Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.


aluminium, silicon and chromium, ionic mobility is low and since these
oxides tend also to be dense they offer good protection against further
oxidation. The first three of these elements tend to have deleterious effects
on the mechanical properties of steels in the quantities necessary to impart
corrosion resistance so that chromium—sometimes assisted by small
amounts of silicon—is used. These elements form barrier films of oxide
which adheres tenaciously to the surface.
13.72 The considerable amount of chromium required to effect oxida-
tion resistance unfortunately also favours rapid grain growth. Hence nickel
is often added to counteract this as in many other steels already dealt with
here.
However, nickel must be absent from those steels which are in con-
tact with atmospheres containing sulphur gases, since brittle grain boun-
dary films of nickel sulphide would be formed (21.20):
Ni-+!Ni
+
+! +2e~
S + 2e--+!S--j
nickel sulphide
13.73 The chromium present contributes to some extent to the high-
temperature strength by ordinary substitutional solid solution. However,
creep strength is generally increased by 'stiffening' the alloy with small
amounts of tungsten, molybdenum, vanadium, titanium, niobium, alu-
minium or nitrogen. Reduction in creep at high temperatures is then
achieved by dispersion hardening provided by the small particles (8.62) of
the carbides of niobium, tungsten, molybdenum, vanadium or titanium;
the nitrides of aluminium, titanium or niobium; and also NiAk.
Tungsten, molybdenum and cobalt also render steels resistant to temper-
ing effects at high temperatures as they do in high-speed steel (14.10), and
thus raise the limiting creep stress.
In some cases precipitation hardening can be used to strengthen the
alloy provided that at the working temperature, non-coherent precipitation
(9.92) does not occur since this would result in loss of strength and an
increase in brittleness. Generally, steels working at high temperatures are
either austenitic or austenitic/ferritic in structure so that brittle martensite
will not form each time the component cools, otherwise cracking in the
martensitic areas would be likely due to internal stress arising from non-
uniform cooling.
13.74 Short-time creep tests (5.72) are often employed in evaluating
heat-resistant alloys. Design is frequently based on the stress which will
produce a creep rate of 0.0001% per hour (1% extension in the gauge
length in 10 000 hours) at the maximum operating temperature. Safe work-
ing stresses are often based on 50% of this stress. However, such a value
can only be applied under conditions of direct axial static loading and
relatively uniform temperature. When impact loading or rapid temperature
changes are involved higher safety factors must be used, ie
33V3%
or even
25%
of the creep stress as derived above. The compositions and properties
of some typical heat-resisting steels are given in Table 13.6.
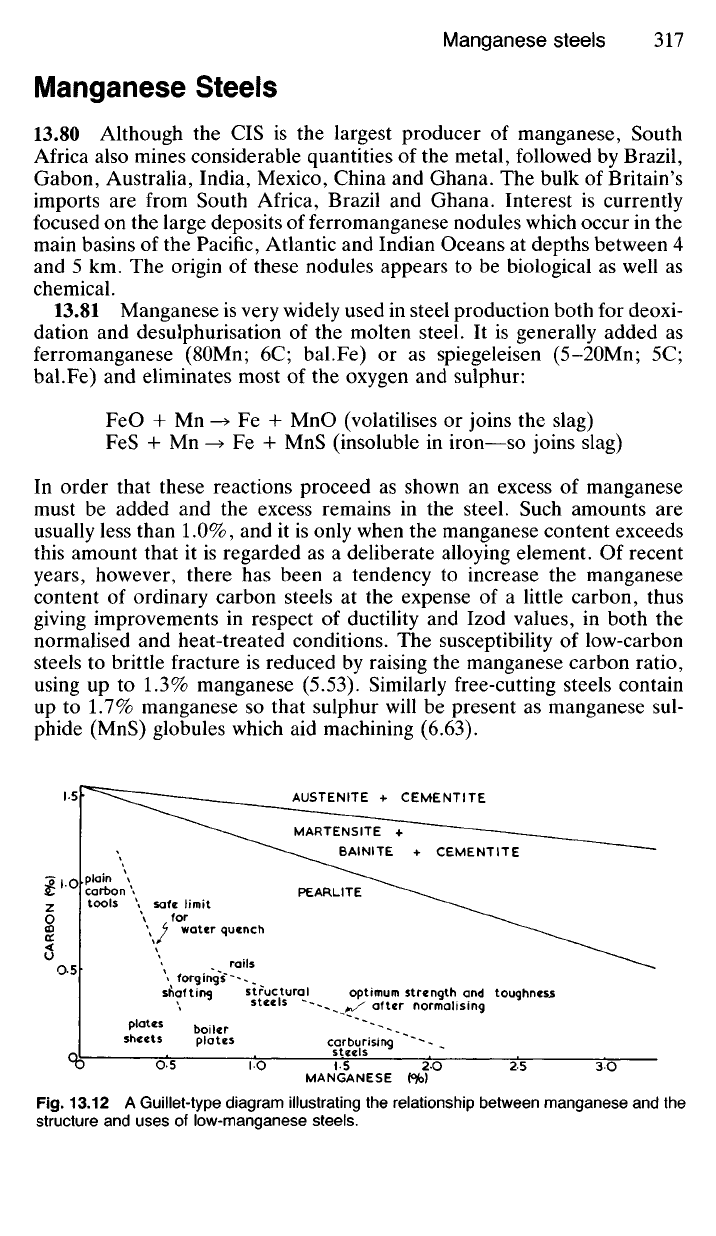
Manganese Steels
13.80 Although the CIS is the largest producer of manganese, South
Africa also mines considerable quantities of the metal, followed by Brazil,
Gabon, Australia, India, Mexico, China and Ghana. The bulk of Britain's
imports are from South Africa, Brazil and Ghana. Interest is currently
focused on the large deposits of ferromanganese nodules which occur in the
main basins of the Pacific, Atlantic and Indian Oceans at depths between 4
and 5 km. The origin of these nodules appears to be biological as well as
chemical.
13.81 Manganese is very widely used in steel production both for deoxi-
dation and desulphurisation of the molten steel. It is generally added as
ferromanganese (80Mn; 6C; bal.Fe) or as spiegeleisen (5-20Mn; 5C;
bal.Fe) and eliminates most of the oxygen and sulphur:
FeO + Mn
—»
Fe 4- MnO (volatilises or joins the slag)
FeS + Mn
—>
Fe + MnS (insoluble in iron—so joins slag)
In order that these reactions proceed as shown an excess of manganese
must be added and the excess remains in the steel. Such amounts are
usually less than 1.0%, and it is only when the manganese content exceeds
this amount that it is regarded as a deliberate alloying element. Of recent
years,
however, there has been a tendency to increase the manganese
content of ordinary carbon steels at the expense of a little carbon, thus
giving improvements in respect of ductility and Izod values, in both the
normalised and heat-treated conditions. The susceptibility of low-carbon
steels to brittle fracture is reduced by raising the manganese carbon ratio,
using up to 1.3% manganese (5.53). Similarly free-cutting steels contain
up to 1.7% manganese so that sulphur will be present as manganese sul-
phide (MnS) globules which aid machining (6.63).
CARBON
fpfo)
MANGANESE ftb)
Fig.
13.12 A Guillet-type diagram illustrating the relationship between manganese and the
structure and uses of low-manganese steels.
AUSTENlTE + CEMENTITE
MARTENSITE +
BAINITE + CEMENTITE
PEARLITE
plain
carbon
tools
safe limit
for
water quench
rails
optimum strength and toughness
after normalising
structural
steels
forgings
shafting
plates
sheets
boiler
plates
carburising
steels
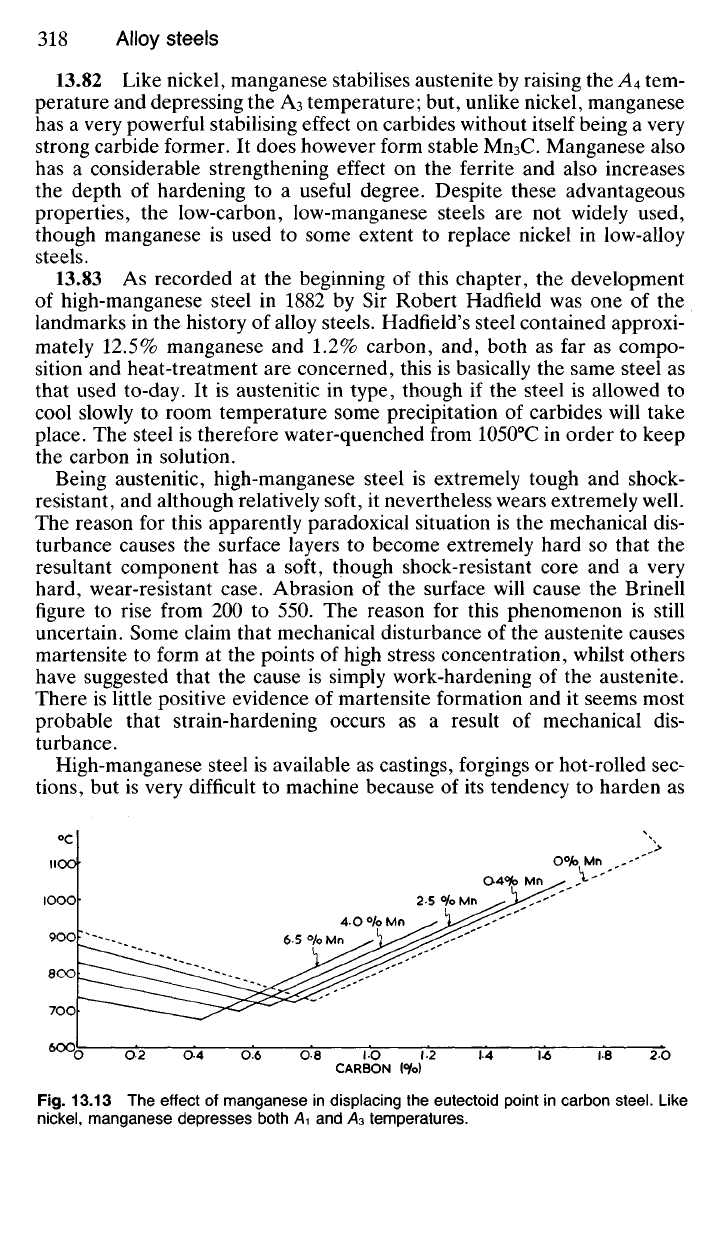
13.82 Like nickel, manganese stabilises austenite by raising the A
4
tem-
perature and depressing the A
3
temperature; but, unlike nickel, manganese
has a very powerful stabilising effect on carbides without itself being a very
strong carbide former. It does however form stable Mn
3
C. Manganese also
has a considerable strengthening effect on the ferrite and also increases
the depth of hardening to a useful degree. Despite these advantageous
properties, the low-carbon, low-manganese steels are not widely used,
though manganese is used to some extent to replace nickel in low-alloy
steels.
13.83 As recorded at the beginning of this chapter, the development
of high-manganese steel in 1882 by Sir Robert Hadfield was one of the
landmarks in the history of alloy steels. Hadfield's steel contained approxi-
mately 12.5% manganese and 1.2% carbon, and, both as far as compo-
sition and heat-treatment are concerned, this is basically the same steel as
that used to-day. It is austenitic in type, though if the steel is allowed to
cool slowly to room temperature some precipitation of carbides will take
place. The steel is therefore water-quenched from 1050
0
C in order to keep
the carbon in solution.
Being austenitic, high-manganese steel is extremely tough and shock-
resistant, and although relatively soft, it nevertheless wears extremely well.
The reason for this apparently paradoxical situation is the mechanical dis-
turbance causes the surface layers to become extremely hard so that the
resultant component has a soft, though shock-resistant core and a very
hard, wear-resistant case. Abrasion of the surface will cause the Brinell
figure to rise from 200 to 550. The reason for this phenomenon is still
uncertain. Some claim that mechanical disturbance of the austenite causes
martensite to form at the points of high stress concentration, whilst others
have suggested that the cause is simply work-hardening of the austenite.
There is little positive evidence of martensite formation and it seems most
probable that strain-hardening occurs as a result of mechanical dis-
turbance.
High-manganese steel is available as castings, forgings or hot-rolled sec-
tions,
but is very difficult to machine because of its tendency to harden as
°c
CARBON (<Yo)
Fig.
13.13 The effect of manganese in displacing the eutectoid point in carbon steel. Like
nickel,
manganese depresses both /\i and A
3
temperatures.
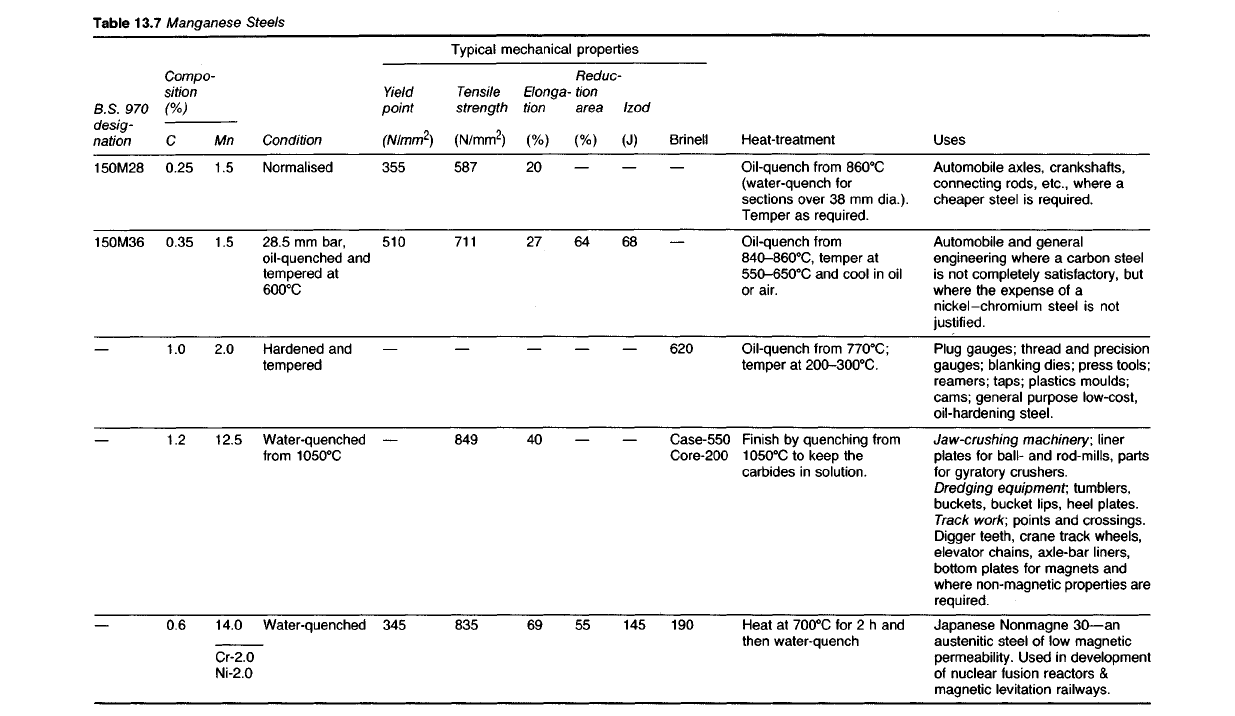
Table
13.7
Manganese
Steels
Uses
Automobile
axles, crankshafts,
connecting rods, etc., where a
cheaper steel is required.
Automobile
and general
engineering where a carbon steel
is
not completely satisfactory, but
where the expense of a
nickel-chromium steel is not
justified.
Plug gauges; thread and precision
gauges; blanking dies; press tools;
reamers; taps; plastics moulds;
cams; general purpose low-cost,
oil-hardening steel.
Jaw-crushing
machinery;
liner
plates for ball- and rod-mills, parts
for
gyratory crushers.
Dredging
equipment;
tumblers,
buckets,
bucket lips, heel plates.
Track
work;
points and crossings.
Digger teeth, crane track wheels,
elevator chains, axle-bar liners,
bottom
plates for magnets and
where non-magnetic properties are
required.
Japanese Nonmagne
30—an
austenitic steel of low magnetic
permeability. Used in development
of
nuclear fusion reactors &
magnetic levitation railways.
Heat-treatment
Oil-quench
from
860
0
C
(water-quench for
sections
over 38 mm dia.).
Temper as required.
Oil-quench from
840-860
0
C,
temper at
550-650
0
C
and cool in oil
or
air.
Oil-quench
from
770
0
C;
temper at
200-300
0
C.
Finish by quenching from
1050
0
C
to keep the
carbides in solution.
Heat at
700
0
C
for 2 h and
then water-quench
Typical mechanical properties
Brinell
620
Case-550
Core-200
190
Izod
(J)
68
145
Reduc-
tion
area
(%)
64
55
Elonga-
tion
(%)
20
27
40
69
Tensile
strength
(N/mm
2
)
587
711
849
835
Yield
point
(N/mm
2
)
355
510
345
Compo-
sition
(%)
Condition
Normalised
28.5 mm bar,
oil-quenched and
tempered at
600
0
C
Hardened and
tempered
Water-quenched
from
1050
0
C
Water-quenched
Mn
1.5
1.5
2.0
12.5
14.0
Cr-2.0
Ni-2.0
C
0.25
0.35
1.0
1.2
0.6
RS. 970
desig-
nation
150M28
150M36
soon as this is attempted. Despite this drawback, no really suitable substi-
tute has been found for Hadfield steel, for applications where toughness
combined with resistance to conditions of extreme abrasion are required,
eg rock-crushing machinery, dredging equipment and track-work points
and crossings. The austenitic core can be further toughened by the addition
of carbide-forming elements, such as chromium and vanadium.
Some manganese steels are shown in Table 13.7.
Steels Containing Tungsten
13.90 Up to the middle of the eighteenth century the name 'tungsten' (or
'heavy stone') implied the mineral scheelite, but in 1781 the renowned
chemist Scheele showed that scheelite contained a peculiar acid which he
called tungstic acid. Two years later metallic tungsten was isolated by J. J.
y Don Fausto d'Elhuyar. To-day tungsten is mined in many countries, the
leading producers being China, the CIS, Bolivia, South Korea, the USA,
North Korea, Thailand, Australia, Portugal and Canada. The bulk of
British imports are from Portugal. The principal ore of tungsten has always
been known as 'wolfram' but IUPAC (International Union of Pure and
Applied Chemistry) suggests that in future this name should be used to
describe the metal
itself.
However, in this edition we have decided to retain
the original name of 'tungsten'.
13.91 Tungsten dissolves in both y- and a-iron but, having a BCC
structure, tends to stabilise a (ferrite). It therefore raises the A
3
tempera-
ture and, like chromium, forms a closed y-loop in the phase diagram.
Unlike chromium, however, it inhibits grain growth and therefore has a
grain-refining effect. It also reduces decarburisation during working and
heat-treatment. Both of these features are useful since high temperatures
are involved during the heat-treatment of all tungsten steels.
Tungsten has a high affinity for carbon forming the extremely hard
and very stable carbides W2C and WC and, in steel, a double carbide
Fe4W2 C. These carbides dissolve very slowly in steel when the latter is
heated, and then only at very high temperatures. Once in solution the
dissolved tungsten renders transformations extremely sluggish. Therefore,
when successfully hardened, steels containing tungsten have a great resist-
ance to tempering conditions and can be heated in the range 600-700
0
C
before carbides begin to precipitate and softening sets in. For this reason
tungsten is an important constituent of most high-speed steels (14.10) and
hot-working die steels in which it develops high-temperature ('red') hard-
ness following suitable heat-treatment.
13.92 Since the carbides of tungsten are so hard, tungsten is commonly
used in small amounts in other tool steels (Table 13.8). It is also used in
heat-resisting steels and alloys in which it assists in raising the creep
strength at high temperatures.
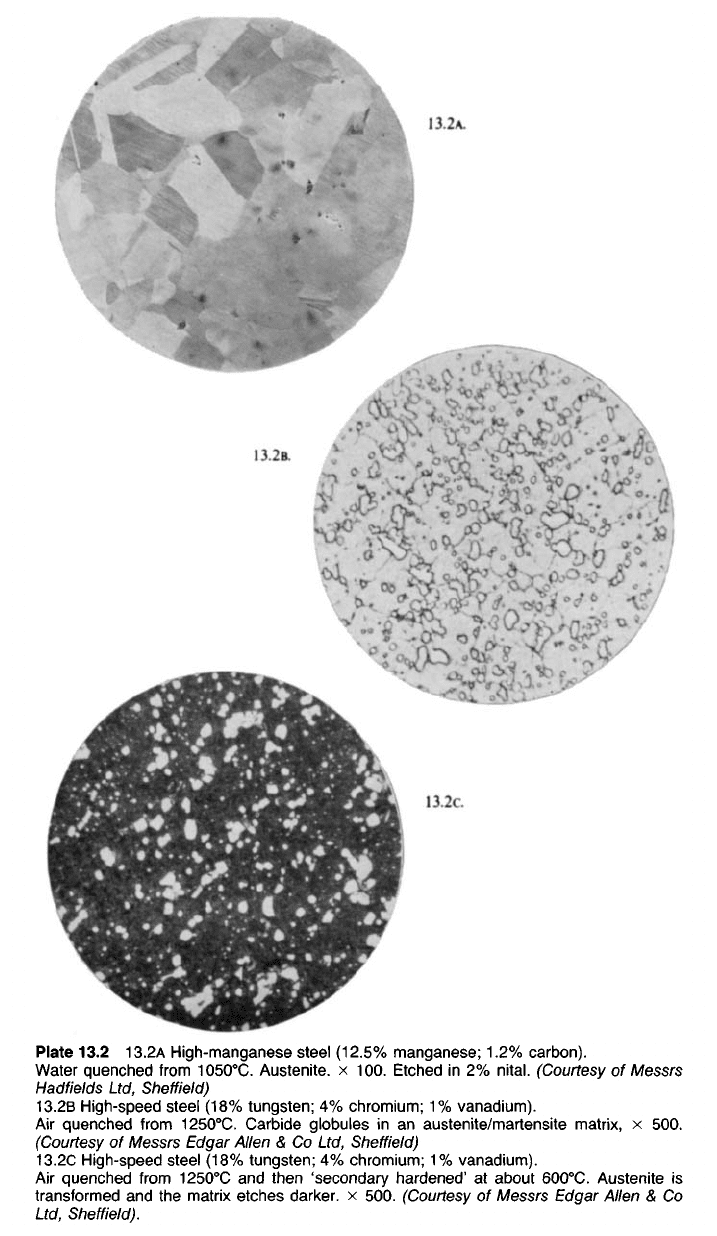
Plate 13.2 13.2A High-manganese steel (12.5% manganese; 1.2% carbon).
Water quenched from 1050
0
C. Austenite. x 100. Etched in 2%
nital.
(Courtesy of Messrs
Hadfields Ltd, Sheffield)
13.2B High-speed steel (18% tungsten; 4% chromium; 1% vanadium).
Air quenched from 1250
0
C. Carbide globules in an austenite/martensite matrix, x 500.
(Courtesy of Messrs Edgar Allen & Co Ltd, Sheffield)
13.2C High-speed steel (18% tungsten; 4% chromium; 1% vanadium).
Air quenched from 1250
0
C and then 'secondary hardened' at about 600
0
C. Austenite is
transformed and the matrix etches darker, x 500. (Courtesy of Messrs Edqar Allen & Co
Ltd, Sheffield).
13.2c
13.2B.
13.2A.
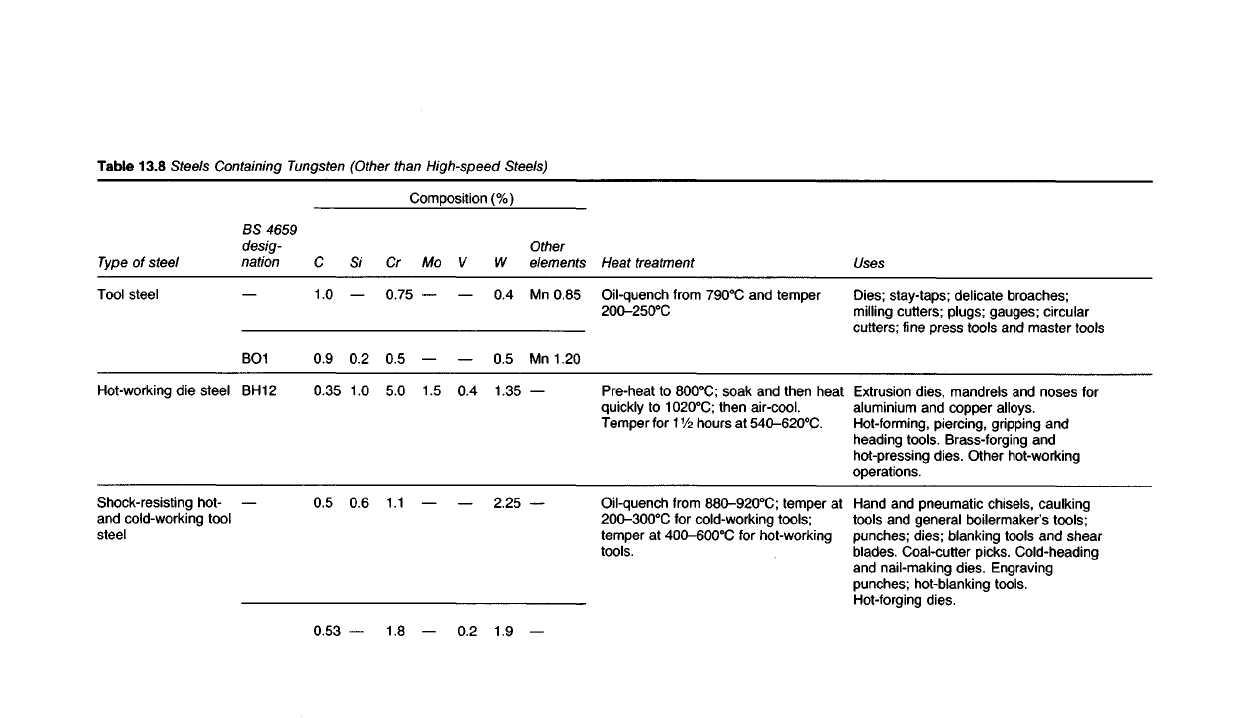
Table
13.8
Steels Containing Tungsten (Other than
High-speed
Steels)
Uses
Dies; stay-taps; delicate broaches;
milling
cutters; plugs;
gauges;
circular
cutters;
fine press tools
and
master tools
Extrusion
dies, mandrels
and
noses for
aluminium
and
copper alloys.
Hot-forming, piercing, gripping
and
heading tools. Brass-forging
and
hot-pressing dies. Other hot-working
operations.
Hand
and
pneumatic chisels, caulking
tools
and
general boilermaker's tools;
punches; dies; blanking tools
and
shear
blades. Coal-cutter picks.
Cold-heading
and nail-making dies. Engraving
punches; hot-blanking tools.
Hot-forging
dies.
Heat treatment
Oil-quench
from
790
0
C
and
temper
200-250°C
Pre-heat
to
800
0
C;
soak
and
then heat
quickly
to
1020
0
C;
then air-cool.
Temper
for
1V2
hours
at 540-620
0
C-
Oil-quench
from
880-920
0
C;
temper
at
200-300
0
C
for cold-working tools;
temper
at 400-600
0
C for
hot-working
tools.
Composition
(%)
Other
elements
Mn
0.85
Mn
1.20
W
0.4
0.5
1.35
2.25
1.9
V
0.4
0.2
Mo
1.5
Cr
0.75
0.5
5.0
1.1
1.8
S/
0.2
1.0
0.6
C
1.0
0.9
0.35
0.5
0.53
BS
4659
desig-
nation
BO1
BH12
Type
of
steel
Tool steel
Hot-working
die
steel
Shock-resisting
hot-
and cold-working tool
steel
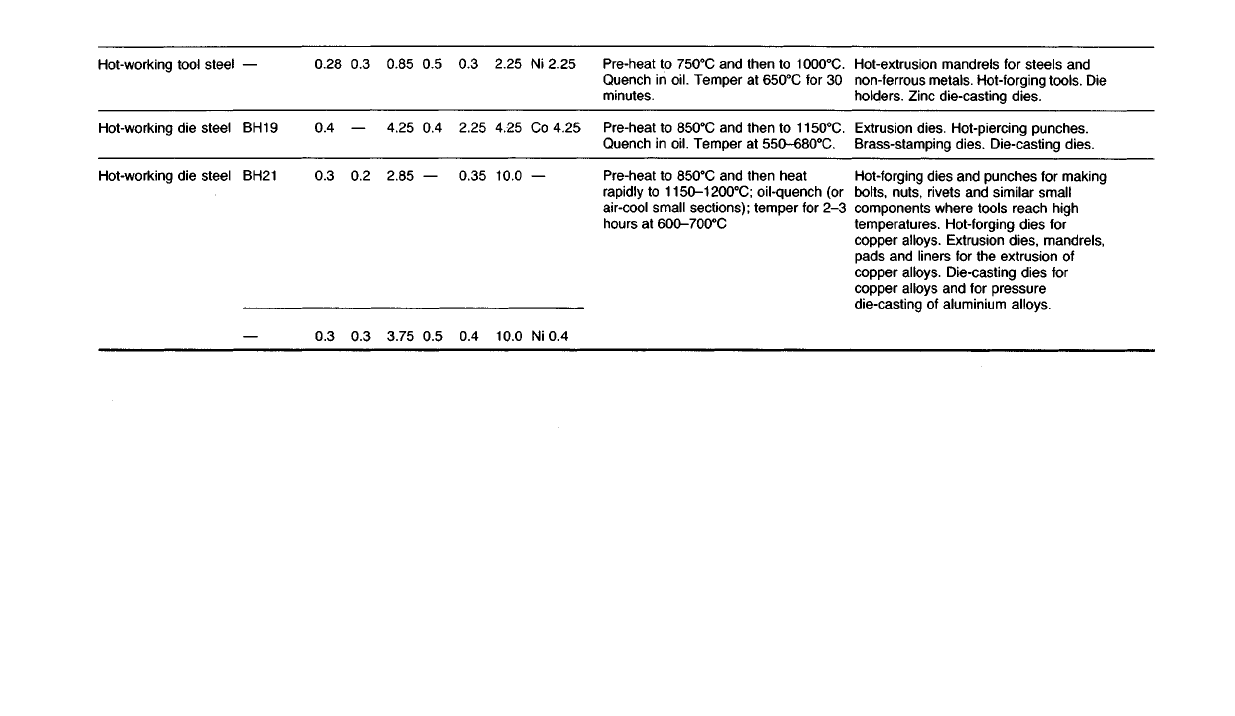
Hot-extrusion
mandrels for steels and
non-ferrous
metals. Hot-forging tools. Die
holders.
Zinc die-casting dies.
Extrusion
dies. Hot-piercing punches.
Brass-stamping dies. Die-casting dies.
Hot-forging
dies and punches for making
bolts,
nuts,
rivets
and similar small
components where tools reach high
temperatures. Hot-forging dies for
copper alloys. Extrusion dies, mandrels,
pads and liners for the extrusion of
copper alloys. Die-casting dies for
copper alloys and for pressure
die-casting of aluminium alloys.
Pre-heat to
750
0
C
and then to
1000
0
C.
Quench in oil.
Temper
at
650
0
C
for 30
minutes.
Pre-heat to
850
0
C
and then to
1150
0
C.
Quench in oil. Temper at
550-680
0
C.
Pre-heat to
850
0
C
and then heat
rapidly
to
1150-1200
0
C;
oil-quench (or
air-cool
small sections); temper for 2-3
hours
at
600-700
0
C
Ni 2.25
Co 4.25
Ni
0.4
2.25
4.25
10.0
10.0
0.3
2.25
0.35
0.4
0.5
0.4
0.5
0.85
4.25
2.85
3.75
0.3
0.2
0.3
0.28
0.4
0.3
0.3
BH19
BH21
Hot-working
tool steel
Hot-working die steel
Hot-working die steel
Steels Containing Cobalt
13.100 Much of the World's supply of cobalt is mined in Zaire whilst
significant amounts are produced in Morocco, Zambia and Canada.
Britain's main sources of supply are Zaire and Zambia.
Cobalt is chemically very similar to nickel and the ores of these metals
are generally associated with each other. Until recently cobalt was used
principally in permanent-magnet alloys (14.36) and in 'super' high-speed
steels (14.11). In the latter it gives a useful increase in red-hardness by
promoting extreme sluggishness in transformation.
13.101 Cobalt is an essential constituent of most 'maraging' steels
developed since the late nineteen-fifties. These are high-strength steels an
important group of which contains 18Ni; 8-12Co; 3-5Mo and small
amounts of titanium and aluminium. Carbon is present in very small
amounts.
If such a steel is solution treated at 820
0
C to absorb precipitated com-
pounds, uniform austenite is produced. On cooling in air, an iron-nickel
martensite is formed, but unlike ordinary tetragonal martensite (12.22)
this is of a lath-like BCC form and is much softer and tougher than ordinary
martensite. If the structure is now 'age-hardened' at 480
0
C for three or
more hours coherent precipitates of intermetallic compounds such as (Ti,
Al or Mo)Ni
3
are formed and a high tensile strength up to 2400N/mm
2
is
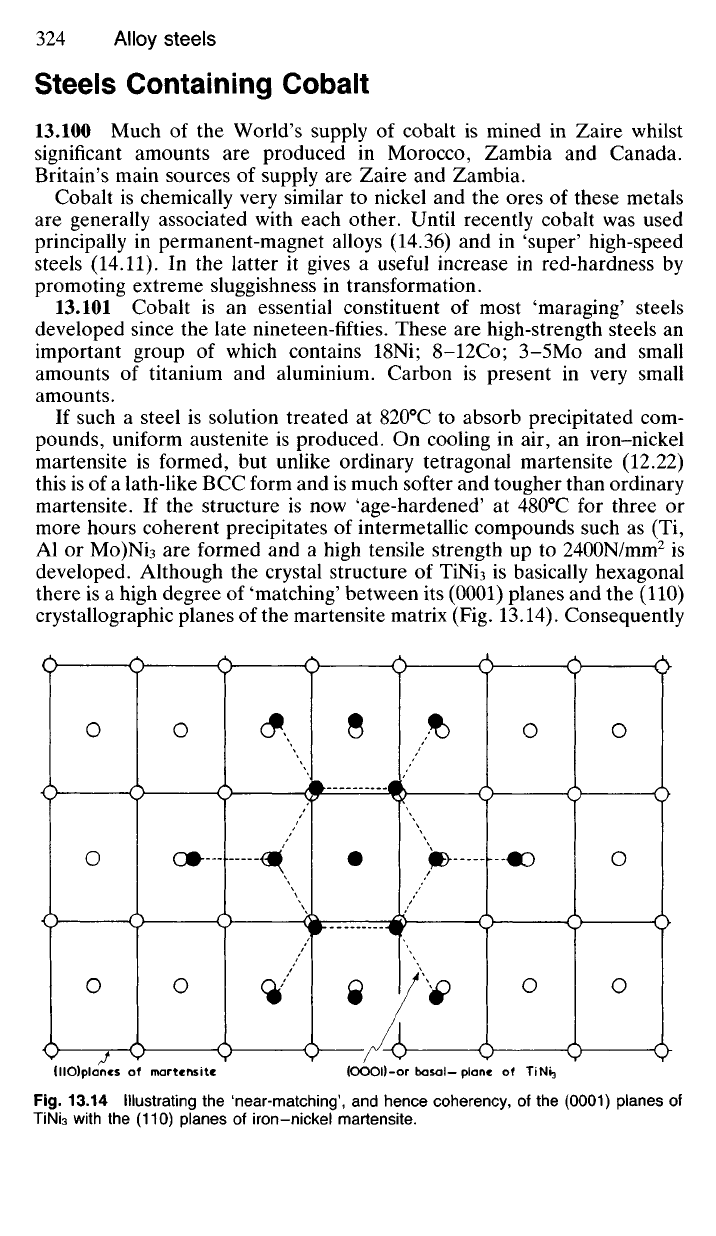
developed. Although the crystal structure of TiNi
3
is basically hexagonal
there is a high degree of 'matching' between its (0001) planes and the (110)
crystallographic planes of the martensite matrix (Fig. 13.14). Consequently
Fig.
13.14 Illustrating the 'near-matching', and hence coherency, of the (0001) planes of
TiNi
3
with the (110) planes of iron-nickel martensite.
(HO)planes of martensite
(OOOD-or basal-plane of
TiNi
3
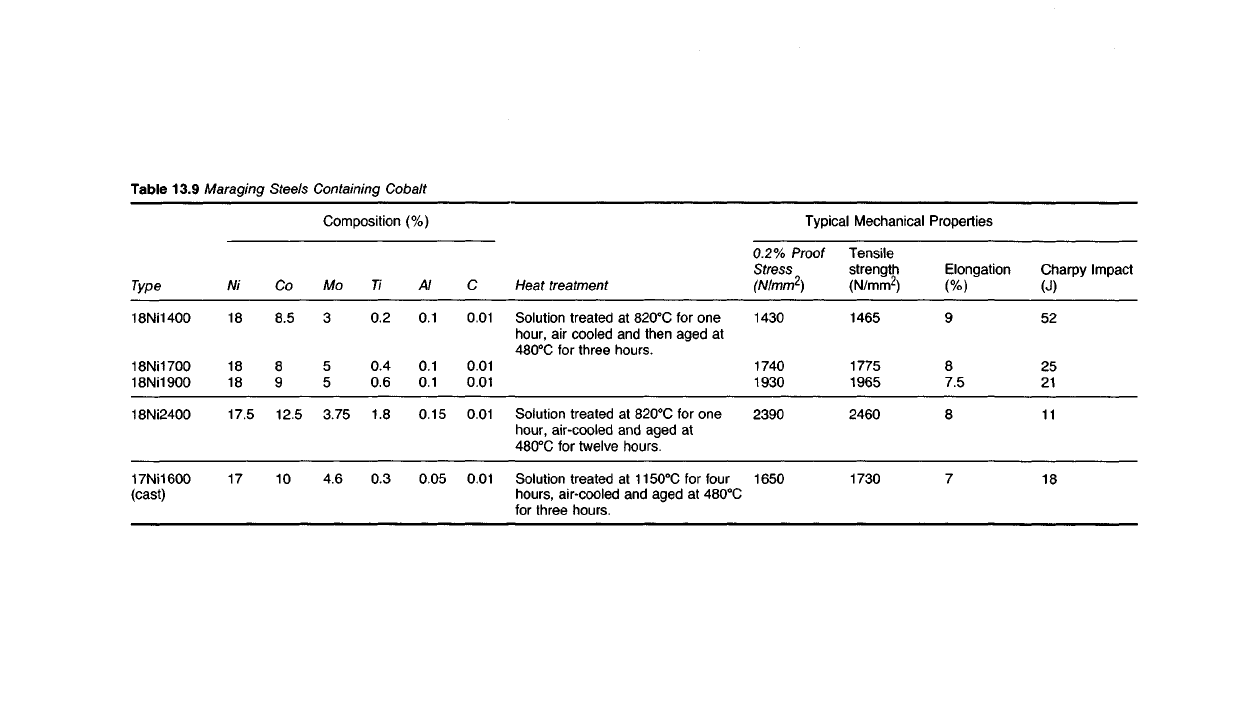
Table
13.9 Maraging
Steels
Containing Cobalt
Typical Mechanical Properties
Charpy Impact
(J)
52
25
21
11
18
Elongation
(%)
9
8
7.5
8
7
Tensile
strength
(N/mm
2
)
1465
1775
1965
2460
1730
0.2%
Proof
Stress
(N/mm
2
)
1430
1740
1930
2390
1650
Heat
treatment
Solution treated at 820
0
C for one
hour,
air cooled and then aged at
480
0
C for three hours.
Solution treated at 820
0
C for one
hour,
air-cooled
and aged at
480
0
C for twelve hours.
Solution treated at 1150
0
C for four
hours,
air-cooled and aged at 480
0
C
for
three hours.
Composition (%)
C
0.01
0.01
0.01
0.01
0.01
Al
0.1
0.1
0.1
0.15
0.05
Ti
0.2
0.4
0.6
1.8
0.3
Mo
3
5
5
3.75
4.6
Co
8.5
8
9
12.5
10
Ni
18
18
18
17.5
17
Type
18NM400
18Ni1700
18NM900
18Ni2400
17Ni1600
(cast)
