Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.

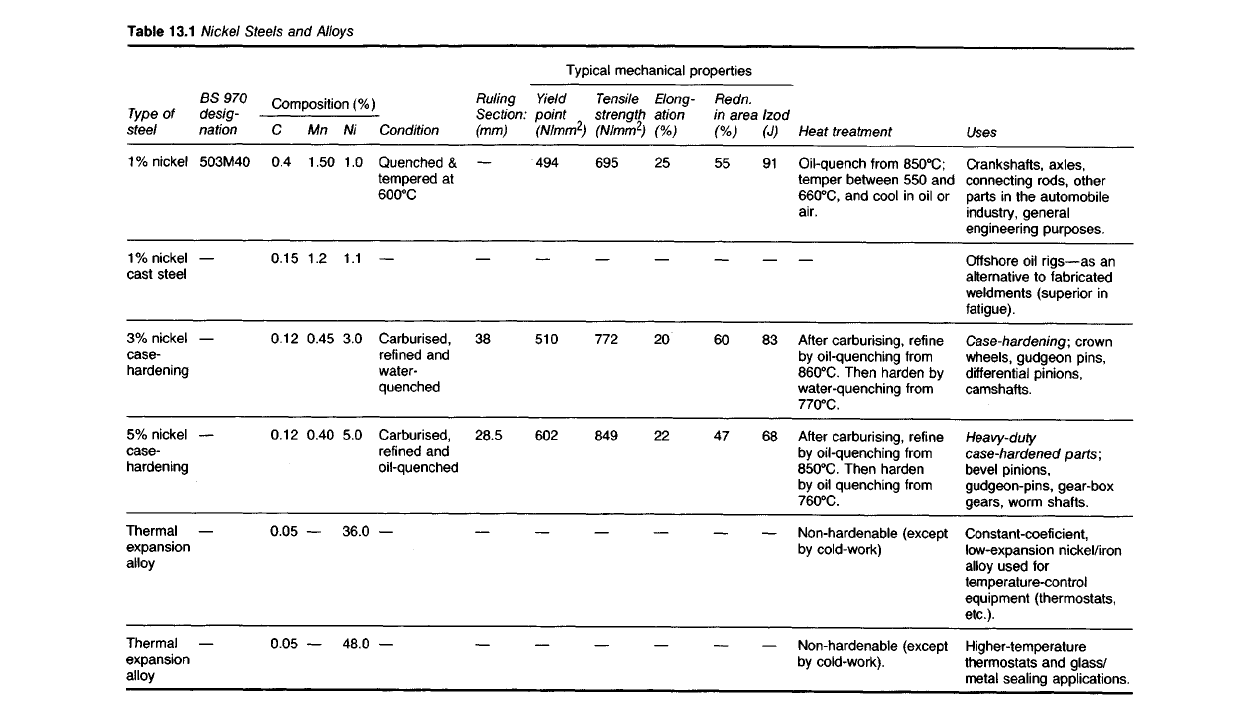
Table
13.1
Nickel
Steels and Alloys
Uses
Crankshafts, axles,
connecting rods, other
parts
in the automobile
industry,
general
engineering purposes.
Offshore
oil rigs—as an
alternative to fabricated
weldments (superior in
fatigue).
Case-hardening;
crown
wheels, gudgeon pins,
differential pinions,
camshafts.
Heavy-duty
case-hardened
parts;
bevel pinions,
gudgeon-pins, gear-box
gears, worm shafts.
Constant-coefi cient,
low-expansion nickel/iron
alloy used for
temperature-control
equipment (thermostats,
etc.).
Higher-temperature
thermostats and glass/
metal
sealing
applications.
Heat treatment
Oil-quench
from
850
0
C;
temper between 550 and
660
0
C,
and cool in oil or
air.
After
carburising, refine
by
oil-quenching
from
860
0
C.
Then
harden by
water-quenching
from
770
0
C.
After
carburising, refine
by
oil-quenching from
850
0
C.
Then harden
by
oil quenching
from
760
0
C.
Non-hardenable (except
by
cold-work)
Non-hardenable (except
by
cold-work).
Typical mechanical properties
Izod
(J)
91
83
68
Redn.
in area
(%)
55
60
47
Elong-
ation
(%)
25
20
22
Tensile
strength
(N/mm
2
)
695
772
849
Yield
point
(N/mm
2
)
494
510
602
Ruling
Section:
(mm)
38
28.5
Composition
(%)
Condition
Quenched &
tempered at
600
0
C
Carburised,
refined
and
water-
quenched
Carburised,
refined and
oil-quenched
Ni
1.0
1.1
3.0
5.0
36.0
48.0
Mn
1.50
1.2
0.45
0.40
C
0.4
0.15
0.12
0.12
0.05
0.05
BS 970
desig-
nation
503M40
Type
of
steel
1% nickel
1% nickel
cast steel
3% nickel
case-
hardening
5% nickel
case-
hardening
Thermal
expansion
alloy
Thermal
expansion
alloy
but these have been largely replaced by nickel-chromium; nickel-molyb-
denum or nickel-chromium-molybdenum steels.
13.25 Nickel reduces the coefficient of thermal expansion of iron-
nickel alloys progressively, until with 36% nickel expansion is extremely
small. Invar' (36Ni; C-less than 0.1) was developed originally for accurate
measuring tapes used in land surveys. In 1920 C. E. Guillaume received
the Nobel Prize for Physics in recognition for its invention. It was also used
for pendulum rods in master clocks and similar alloys are now employed for
such diverse applications as the lining of tanks in vessels carrying 'liquid
natural gas' and in the many types of thermostat in modern heating
equipment.
Another valuable property of the high-nickel alloys is high magnetic
permeability (14.30). An alloy containing 51 Ni-49Fe has a similar
coef-
ficient of expansion to that of glass making it useful in the production of
'reed switches'. In this simple device (Fig. 13.9) two 'reeds' are aligned
and then sealed in a closed glass envelope containing an inert gas. To close
the switch a magnetic field is applied from outside the tube, the reeds
operating as simple cantilever springs. The switch is used extensively in
semi-electronic telephone exchanges.
Fig.
13.9 A miniature 'reeef switch using a 51 Ni-49 Fe alloy.
Chromium Steels
13.30 The bulk of metallic chromium produced is used in the manufacture
of alloy steels and in the electro-plating industry. The main producers
of chromium are South Africa, CIS, Albania, Turkey, Zimbabwe, the
Philippines and India. Britain's chief imports of the metal are from South
Africa and the Philippines.
13.31 It is often assumed that the addition of chromium to a steel will
automatically increase its hardness, but this can only take place when
sufficient carbon is present. The increase in hardness is due mainly to the
fact that chromium is a carbide stabiliser and forms the hard carbides Cr
7
C
3
or O23C6 or, alternatively, double carbides with iron. All of these carbides
are harder than ordinary cementite. In low-carbon steels the addition of
chromium increases strength, with some loss in ductility, due to its forming
a solid solution in ferrite.
13.32 Chromium lowers the
AA
temperature and raises the A3 tempera-
ture,
forming the closed y-loop already mentioned. In this way it stabilises
the a-phase at the expense of the y-phase. The latter is eliminated entirely,
as shown in Fig. 13.10 if more than 11% chromium is added to pure
iron, though with carbon steels a greater amount of chromium would be
'reeds
glass tube
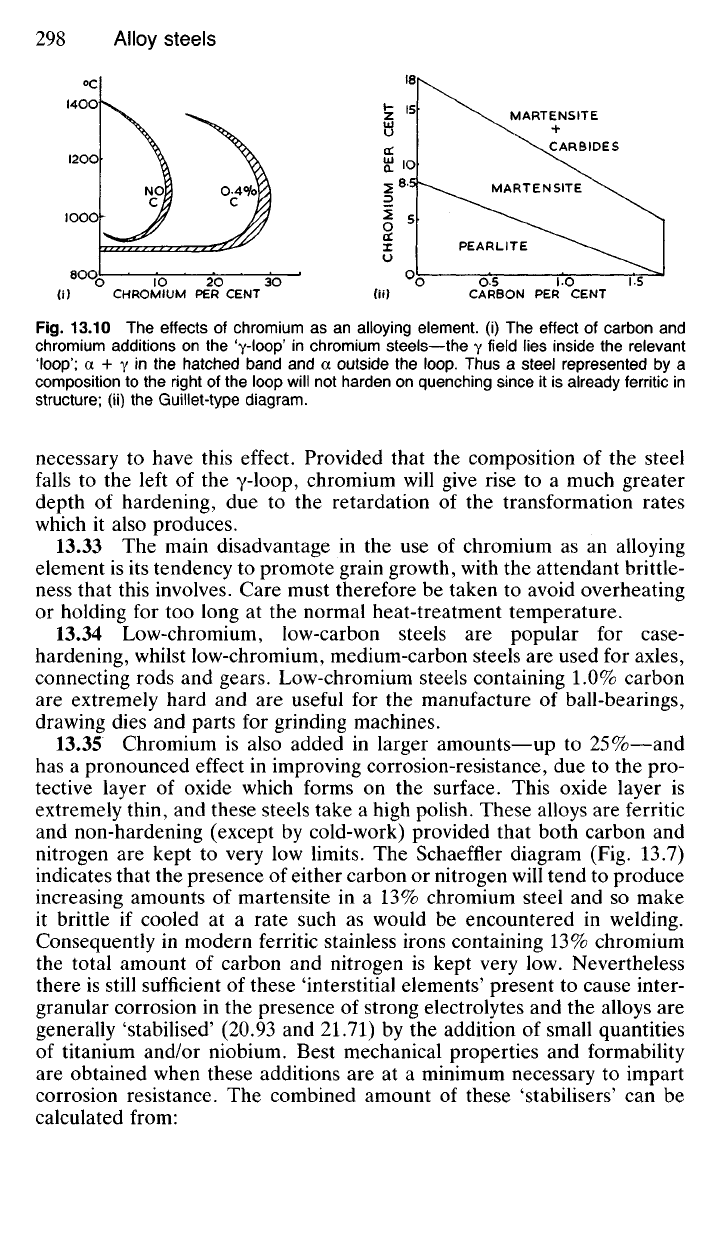
Fig.
13.10 The effects of chromium as an alloying element, (i) The effect of carbon and
chromium additions on the yioop' j
n
chromium steels—the y field lies inside the relevant
'loop';
a + Y in the hatched band and a outside the loop. Thus a steel represented by a
composition to the right of the loop will not harden on quenching since it is already ferritic in
structure; (ii) the Guillet-type diagram.
necessary to have this effect. Provided that the composition of the steel
falls to the left of the y-loop, chromium will give rise to a much greater
depth of hardening, due to the retardation of the transformation rates
which it also produces.
13.33 The main disadvantage in the use of chromium as an alloying
element is its tendency to promote grain growth, with the attendant brittle-
ness that this involves. Care must therefore be taken to avoid overheating
or holding for too long at the normal heat-treatment temperature.
13.34 Low-chromium, low-carbon steels are popular for case-
hardening, whilst low-chromium, medium-carbon steels are used for axles,
connecting rods and gears. Low-chromium steels containing 1.0% carbon
are extremely hard and are useful for the manufacture of ball-bearings,
drawing dies and parts for grinding machines.
13.35 Chromium is also added in larger amounts—up to 25%—and
has a pronounced effect in improving corrosion-resistance, due to the pro-
tective layer of oxide which forms on the surface. This oxide layer is
extremely thin, and these steels take a high polish. These alloys are ferritic
and non-hardening (except by cold-work) provided that both carbon and
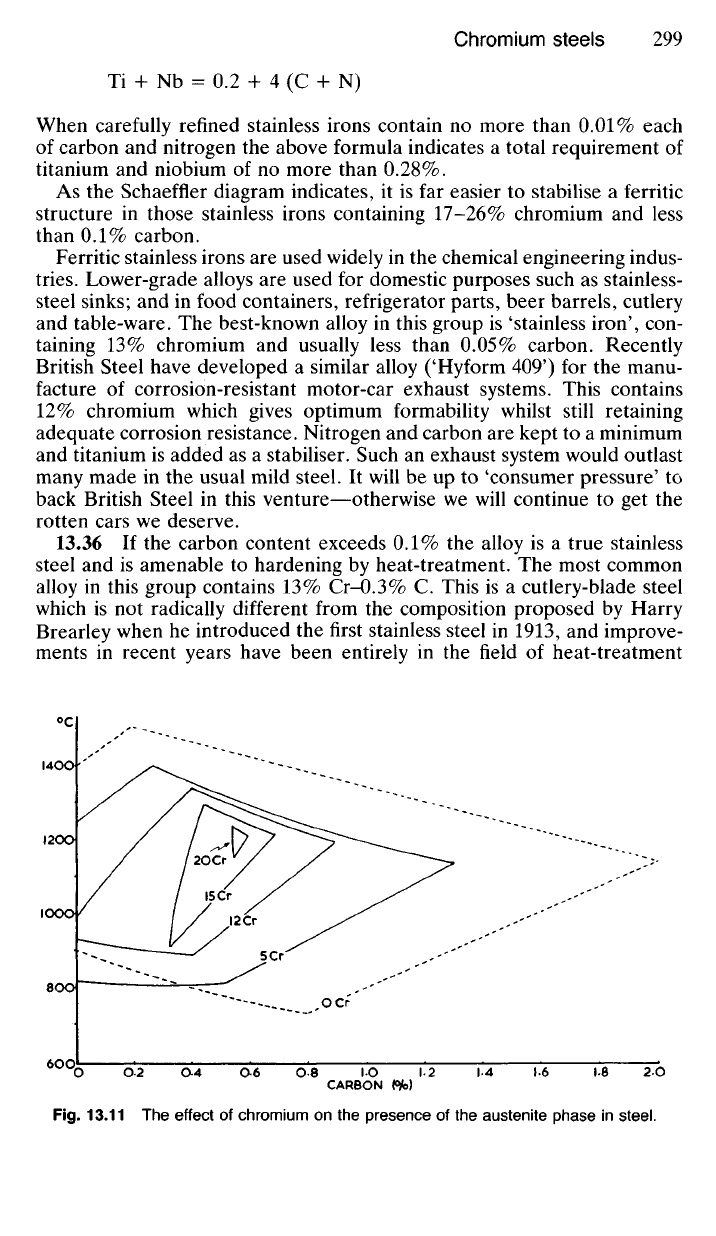
nitrogen are kept to very low limits. The Schaeffler diagram (Fig. 13.7)
indicates that the presence of either carbon or nitrogen will tend to produce
increasing amounts of martensite in a 13% chromium steel and so make
it brittle if cooled at a rate such as would be encountered in welding.
Consequently in modern ferritic stainless irons containing 13% chromium
the total amount of carbon and nitrogen is kept very low. Nevertheless
there is still sufficient of these 'interstitial elements' present to cause inter-
granular corrosion in the presence of strong electrolytes and the alloys are
generally 'stabilised' (20.93 and 21.71) by the addition of small quantities
of titanium and/or niobium. Best mechanical properties and formability
are obtained when these additions are at a minimum necessary to impart
corrosion resistance. The combined amount of these 'stabilisers' can be
calculated from:
CHROMIUM PER
CENT
CHROMIUM PER CENT
oc
MARTENSITE
CARBIDES
MARTENSITE
PEARLITE
CARBON PER CENT
When carefully refined stainless irons contain no more than 0.01% each
of carbon and nitrogen the above formula indicates a total requirement of
titanium and niobium of no more than 0.28%.
As the Schaeffler diagram indicates, it is far easier to stabilise a ferritic
structure in those stainless irons containing 17-26% chromium and less
than 0.1% carbon.
Ferritic stainless irons are used widely in the chemical engineering indus-
tries.
Lower-grade alloys are used for domestic purposes such as stainless-
steel sinks; and in food containers, refrigerator parts, beer barrels, cutlery
and table-ware. The best-known alloy in this group is 'stainless iron', con-
taining 13% chromium and usually less than 0.05% carbon. Recently
British Steel have developed a similar alloy ('Hyform 409') for the manu-
facture of corrosion-resistant motor-car exhaust systems. This contains
12%
chromium which gives optimum formability whilst still retaining
adequate corrosion resistance. Nitrogen and carbon are kept to a minimum
and titanium is added as a stabiliser. Such an exhaust system would outlast
many made in the usual mild steel. It will be up to 'consumer pressure' to
back British Steel in this venture—otherwise we will continue to get the
rotten cars we deserve.
13.36 If the carbon content exceeds 0.1% the alloy is a true stainless
steel and is amenable to hardening by heat-treatment. The most common
alloy in this group contains 13%
Cr-0.3%
C. This is a cutlery-blade steel
which is not radically different from the composition proposed by Harry
Brearley when he introduced the first stainless steel in 1913, and improve-
ments in recent years have been entirely in the field of heat-treatment
0
C
CARBON (Ph)
Fig.
13.11 The effect of chromium on the presence of the austenite phase in steel.
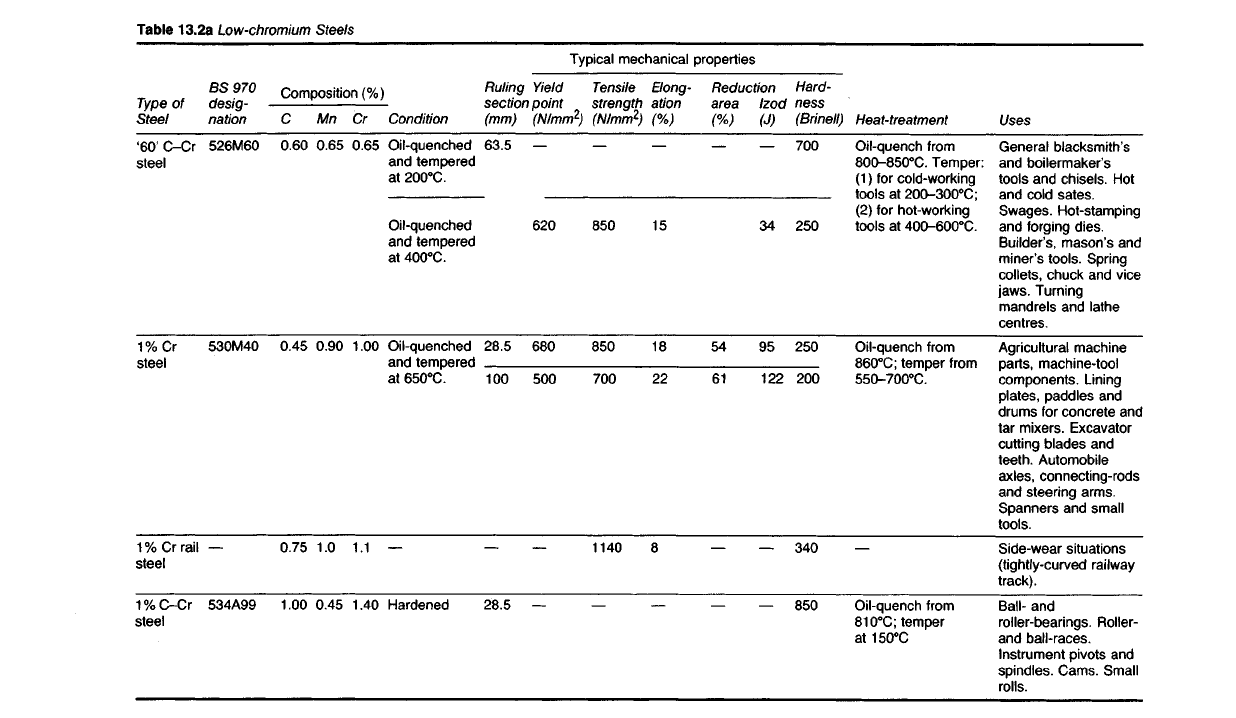
Table
13.2a
Low-chromium Steels
Uses
General blacksmith's
and boilermaker's
tools
and
chisels.
Hot
and cold sates.
Swages. Hot-stamping
and forging dies.
Builder's, mason's
and
miner's tools. Spring
collets,
chuck
and
vice
jaws.
Turning
mandrels
and
lathe
centres.
Agricultural
machine
parts,
machine-tool
components. Lining
plates, paddles
and
drums for concrete
and
tar
mixers. Excavator
cutting blades
and
teeth. Automobile
axles, connecting-rods
and steering arms.
Spanners
and
small
tools.
Side-wear situations
(tightly-curved railway
track).
Ball-
and
roller-bearings. Roller-
and ball-races.
Instrument pivots
and
spindles. Cams. Small
rolls.
Heat-treatment
Oil-quench
from
800-850
0
C.
Temper:
(1)
for cold-working
tools
at 200-300
0
C;
(2) for hot-working
tools
at 400-600
0
C.
Oil-quench
from
860
0
C;
temper from
550-700
0
C.
Oil-quench
from
810
0
C;
temper
at
150
0
C
Typical mechanical properties
Hard-
ness
(Brinell)
700
250
250
~200
340
850
Reduction
area Izod
(%)
(J)
34
54
95
61
122
Elong-
ation
15
18
22
8
Tensile
strength
850
850
700
1140
Yield
point
(N/mm
2
)
620
680
500
Ruling
section
(mm)
63.5
28.5
100
28.5
Condition
Oil-quenched
and tempered
at
200
0
C.
Oil-quenched
and tempered
at
400
0
C.
Oil-quenched
and tempered
at
650
0
C.
Hardened
Composition
(%)
C
Mn Cr
0.60
0.65 0.65
0.45
0.90 1.00
0.75
1.0 1.1
1.00
0.45 1.40
BS
970
desig-
nation
526M60
530M40
534A99
Type
of
Steel
'60'
C-Cr
steel
1%Cr
steel
1%
Cr
rail
steel
1%C-Cr
steel
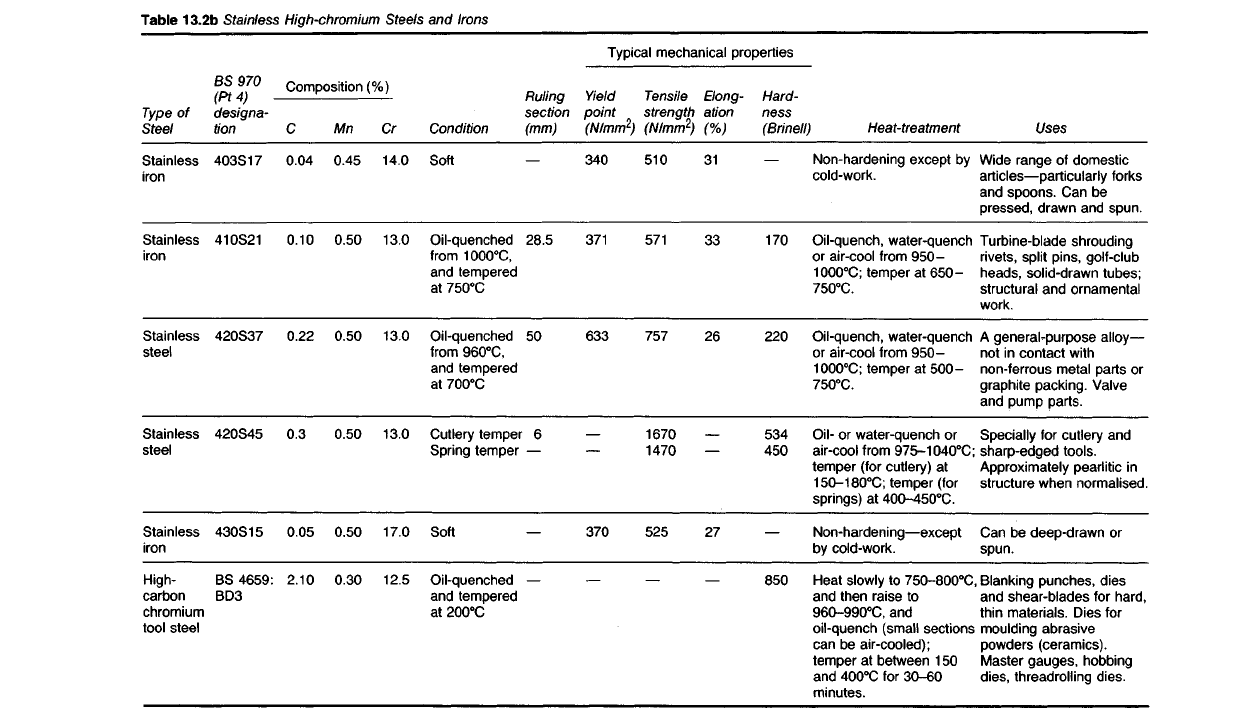
Table
13.2b Stainless
High-chromium
Steels and Irons
Uses
Wide range of domestic
articles—particularly
forks
and spoons. Can be
pressed,
drawn and spun.
Turbine-blade shrouding
rivets,
split
pins, golf-club
heads, solid-drawn tubes;
structural
and ornamental
work.
A
general-purpose alloy—
not
in contact with
non-ferrous
metal parts or
graphite packing. Valve
and pump parts.
Specially for cutlery and
sharp-edged tools.
Approximately
pearlitic in
structure
when normalised.
Can be deep-drawn or
spun.
Blanking punches, dies
and shear-blades for hard,
thin
materials. Dies for
moulding abrasive
powders
(ceramics).
Master
gauges,
hobbing
dies, threadrolling dies.
Heat-treatment
Non-hardening except by
cold-work.
Oil-quench, water-quench
or
air-cool
from
950-
1000
0
C;
temper at 650-
75O
0
C.
Oil-quench, water-quench
or
air-cool
from
950-
1000
0
C;
temper at 500-
750
0
C.
Oil- or water-quench or
air-cool
from
975-1040
0
C;
temper
(for cutlery) at
150-180
0
C;
temper (for
springs)
at
400-450
0
C.
Non-hardening—except
by
cold-work.
Heat slowly to
750-800
0
C
1
and then raise to
960-990
0
C,
and
oil-quench (small sections
can be air-cooled);
temper
at
between
150
and
400
0
C
for
30-60
minutes.
Typical mechanical properties
Hard-
ness
(Brinell)
170
220
534
450
850
Elong-
ation
(%)
31
33
26
27
Tensile
strength
(N/mm
2
)
510
571
757
1670
1470
525
Yield
point
(N/mm
2
)
340
371
633
370
Ruling
section
(mm)
28.5
50
6
Condition
Soft
Oil-quenched
from
1000
0
C,
and tempered
at
750
0
C
Oil-quenched
from
960
0
C,
and tempered
at
700
0
C
Cutlery
temper
Spring
temper
Soft
Oil-quenched
and tempered
at
200
0
C
Composition (%)
Cr
14.0
13.0
13.0
13.0
17.0
12.5
Mn
0.45
0.50
0.50
0.50
0.50
0.30
C
0.04
0.10
0.22
0.3
0.05
2.10
BS 970
(Pt
4)
designa-
tion
403S17
410S21
420S37
420S45
430S15
BS
4659:
BD3
Type
of
Steel
Stainless
iron
Stainless
iron
Stainless
steel
Stainless
steel
Stainless
iron
High-
carbon
chromium
tool steel
enabling much better cutting edges to be obtained. Consequently in post-
war years these steels have been developed for razor blades, hand tools,
garden tools and food-processing equipment as well as for knife blades.
Due to displacement of the eutectoid point to the left, this 13Cr-0.3 C
steel is of approximately eutectoid composition when annealed and slowly
cooled. It has a martensitic structure when air-hardened but it is essential
that the hardening temperature is high enough (975-1040
0
C) or undis-
solved carbides may be present after hardening, giving rise to electrolytic
corrosion. With higher carbon contents the formation of brittle carbide
networks is more likely particularly in structures which have been annealed
and then inadequately solution treated. This type of carbide precipitation
leaves the matrix locally depleted in chromium so that it becomes anodic
(21.71) to the remainder of the chromium-rich structure and electrolytic
corrosion follows.
Compositions and properties of the more important chromium steels are
given in Tables 13.2a and b.
Nickel-Chromium Steels
13.40 The addition of either nickel or chromium singly to a steel can have
some adverse effects. Whilst nickel tends to inhibit grain growth during
heat-treatment, chromium accelerates it, thus producing brittleness under
shock. Meanwhile, chromium tends to form stable carbides, making it
possible to produce high-chromium, high-carbon steels, whilst nickel has
the reverse effect in promoting graphitisation. The deleterious effects of
each element can be overcome, therefore, if we add them in conjunction
with each other. Then, the tendency of chromium to cause grain growth
is nullified by the grain-refining effect of the nickel, whilst the tendency of
nickel to favour graphitisation of the carbides is counteracted by the strong
carbide-forming tendency of the chromium.
13.41 At the same time other physical effects of each element are
additive, as for example, increase in strength due to the formation of
substitutional solid solutions in the ferrite; increase in corrosion resistance
and also the retardation of transformation rates during heat-treatment.
This latter effect is particularly useful in rendering drastic water-quenches
avoidable.
13.42 In general, the low-nickel, low-chromium steels contain rather
more than two parts of nickel to one part of chromium. Those with up to
3.5Ni and 1.5Cr are oil-hardened from 810-850
0
C, followed by tempering
at 150-650
0
C according to the properties required. Some of these steels
suffer from 'temper brittleness' when tempered in the range 250-400
0
C.
This is shown by low resistance to shock in impact tests. If tempered above
400
0
C,
therefore, it is necessary to cool the steel quickly in oil through the
range in which temper-brittleness develops. The effect is minimised but
not completely eliminated by adding small amounts of molybdenum
(13.50),
thus producing the range of well-known 'nickel-chrome-moly'
steels.
In fact most of the steels in this group now contain molybdenum.

13.43 The nickel-chromium steels are very adaptable and useful alloys.
They forge well and also machine well in the softened condition whilst
their mechanical properties can be varied considerably by the treatment
given. When the nickel content is increased to about 4% and chromium
to about 1.5% an air-hardening steel is obtained. Such a steel is very useful
for the manufacture of complex shapes which have to be hardened, and
which would be likely to distort if water- or oil-quenching were attempted.
13.44 The high-nickel, high-chromium steels are all stainless alloys con-
taining less than 0.1% carbon which is virtually a troublesome impurity,
expensive to reduce below 0.04%. The most popular is the
'18-8'
stainless
steel with 18Cr-8Ni. The introduction of nickel to a ferritic 18% Cr alloy
considerably enlarges the y-loop of the phase diagram. It also decreases the
M
s
temperature so that with 8% Ni the M
s
temperature is below ambient
temperatures and austenite is therefore retained after slow cooling. 18-8
stainless steel takes a good polish and resists corrosion by many relatively
corrosive organic and inorganic reagents. When cold-worked these austen-
itic steels strain harden quickly and it was more than twenty years after
their introduction before they became widely used for domestic purposes.
Improved tool design and shaping methods solved many of the production
problems but even so a 12Cr-12Ni alloy is far more ductile and still suf-
ficiently corrosion resistant for use as table ware.
13.45 Chromium has a relatively high affinity for oxygen and conse-
quently oxidises very easily. Nevertheless the oxide film which forms
rapidly on the surface of chromium, though extremely thin, is also very
stable and strongly adherent to the surface which it therefore protects from
further attack. When in solid solution, either in ferrite or in austenite, it
bestows these corrosion-resisting properties upon iron particularly when
more than 12% chromium is present. Although the film is extremely thin
it builds up immediately the surface is polished.
In the presence of concentrated nitric acid, a powerful oxidising agent,
it has been shown that stainless steel begins to dissolve almost as quickly
as would mild steel but that, immediately, a thin oxide film is formed
providing a protective passive layer. As long as oxidising conditions are
present in the environment the film repairs itself should abrasion take
place, but in the presence of non-oxidising corrosive media such as concen-
trated hydrochloric acid or strong chloride solutions, corrosion may occur,
particularly in impingement attack where the oxide film is broken and
is unable to repair itself because of the absence of oxidising conditions.
Fortunately 8-10% Ni renders stainless steel more resistant to attack by
hydrochloric acid, chloride solutions and other non-oxidising media—yet
another instance where the action of nickel and chromium is complemen-
tary and indicating why 18-8 stainless steels are so widely used both in the
chemical industries and elsewhere.
13.46 The carbon content is kept as low as is economically possible
since the presence of precipitated carbides in the microstructure reduces
corrosion-resistance. Even with a carbon content below 0.1% slow cooling
of the steel to ambient temperature will cause carbide precipitation to
take place, and this considerably reduces corrosion-resistance, because the
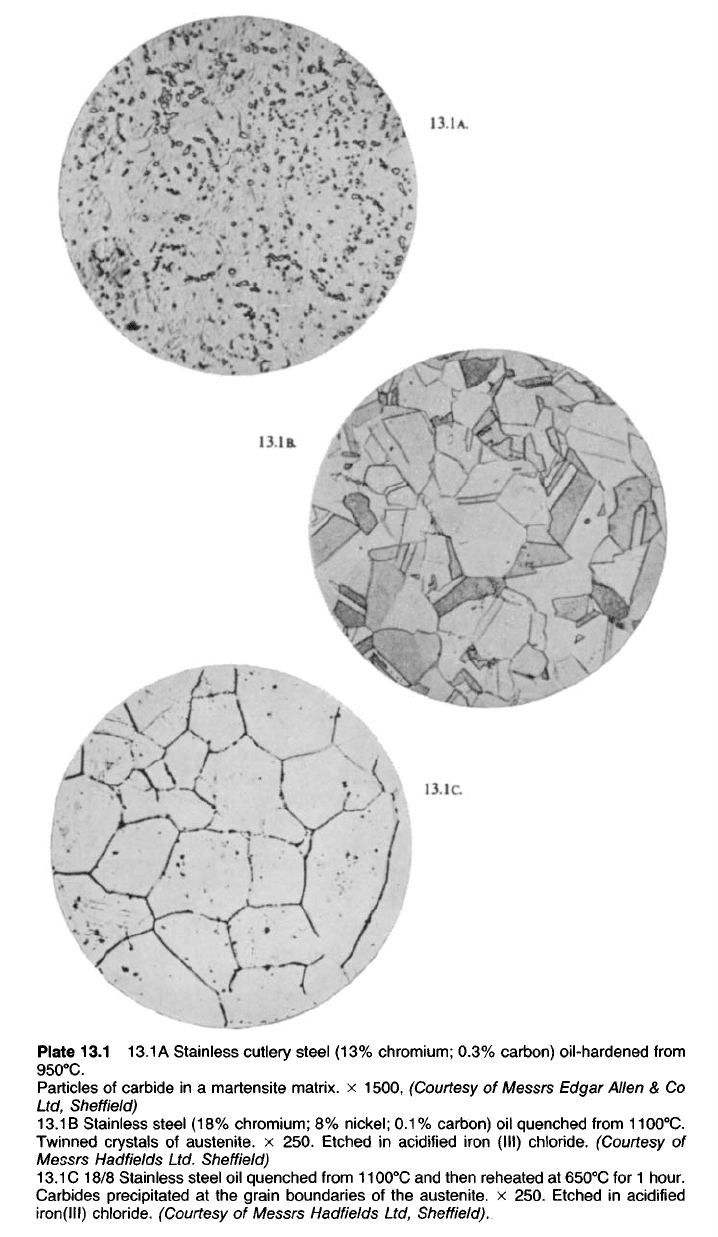
Plate 13.1
13.1
A Stainless cutlery steel (13% chromium; 0.3% carbon) oil-hardened from
950
0
C.
Particles of carbide in a martensite matrix, x 1500, (Courtesy of Messrs Edgar Allen & Co
Ltd, Sheffield)
13.1 B Stainless steel (18% chromium; 8% nickel; 0.1% carbon) oil quenched from 1100
0
C.
Twinned crystals of austenite. x 250. Etched in acidified iron (III) chloride. (Courtesy of
Messrs Hadfields Ltd. Sheffield)
13.1
C 18/8 Stainless steel oil quenched from 1100
0
C and then reheated at 650
0
C for 1 hour.
Carbides precipitated at the grain boundaries of the austenite. x 250. Etched in acidified
iron(lll) chloride. (Courtesy of Messrs Hadfields Ltd, Sheffield).
13.1c
13.1a
13.1A.
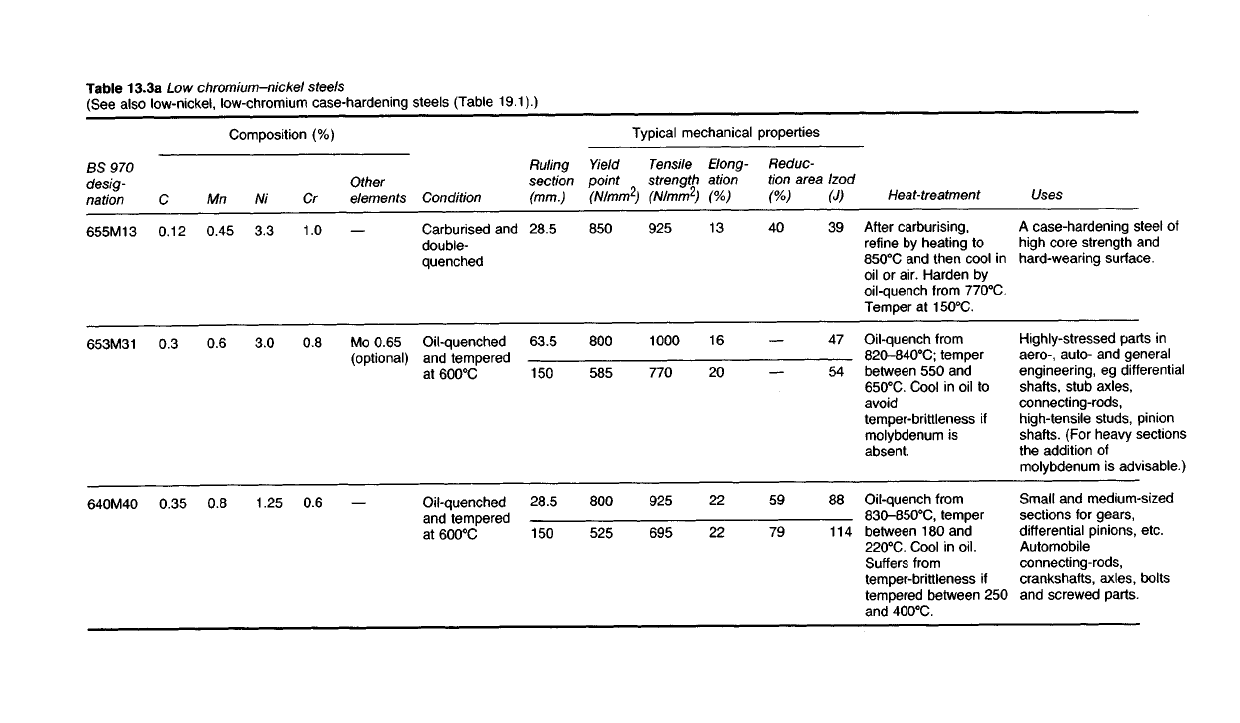
Table 13.3a Low chromium-nickel
steels
(See
also low-nickel, low-chromium case-hardening steels (Table 19.1).)
Uses
A
case-hardening steel of
high core strength and
hard-wearing surface.
Highly-stressed parts in
aero-, auto- and general
engineering, eg differential
shafts,
stub axles,
connecting-rods,
high-tensile
studs, pinion
shafts.
(For heavy sections
the addition of
molybdenum is advisable.)
Small
and medium-sized
sections for gears,
differential pinions, etc.
Automobile
connecting-rods,
crankshafts, axles, bolts
and screwed parts.
Heat-treatment
After
carburising,
refine
by heating to
850
0
C and then cool in
oil or air. Harden by
oil-quench
from
770
0
C.
Temper at 150
0
C.
Oil-quench from
820-840
0
C;
temper
between
550 and
650
0
C. Cool in oil to
avoid
temper-brittleness if
molybdenum is
absent.
Oil-quench
from
830-850
0
C,
temper
between
180 and
220
0
C. Cool in oil.
Suffers
from
temper-brittleness if
tempered between 250
and 400
0
C.
Typical
mechanical properties
Izod
(J)
39
47
88
114
Reduc-
tion
area
(%)
40
59
79
Elong-
ation
13
16
20
22
22
Tensile
strength
(N/mm
2
)
925
1000
770
925
695
Yield
point
(N/mm
2
)
850
800
585
800
525
Ruling
section
(mm.)
28.5
63.5
150
28.5
150
Condition
Carburised and
double-
quenched
Oil-quenched
and tempered
at
600
0
C
Oil-quenched
and tempered
at
600
0
C
Composition (%)
Other
elements
Mo
0.65
(optional)
Cr
1.0
0.8
0.6
Ni
3.3
3.0
1.25
Mn
0.45
0.6
0.8
C
0.12
0.3
0.35
BS
970
desig-
nation
655M13
653M31
640M40
