Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.


Thus the addition of small quantities of nickel and chromium will produce
a general improvement in the basic mechanical properties of strength and
toughness, whilst larger amounts of these elements will introduce new
phenomena such as the stabilisation of austenite at ambient temperatures,
accompanied by the loss of ferromagnetism and, of course, a very high
resistance to corrosion. The alloying elements added may either simply
dissolve in the ferrite or they may combine with some of the carbon,
forming carbides, which associate with the iron carbide already present.
The decision to use alloy steels will not be taken lightly since they are
expensive materials as compared with plain carbon steels. This is to be
expected when it is realised that the unit costs of metals like nickel and
chromium are many times that of ordinary medium carbon steel. Neverthe-
less this extra cost may often be partly offset by the greater ease with
which most alloy steels can be heat-treated, making possible automatic
programming of the heat-treatment cycle with the use of relatively
unskilled labour.
The principal effects which alloying elements have on the microstructure
and properties of a steel can be classified as follows:
13.11 The Effect on the Polymorphic* Transformation Tempera-
tures The polymorphic transformation temperatures which concern us
here are those at 910
0
C where the a ^± y transformation occurs; and at
1400
0
C where the y ^ 6 change takes place. That is, when BCC (a) iron
is heated above 910
0
C it transforms to FCC (y) iron and if heated further
to 1400
0
C it changes again to BCC (6) iron. These transformations are
reversible on cooling. The temperatures 910
0
C and 1400
0
C are designated
A
3
and A
4
respectively (Fig. 13.1). (The A\ temperature is at 723°C—the
'lower critical temperature'—where the austenite ^± pearlite transforma-
tion occurs in plain-carbon steels; whilst the A
2
temperature is at 769°C,
the Curie point, above which pure iron ceases to be ferromagnetic. The
Curie point has no metallographic significance.)
Some elements, notably nickel, manganese, cobalt and copper, raise the
A
4
temperature and lower A
3
as shown in Fig. 13.1A. Therefore these
elements, when added to a carbon steel tend to stabilise austenite (y) still
further and increase the range of temperature over which austenite can
exist as a stable phase. Other elements, the most important of which
include chromium, tungsten, vanadium, molybdenum, aluminium and sili-
con, have the reverse effect, in that they tend to stabilise ferrite (a) by
raising the A
3
temperature and lowering the A
4
, as indicated in Fig. 13.1B.
Such elements restrict the field over which austenite may exist, and thus
form what is commonly called a 'gamma (y) loop'.
Many of the elements of the austenite-stabilising group have a FCC
crystal structure like that of austenite. They therefore dissolve substi-
tutionally with ease in austenite and consequently resist and retard the
transformation of austenite to ferrite. Carbon itself has the same effect on
the y
—>
a transformation in iron, as indicated in the iron-carbon diagram,
* The term 'allotropic' is often used to describe transformations of this
type.
However, 'polymorphic'
is
more precise since we refer to changes in
crystal
structures only, in this instance.
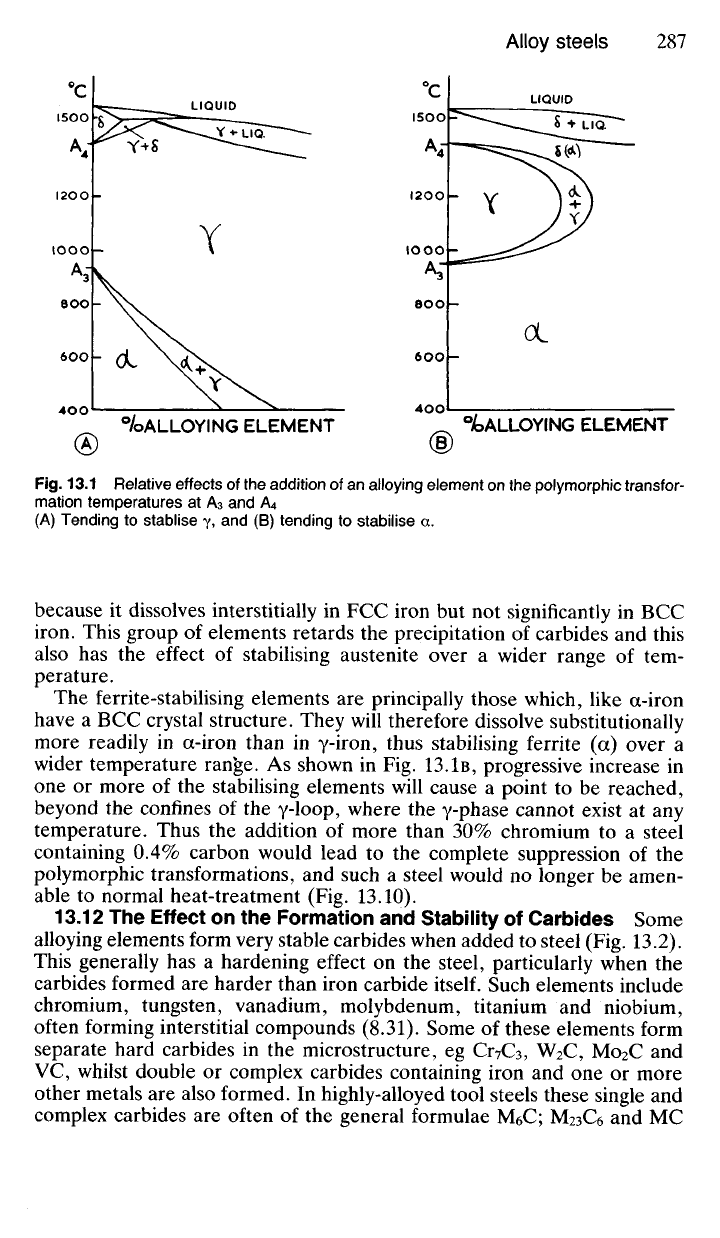
Fig.
13.1 Relative effects
of
the addition
of an
alloying element
on the
polymorphic transfor-
mation
temperatures
at A
3
and A
4
(A)
Tending
to
stablise
y, and (B)
tending
to
stabilise
a.
because
it
dissolves interstitially
in FCC
iron
but not
significantly
in BCC
iron. This group
of
elements retards
the
precipitation
of
carbides
and
this
also
has the
effect
of
stabilising austenite over
a
wider range
of tem-
perature.
The ferrite-stabilising elements
are
principally those which, like a-iron
have
a BCC
crystal structure. They will therefore dissolve substitutionally
more readily
in
a-iron than
in
y-iron, thus stabilising ferrite
(a)
over
a
wider temperature range.
As
shown
in Fig. 13.1B,
progressive increase
in
one
or
more
of the
stabilising elements will cause
a
point
to be
reached,
beyond
the
confines
of the
yloop, where
the
y-phase cannot exist
at any
temperature. Thus
the
addition
of
more than
30%
chromium
to a
steel
containing
0.4%
carbon would lead
to the
complete suppression
of the
polymorphic transformations,
and
such
a
steel would
no
longer
be
amen-
able
to
normal heat-treatment
(Fig.
13.10).
13.12
The
Effect
on the
Formation
and
Stability
of
Carbides Some
alloying elements form very stable carbides when added
to
steel (Fig. 13.2).
This generally
has a
hardening effect
on the
steel, particularly when
the
carbides formed
are
harder than iron carbide
itself.
Such elements include
chromium, tungsten, vanadium, molybdenum, titanium
and
niobium,
often forming interstitial compounds (8.31). Some
of
these elements form
separate hard carbides
in the
microstructure,
eg
Cr
7
Cs,
W
2
C,
Mo
2
C
and
VC,
whilst double
or
complex carbides containing iron
and one or
more
other metals
are
also formed.
In
highly-alloyed tool steels these single
and
complex carbides
are
often
of the
general formulae
M
6
C;
M
23
C
6
and MC
0
^ALLOYING ELEMENT
°/oALLOYING
ELEMENT
0
C
LIQUID
°c
LIQUID
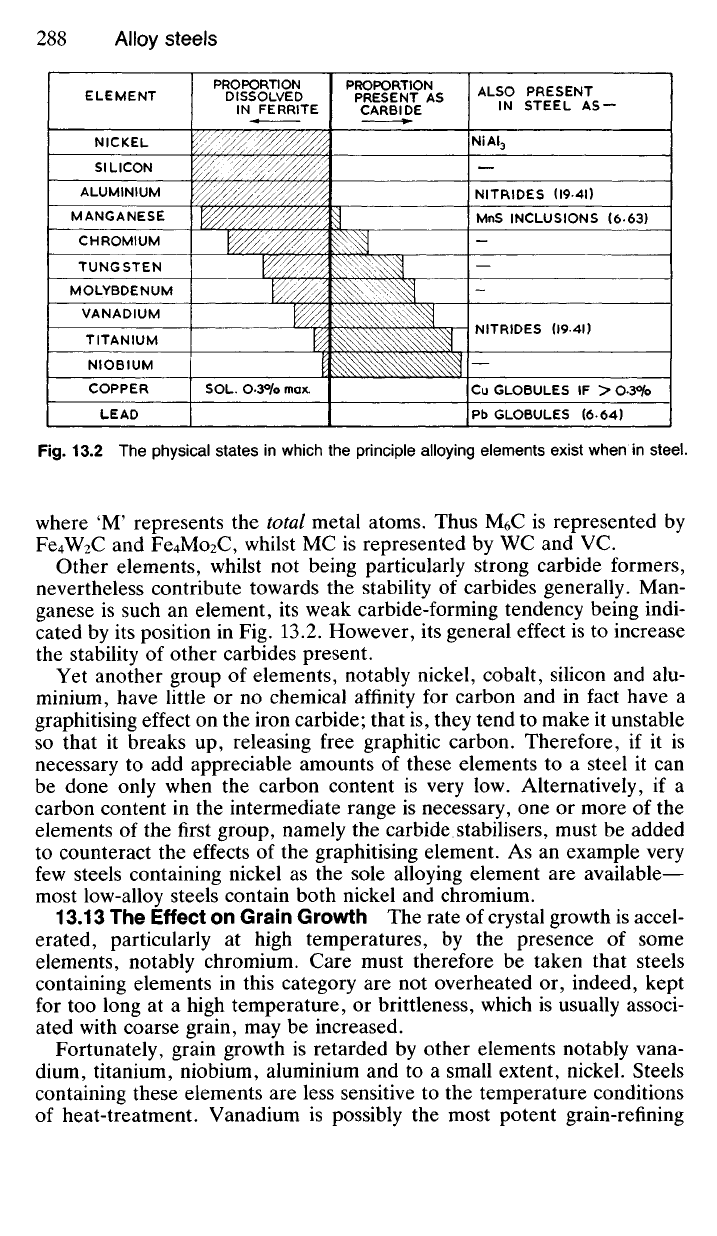
Fig.
13.2 The physical states in which the principle alloying elements exist when in steel.
where 'M' represents the total metal atoms. Thus M
6
C is represented by
Fe
4
W
2
C and Fe
4
Mo
2
C, whilst MC is represented by WC and VC.
Other elements, whilst not being particularly strong carbide formers,
nevertheless contribute towards the stability of carbides generally. Man-
ganese is such an element, its weak carbide-forming tendency being indi-
cated by its position in Fig. 13.2. However, its general effect is to increase
the stability of other carbides present.
Yet another group of elements, notably nickel, cobalt, silicon and alu-
minium, have little or no chemical affinity for carbon and in fact have a
graphitising effect on the iron carbide; that is, they tend to make it unstable
so that it breaks up, releasing free graphitic carbon. Therefore, if it is
necessary to add appreciable amounts of these elements to a steel it can
be done only when the carbon content is very low. Alternatively, if a
carbon content in the intermediate range is necessary, one or more of the
elements of the first group, namely the carbide stabilisers, must be added
to counteract the effects of the graphitising element. As an example very
few steels containing nickel as the sole alloying element are available—
most low-alloy steels contain both nickel and chromium.
13.13 The Effect on Grain Growth The rate of crystal growth is accel-
erated, particularly at high temperatures, by the presence of some
elements, notably chromium. Care must therefore be taken that steels
containing elements in this category are not overheated or, indeed, kept
for too long at a high temperature, or brittleness, which is usually associ-
ated with coarse grain, may be increased.
Fortunately, grain growth is retarded by other elements notably vana-
dium, titanium, niobium, aluminium and to a small extent, nickel. Steels
containing these elements are less sensitive to the temperature conditions
of heat-treatment. Vanadium is possibly the most potent grain-refining
ALSO PRESENT
IN STEEL AS —
NiAI
3
NITRIDES (I94I)
MnS INCLUSIONS (663)
NITRIDES (I9-4I)
Cu GLOBULES IF > O3°/o
Pb GLOBULES (664)
ELEMENT
NICKEL
SILICON
ALUMINIUM
MANGANESE
CHROMIUM
TUNGSTEN
MOLYBDENUM
VANADIUM
TITANIUM
NIOBIUM
COPPER
LEAD
PROPORTION
DISSOLVED
IN FERRITE
PROPORTION
PRESENT AS
CARBIDE
SOL. O.3°/o max.
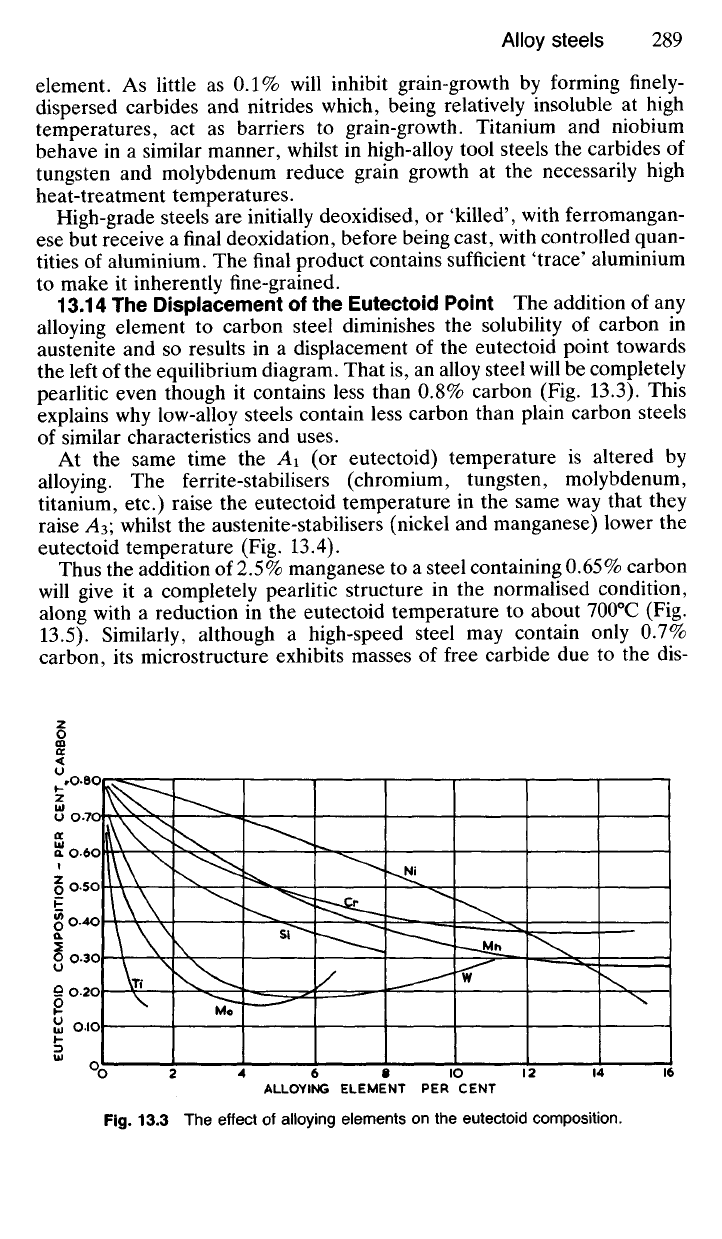
element. As little as 0.1% will inhibit grain-growth by forming finely-
dispersed carbides and nitrides which, being relatively insoluble at high
temperatures, act as barriers to grain-growth. Titanium and niobium
behave in a similar manner, whilst in high-alloy tool steels the carbides of
tungsten and molybdenum reduce grain growth at the necessarily high
heat-treatment temperatures.
High-grade steels are initially deoxidised, or 'killed', with ferromangan-
ese but receive a final deoxidation, before being cast, with controlled quan-
tities of aluminium. The final product contains sufficient 'trace' aluminium
to make it inherently fine-grained.
13.14 The Displacement of the Eutectoid Point The addition of any
alloying element to carbon steel diminishes the solubility of carbon in
austenite and so results in a displacement of the eutectoid point towards
the left of the equilibrium diagram. That is, an alloy steel will be completely
pearlitic even though it contains less than 0.8% carbon (Fig. 13.3). This
explains why low-alloy steels contain less carbon than plain carbon steels
of similar characteristics and uses.
At the same time the Ai (or eutectoid) temperature is altered by
alloying. The ferrite-stabilisers (chromium, tungsten, molybdenum,
titanium, etc.) raise the eutectoid temperature in the same way that they
raise A
3
; whilst the austenite-stabilisers (nickel and manganese) lower the
eutectoid temperature (Fig. 13.4).
Thus the addition of 2.5% manganese to a steel containing 0.65% carbon
will give it a completely pearlitic structure in the normalised condition,
along with a reduction in the eutectoid temperature to about 700
0
C (Fig.
13.5).
Similarly, although a high-speed steel may contain only 0.7%
carbon, its microstructure exhibits masses of free carbide due to the dis-
EUTECTOlD
COMPOSITION - PER CENT.
CARBON
ALLOYING ELEMENT PER CENT
Fig.
13.3 The effect of alloying elements on the eutectoid composition.
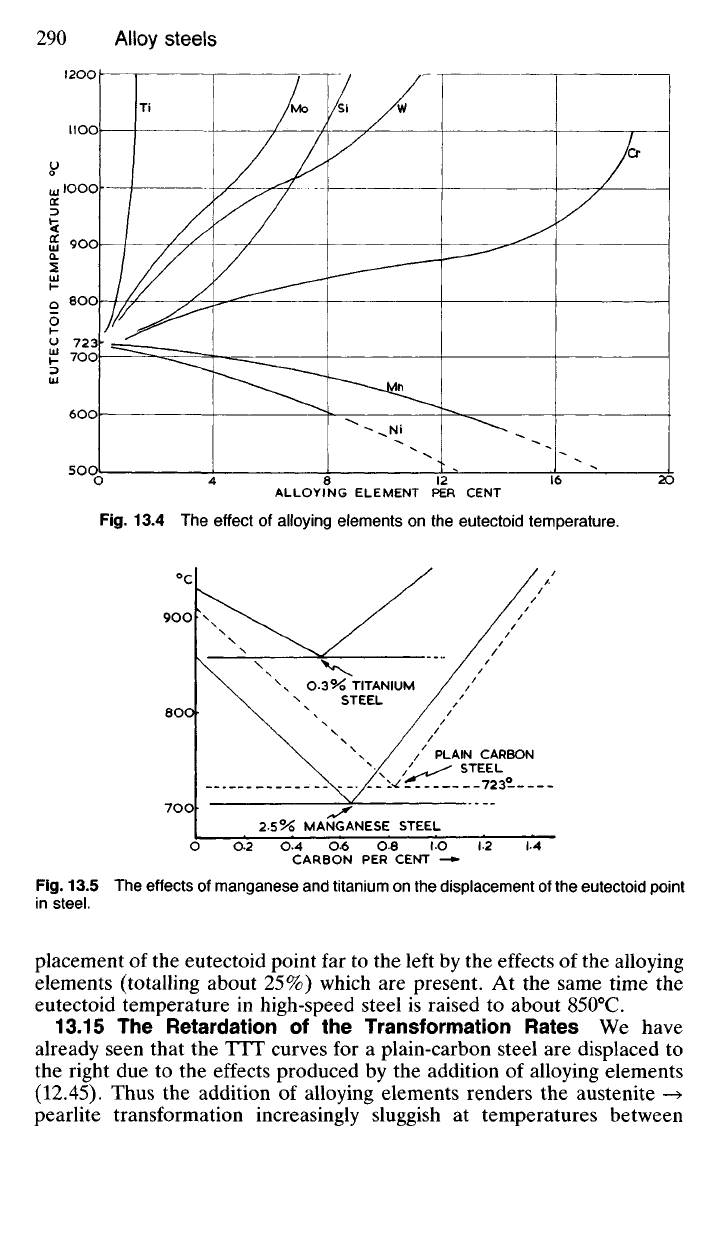
ALLOYING ELEMENT PER CENT
Fig.
13.4 The
effect
of
alloying elements
on the
eutectoid temperature.
Fig.
13.5 The
effects
of
manganese
and
titanium
on the
displacement
of the
eutectoid point
in steel.
placement
of the
eutectoid point
far to the
left
by the
effects
of the
alloying
elements (totalling about
25%)
which
are
present.
At the
same time
the
eutectoid temperature
in
high-speed steel
is
raised
to
about 850
0
C.
13.15
The
Retardation
of the
Transformation Rates
We
have
already seen that
the TTT
curves
for a
plain-carbon steel
are
displaced
to
the right
due to the
effects produced
by the
addition
of
alloying elements
(12.45).
Thus
the
addition
of
alloying elements renders
the
austenite
-»
pearlite transformation increasingly sluggish
at
temperatures between
EUTECTOID
TEMPERATURE
0
C
0
C
O3% TITANIUM
STEEL
PLAIN CARBON
STEEL
MANGANESE STEEL
CARBON PER CENT

500
0
C and 700
0
C and so reduces the critical cooling rate necessary to obtain
martensite. This feature of alloying in steels has obvious advantages and all
alloying elements, with the exception of cobalt, will reduce transformation
rates.
In order to obtain a completely martensitic structure in the case of a
plain 0.8% carbon steel, we must cool it from above 723°C to room tem-
perature in approximately one second. This treatment involves a very dras-
tic quench, generally leading to distortion or cracking of the component.
By adding small amounts of suitable alloying elements such as nickel and
chromium, we reduce this critical cooling rate to such an extent that a less
drastic oil-quench is rapid enough to produce a fully martensitic structure.
Further increases in the amounts of alloying elements will so reduce the
rate of transformation that such a steel can be hardened by cooling in air.
'Air-hardening' steels have the particular advantage that comparatively
little distortion is produced during hardening. Alternatively, such a steel,
containing
4Vi %
nickel and 1V4% chromium and which will air-harden in
thin sections, can be hardened completely through sections up to 0.15 m
diameter by oil-quenching. This aspect of alloying is one of the greatest
value since it makes possible uniform hardening of mass-produced
components by conveyor-belt methods operated by unskilled labour.
A minor disadvantage is that all alloying elements, except cobalt, also
lower the M
s
and M/ temperatures to the extent that for many alloy steels,
Mf is well below ambient temperatures resulting in the retention of some
austenite in the quenched structure.
13.16 The Improvement in Corrosion-resistance The corrosion
resistance of steels is substantially improved by the addition of elements
such as aluminium, silicon and chromium. These elements form thin but
dense and adherent oxide films which protect the surface of the steel from
further attack. Of the elements mentioned, chromium is the most useful
when mechanical properties have to be considered. When nickel also is
added in sufficient quantities, the austenitic structure is maintained at room
temperature, so that, provided the carbon content is kept low, a completely
solid-solution type of structure prevails. This also helps in maintaining
a high corrosion-resistance limiting the possibility of electrolytic attack
(21.30).
13.17 Effects on the Mechanical Properties One of the main reasons
for alloying is to effect improvements in the mechanical properties of a
steel. These improvements are generally the results of physical changes
already referred to. For example, hardness is increased by elements which
stabilise the carbides; strength is increased by all alloying elements since
they dissolve in ferrite; and toughness is improved by elements which refine
the grain. Many formulae have been devised, by the use of which the
approximate tensile strength of an alloy steel may be estimated from its
known composition. For example, Walters takes a basic tensile strength
for pure iron of 250 N/mm
2
and multiplies this by factors for each alloying
element present. The factor for each element changes as its quantity
changes. Such factors for most of the principal alloying elements are shown
in Fig. 13.6 and are true for pearlitic steels in the normalised condition.
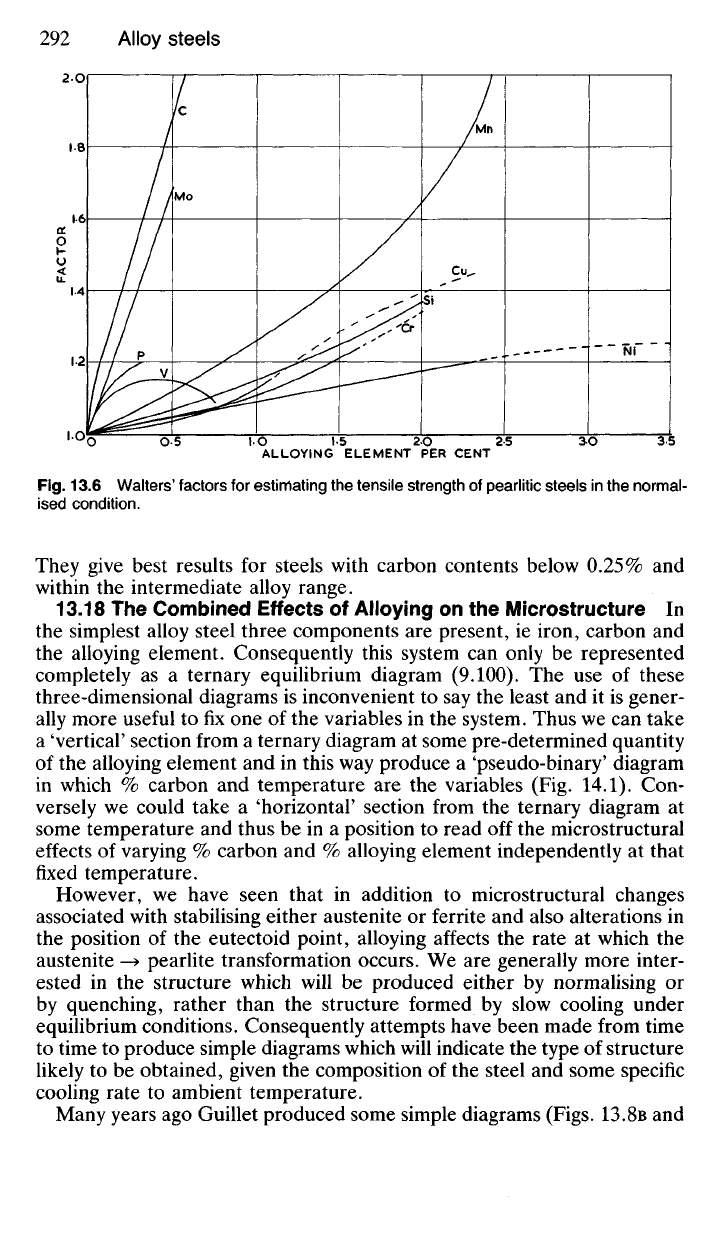
Fig.
13.6 Walters' factors for estimating the tensile strength of pearlitic steels in the normal-
ised condition.
They give best results for steels with carbon contents below 0.25% and
within the intermediate alloy range.
13.18 The Combined Effects of Alloying on the Microstructure In
the simplest alloy steel three components are present, ie iron, carbon and
the alloying element. Consequently this system can only be represented
completely as a ternary equilibrium diagram (9.100). The use of these
three-dimensional diagrams is inconvenient to say the least and it is gener-
ally more useful to fix one of the variables in the system. Thus we can take
a 'vertical' section from a ternary diagram at some pre-determined quantity
of the alloying element and in this way produce a 'pseudo-binary' diagram
in which % carbon and temperature are the variables (Fig. 14.1). Con-
versely we could take a 'horizontal' section from the ternary diagram at
some temperature and thus be in a position to read off the microstructural
effects of varying % carbon and % alloying element independently at that
fixed temperature.
However, we have seen that in addition to microstructural changes
associated with stabilising either austenite or ferrite and also alterations in
the position of the eutectoid point, alloying affects the rate at which the
austenite
—>
pear
lite
transformation occurs. We are generally more inter-
ested in the structure which will be produced either by normalising or
by quenching, rather than the structure formed by slow cooling under
equilibrium conditions. Consequently attempts have been made from time
to time to produce simple diagrams which will indicate the type of structure
likely to be obtained, given the composition of the steel and some specific
cooling rate to ambient temperature.
Many years ago Guillet produced some simple diagrams (Figs.
13.8B
and
FACTOR
ALLOYING ELEMENT PER CENT
13.10B)
which showed the general relationship between composition and
microstructure for alloy steels cooled slowly to ambient temperatures.
These diagrams were later developed to give rather fuller information of
a practical nature (Fig. 13.12). Guillet diagrams allowed for the effects of
only one alloying element to be considered and modern alloy steels contain
as many as three or more elements in addition to iron and carbon. To
represent the structures of such steels graphically the most satisfactory
method is to place the alloying elements into two groups according to
whether they stabilise austenite or ferrite and to ascribe factors to each
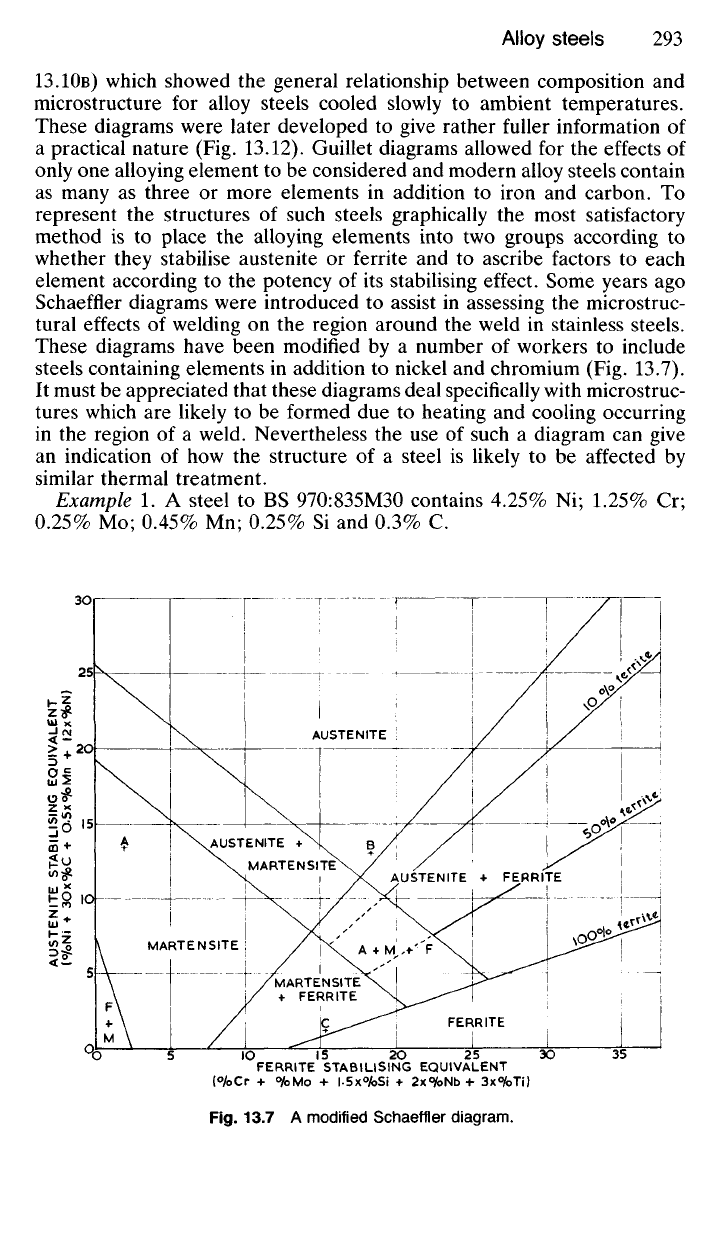
element according to the potency of its stabilising effect. Some years ago
Schaeffler diagrams were introduced to assist in assessing the microstruc-
tural effects of welding on the region around the weld in stainless steels.
These diagrams have been modified by a number of workers to include
steels containing elements in addition to nickel and chromium (Fig. 13.7).
It must be appreciated that these diagrams deal specifically with microstruc-
tures which are likely to be formed due to heating and cooling occurring
in the region of a weld. Nevertheless the use of such a diagram can give
an indication of how the structure of a steel is likely to be affected by
similar thermal treatment.
Example 1. A steel to BS 970:835M30 contains 4.25% Ni; 1.25% Cr;
0.25%
Mo; 0.45% Mn; 0.25% Si and 0.3% C.
AUSTENITE
STABILISING
EQUIVALENT
(°/oNi
+
3Ox°/bC
+ O.5x%Mn +
|2x<fcN)
FERRITE STABILISING EQUIVALENT
(o/oCr + %Mo + l5x%>Si + 2x<fcNb + 3x°/oTi)
Fig.
13.7 A modified Schaeffler
diagram.
FERRITE
MARTENSITE
+ FERRITE
MARTENSITE j
AUSTENITE + FERRITE
AUSTENITE +
MARTENSITE
AUSTENITE !
Austenite stabilising
equivalent (from Fig. 13.7) = 4.25(Ni) + (0.45 X 0.5(Mn)) + (0.3 x
30(C))
= 4.25 4- 0.225 4- 9.0
= 13.475
Ferrite stabilising
equivalent =
1.25(Cr)
4- 0.25(Mo) 4- (0.25 X
1.5(Si))
= 1.25 4- 0.25 4- 0.375
= 1.875
These coordinates are represented by point A in Fig. 13.7 indicating that
the steel will have a martensitic structure after air-cooling from its austen-
itic condition. It is in fact an air-hardening steel.
Example 2. A stainless steel (304S15) contains 17.5% Cr; 11.0% Ni;
1.2% Mn; 0.6% Si and 0.05% C.
Austenite stabilising equiv. = 11.0(Ni) 4- (1.2 x 0.5(Mn)) 4- (0.05 X
30(C))
= 11.0 4- 0.6 4- 1.5
= 13.1
Ferrite stabilising equiv. = 17.5(Cr) 4- (0.6 X
1.5(Si))
= 17.5 4- 0.9
= 18.4
which is represented by B in Fig. 13.7 showing that the steel will have an
austenitic structure following air-cooling to ambient temperatures.
Example 3. A stainless iron (403S17) contains 14.0% Cr; 0.03% C; 0.8%
Si and 0.5% Mn.
Austenite stabilising equiv. = (0.03 x 30(C)) 4 (0.5 x 0.5(Mn))
= 1.15
Ferrite stabilising equiv. = 14.0(Cr) 4- (0.8 x
1.5(Si))
= 15.2
These coordinates are represented by point C suggesting that the struc-
ture will be mainly ferritic but that it may also contain a little martensite
when air-cooled from a high temperature.
Nickel Steels
13.20 Nickel is extensively used in alloy steels for engineering purposes,
generally in quantities up to about 5.0%. When so used, its purpose is to
increase strength and toughness. It is also used in larger amounts in stain-
less steels (13.44) and in maraging steels (13.101). Nickel is mined exten-
sively in CIS; the Pacific island of New Caledonia; Australia; Cuba and
the Philippines, but by far the major producing country is Canada from
whence Britain imports her supplies.
13.21 The addition of nickel to a plain-carbon steel tends to stabilise
the austenite phase over an increasing temperature range, by raising the
A
4
temperature and depressing the A3. Thus, the addition of 25% nickel
to pure iron renders it austenitic, and so non-magnetic, even after slow
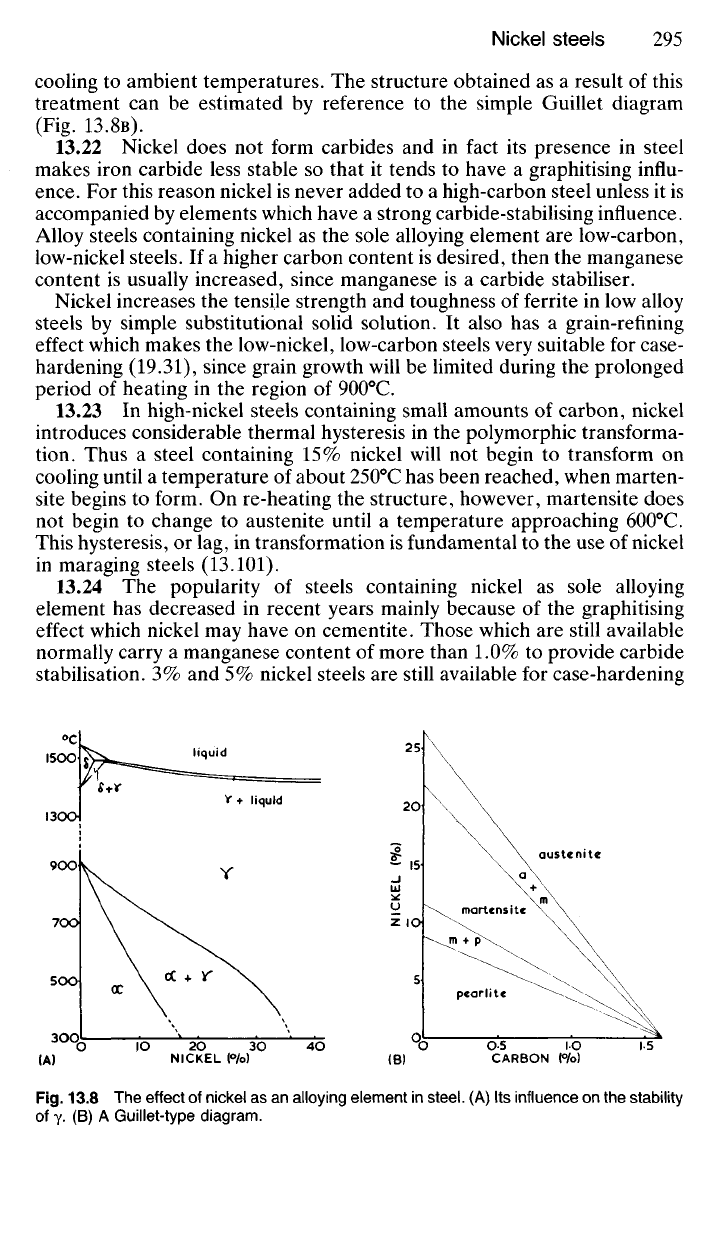
cooling to ambient temperatures. The structure obtained as a result of this
treatment can be estimated by reference to the simple Guillet diagram
(Fig. 13.8B).
13.22 Nickel does not form carbides and in fact its presence in steel
makes iron carbide less stable so that it tends to have a graphitising influ-
ence.
For this reason nickel is never added to a high-carbon steel unless it is
accompanied by elements which have a strong carbide-stabilising influence.
Alloy steels containing nickel as the sole alloying element are low-carbon,
low-nickel steels. If a higher carbon content is desired, then the manganese
content is usually increased, since manganese is a carbide stabiliser.
Nickel increases the tensile strength and toughness of ferrite in low alloy
steels by simple substitutional solid solution. It also has a grain-refining
effect which makes the low-nickel, low-carbon steels very suitable for case-
hardening (19.31), since grain growth will be limited during the prolonged
period of heating in the region of 900
0
C.
13.23 In high-nickel steels containing small amounts of carbon, nickel
introduces considerable thermal hysteresis in the polymorphic transforma-
tion. Thus a steel containing 15% nickel will not begin to transform on
cooling until a temperature of about 250
0
C has been reached, when marten-
site begins to form. On re-heating the structure, however, martensite does
not begin to change to austenite until a temperature approaching 600
0
C.
This hysteresis, or lag, in transformation is fundamental to the use of nickel
in maraging steels (13.101).
13.24 The popularity of steels containing nickel as sole alloying
element has decreased in recent years mainly because of the graphitising
effect which nickel may have on cementite. Those which are still available
normally carry a manganese content of more than 1.0% to provide carbide
stabilisation. 3% and 5% nickel steels are still available for case-hardening
NICKEL
Wo)
0
C
liquid
V+
liquid
austenite
martensite
pearlite
Fig.
13.8 The effect of nickel as an alloying element in
steel.
(A) Its influence on the stability
of
Y- (B) A Guillet-type
diagram.
CARBON
(%>)
(B)
NICKEL
(%>)
(A)
