetism.
Outline
the basic theory which seeks to explain ferromagnetic properties. (14.30 to
14.33)
7.
Explain why the value
BH
mdX
is important in assessing the suitability of a perma-
nent magnet material for service.
Outline developments which have taken place in the formulation of permanent
magnet materials in recent years. (14.35 to 14.37)
8. Define
magnetic permeability
(\i) and sketch representative hysteresis curves for
both magnetically 'hard' and 'soft' materials.
For what purposes are magnetically 'soft' materials used? (14.38)
9. Some magnetic materials are said to give a 'square' hysteresis loop.
Explain what this means and say for what purposes such materials are used.
(14.39)
Bibliography
Berkowski, A. E. and Kellner, E., Magnetism and Metallurgy, Academic Press,
1970.
Hoyle, G., High Speed Steels, Butterworths, 1988.
Rosenberg, H. M., The Solid State, Oxford University Press, 1978.
Thelning, K-E., Steel and its Heat-treatment: Bofors Handbook, Butterworths,
1984.
Wilson, R., Metallurgy and
Heat-treatment
of Tool Steels, McGraw-Hill, 1975.

Cast Irons and Alloy
Cast Irons
15.10 In Victorian times almost anything was likely to be made of cast
iron—street lamps, domestic fireplaces, railings, mock-gothic church
window frames and the ornamental water fountain in the local park. Sadly
many of these relics of the nineteenth century foundryman's art are no
more since most fell victim to the urgent need for steel during the Second
World War. Even as late as the author's childhood toy 'six-shooters' were
of white cast iron, whereas to-day's children play with 'space-age' guns
made of plastics materials. One important reason for the widespread use
of cast iron in Victorian times was the fact that in those days not all
pig iron produced was suitable for conversion into merchantable steel. In
particular those pig irons high in phosphorus and sulphur were suitable
only for ornamental castings which required little strength. Today cast iron
is used exclusively for engineering purposes and its technology, like that
of other alloys, continues to be developed at highly sophisticated levels.
'Spheroidal graphite' cast iron and, more recently, 'compacted graphite'
irons are of this type.
15.11 Ordinary cast iron is very similar in composition and structure
to the crude pig iron from the blast furnace. In most foundries the 'pigs'
are melted, often with the addition of low-grade steel scrap, in a cupola,
any necessary adjustments in composition being made during this melting
process. The relative scarcity and consequent high cost of metallurgical
coke has led some foundries to adopt electric melting, usually by high-
frequency induction furnaces. An obvious advantage of electric melting is
its chemical cleanliness and lack of sulphur contamination as compared
with cupola melting where the iron is in intimate contact with the burning
coke.
Consequently many of the high quality grey cast irons, as well as
spheroidal-graphite irons and alloy cast irons are now melted electrically.
Because production costs of pig iron are relatively low as compared with
other alloys, and since no expensive refining process is necessary, cast iron
is a cheap metallurgical material which is particularly useful where a casting
15
requiring rigidity, resistance to wear or high compressive strength is neces-
sary. Other useful properties of cast iron include:
(i) good machinability when a suitable composition is selected;
(ii) high fluidity and the ability to make good casting impressions;
(iii) fairly low melting range (1130-1250
0
C) as compared with steel;
(iv) the availability of high strengths when additional treatment is given
to suitable irons, eg spheroidal-graphite iron, compacted-graphite
irons or pearlitic malleable irons.
The structure and physical properties of a cast iron depend upon both
chemical composition and the rate at which it solidifies following casting.
The Effects of Composition on the Structure of
Cast Iron
15.20 Ordinary cast iron is a complex alloy containing a total of up to
10%
of the elements carbon, silicon, manganese, sulphur and phosphorus;
the balance being iron. Alloy cast irons, which will be dealt with later in this
chapter, contain also varying amounts of nickel, chromium, molybdenum,
vanadium and copper.
15.21 Carbon can exist in two forms in cast iron, namely as free graph-
ite or combined with some of the iron to form iron carbide (cementite).
These two varieties are usually referred to as graphic carbon and combined
carbon respectively, and the total amount of both types in the specimen
of iron as total carbon. In ordinary engineering cast iron the form in which
carbon is present depends largely upon the cooling rate during solidification
and upon the quantity of silicon present. In alloy cast irons those elements
mentioned above will also affect the resultant structure. In fact the effects
of various elements on the microstructure of cast iron is generally the same
as in steels (13.12).
Cementite is a hard, white, brittle compound, so that irons which contain
much of it will present a white fracture when broken, and will have a low
resistance to shock. At the same time they will possess a high resistance
to wear. Such irons are called white irons. The fractured surface of a cast
iron containing graphite, however, will appear grey, and the iron will be
termed a grey iron.
Although the form of the iron-carbon diagram shown in Fig. 11.1 is of
great value to the practical metallurgist it is not a true equilibrium diagram
because in fact cementite is not an 'equilibrium phase'. Cementite is said
to be a metastable phase, that is, it has a natural tendency to decompose
forming a mixture of iron (ferrite) and carbon (graphite). In ordinary steels
this decomposition almost never occurs because in iron supersaturated with
carbon the nucleation of cementite takes place much more readily than
the nucleation of graphite. Once cementite has formed it is quite stable
and for practical purposes can be regarded as an equilibrium phase.
In a simple iron-carbon alloy containing, say, 3% carbon, solidification
would begin with the formation of primary austenite and at 1131°C a

Fig.
15.1 The formation of 'flake' graphite resulting from the growth of eutectic cells. In (iv)
some free cementite will also be present as the solubility of carbon in austenite decreases
from 2.0% at 1131
0
C to 0.8% at 723°C.
eutectic consisting of austenite and cementite would form. However, engin-
eering cast irons contain sufficient silicon to increase the instability of
cementite to the point where graphite is precipitated from solution instead.
Therefore primary austenite will separate out first until the eutectic tem-
perature (1131°C) is reached, but at that temperature the eutectic which
forms consists of austenite and graphite. This eutectic develops from nuclei
and is in the form of approximately spherical particles known as eutectic
cells. The layers of austenite and graphite develop in roughly radial form,
both being deposited directly from the liquid phase (Fig. 15.1). In Fig.
15.1 and Plate
15.
IA graphite appears to be in the form of separate flakes,
but in fact the eutectic cells are three-dimensional and roughly spherical
in shape so that the graphite layers can be regarded as something like those
shown in Fig. 15.2. That is, rather like a cluster of potato crisps growing
out from a central nucleus.
As in the case of the eutectoid point in steels (13.14) the eutectic point
in cast irons may be displaced to the left by the presence of other elements,
notably silicon. Thus, a cast iron containing less than 4.3% carbon may be
Plate 15.1 15.1A Grey cast
iron.
Graphite flakes in a matrix of pearlite. x 60. Etched in 4% picral.
15.1 B White cast
iron.
Primary cementite (white) and pearlite. x 100. Etched in 4% picral. (Courtesy of BCIRA).
liquid
austenite
pearlite
(i)Eutectic cell nuclei
form.
(it)Eutectic cells grow.
ill)Solidification complete
-graphite in an
austenite matrix.
(iv) Cooled to room temp.
-the austenite matrix
transforms to pearlite.
