Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.

The effect of this prolonged annealing is to cause the cementite to break
down, but instead of forming coarse graphite flakes, the carbon is precipi-
tated in the form of small 'rosettes' of 'temper carbon'. A fractured section
will thus be black, hence the term 'blackheart'. The final structure, which
consists entirely of ferrite and finely divided temper carbon, is soft, readily
machinable and almost as ductile as cast steel. Blackheart malleable cast-
ings find particular application in the automobile industries because of the
combination of castability, shock-resistance and ease of machining they
afford. Typical uses include rear-axle housings, wheel hubs, brake shoes,
pedals, levers and door hinges.
15.72.2.
Whiteheart Malleable
Iron castings are manufactured from white
iron of which the following composition is typical:
Carbon 3.3%
Silicon 0.6%
Manganese 0.5%
Sulphur 0.25%
Phosphorus 0.1%
In this process the castings are heated in contact with some oxidising
material, such as haematite ore, for between 70 and 100 hours at a tempera-
ture of about 1000
0
C.
During annealing, the carbon at the surface of the casting is oxidised by
contact with the hematite ore, and lost as carbon dioxide. This causes more
carbon to diffuse outwards from the core, and, in turn, this is lost by
oxidation. Thus, after treatment a thin section may be completely ferritic,
and on fracture present a steely white appearance; hence the name
'whiteheart'. Heavier sections will not be completely decarburised, and so,
whilst the outer layer is ferritic, this will merge into an inner zone contain-
ing some pearlite, and at the extreme core some nodules of temper carbon.
The surface layer may exhibit some oxide penetration.
Thin-sectioned components requiring high ductility are often made in
the form of whiteheart malleable castings. Examples include fittings for
gas,
water, air and steam pipes; bicycle- and motorcycle-frame fittings;
parts for agricultural machinery; switchgear equipment; and parts for tex-
tile machinery.
15.72.3.
Pearlitic Malleable Iron is produced from raw material similar
in composition to that of blackheart malleable iron, though in some cases
alloying elements may be used to stabilise the required pearlitic matrix in
the final structure. When an unalloyed iron is used it is first malleabilised
either fully or partially at about 950
0
C to cause adequate breakdown of
the primary cementite. It is then reheated to 900
0
C so that carbon will
dissolve in the austenite present at that temperature. Subsequent treatment
consists either of air-cooling (to produce a pearlitic matrix) or some other
form of heat treatment designed to give a bainitic or tempered-martensite
type of matrix.
If an alloyed iron is used it is often given a normal malleabilising treat-
*Whiteheart malleable castings of greater cross-section are likely to be stronger since the core will
contain more pearlite.
ment, the presence of carbide-stabilising elements causing retention of the
pearlitic matrix during this process.
The final structure will consist of rosettes of temper carbon in a matrix
of pearlite, bainite or tempered martensite according to the final heat-
treatment given. Tensile strengths in the region of 775 N/mm
2
are possible
and in many respects the product can compete with cast steel, despite the
extra cost of the heat-treatment processes.
15.73 Compacted-graphite (CG) Irons These materials have their
origins in work carried out by Morrogh at BCIRA* many years ago and
are characterised by graphite structures (Pl.
15.4B)—and
consequently also
physical properties—which are intermediate between those of ordinary
grey flake-graphite irons and those of SG iron. With appropriate chemical
treatment the graphite flakes produced are short and stubby and have
rounded edges. This has led to the term 'vermicular iron' being used in the
USA, though the flakes are not quite 'wormlike' as the title implies.
CG iron is produced when molten iron of near-eutectic composition is
first de-sulphurised in the ladle and then treated at 1400
0
C with a single
alloy containing appropriate amounts of magnesium, titanium and cerium
so that the resultant iron contains Mg (0.015-0.03%), Ti (0.06-0.13%)
and Ce (a trace). The presence of magnesium tends to produce spheroidal
graphite (as it does in SG iron) and this is controlled by the restraining
tendency of titanium which makes the amount of the magnesium less
critical.
The mechanical properties are roughly intermediate between those of
grey iron and those of SG iron, whilst the resistance to scaling and 'growth'
at high temperatures are very good. Since in cast irons generally graphite
* British Cast Iron Research Association, Alvechurch, Birmingham.
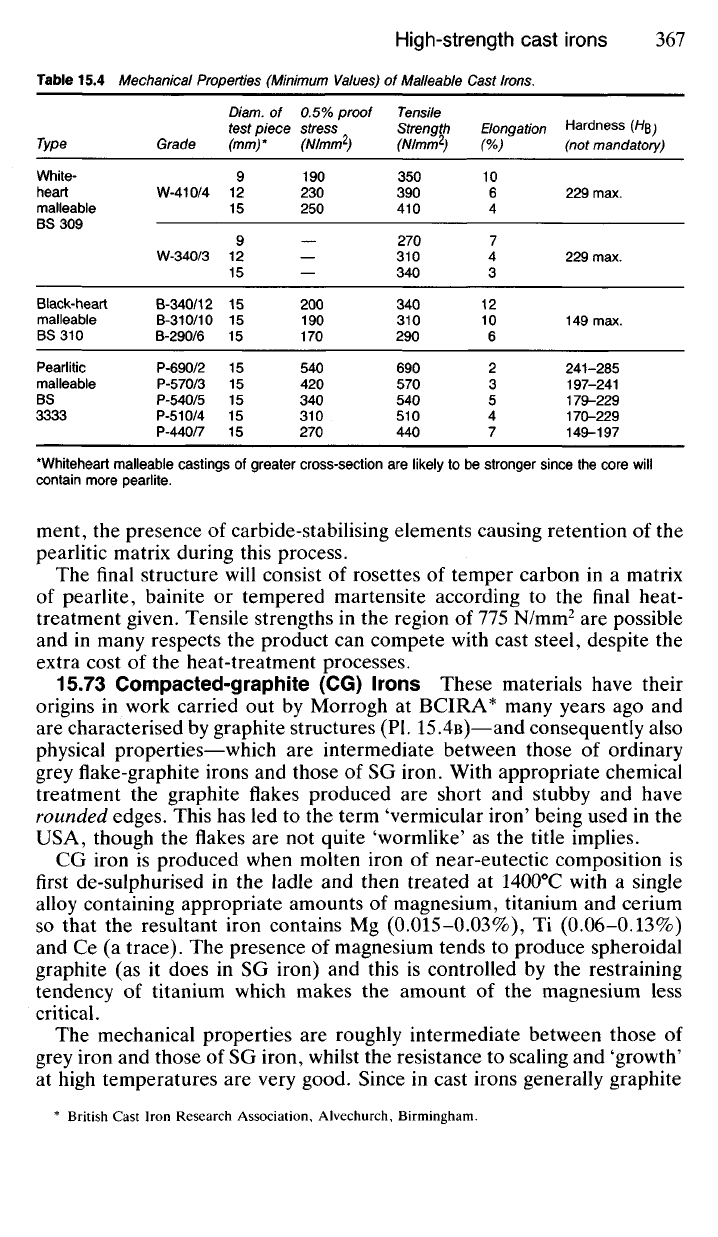
Table 15.4 Mechanical Properties (Minimum Values) of Malleable Cast Irons.
Type
White-
heart
malleable
BS 309
Black-heart
malleable
BS 310
Pearlitic
malleable
BS
3333
Grade
W-410/4
W-340/3
B-340/12
B-310/10
B-290/6
P-690/2
P-570/3
P-540/5
P-510/4
P-440/7
Diam. of
test piece
(mm)*
9
12
15
9
12
15
15
15
15
15
15
15
15
15
0.5% proof
stress
(N/mm
2
)
190
230
250
200
190
170
540
420
340
310
270
Tensile
Strength
(N/mm
2
)
350
390
410
270
310
340
340
310
290
690
570
540
510
440
Elongation
(%)
10
6
4
7
4
3
12
10
6
2
3
5
4
7
Hardness (H$ \
(not mandatory)
229 max.
229 max.
149 max.
241-285
197-241
179-229
170-229
149-197
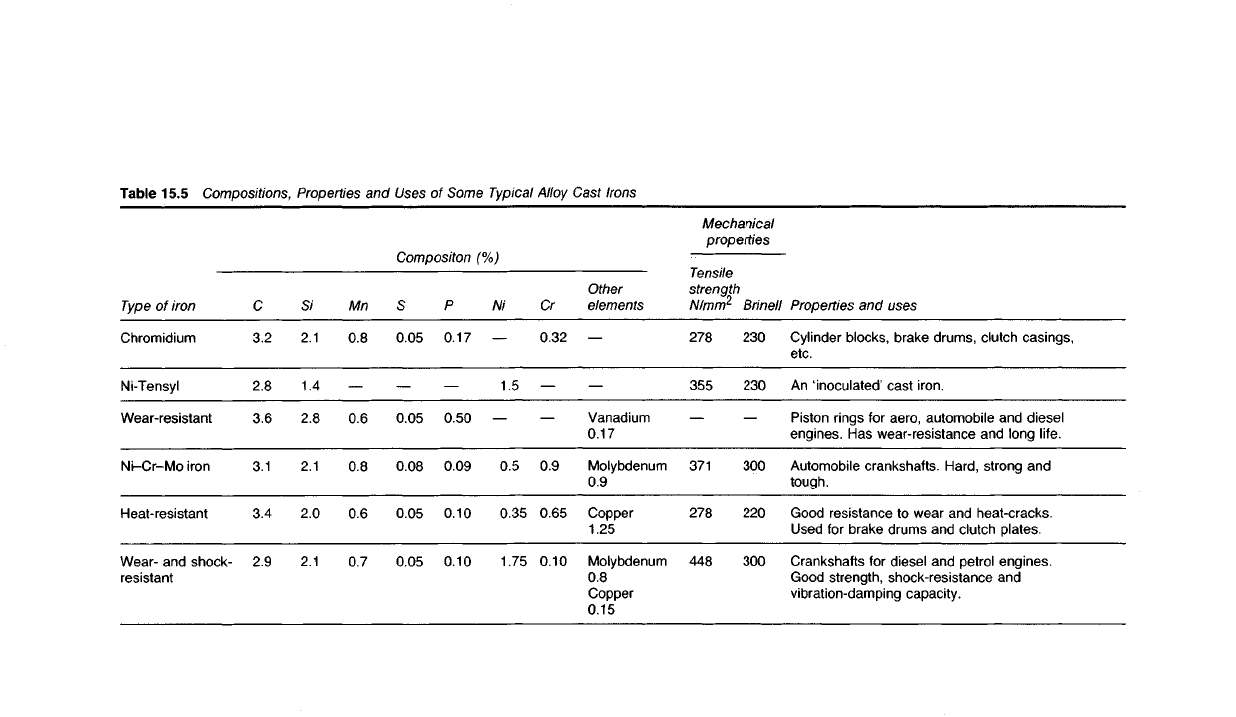
Table
15.5 Compositions, Properties and
Uses
of Some
Typical
Alloy Cast
Irons
Properties
and uses
Cylinder blocks, brake drums, clutch
casings,
etc.
An
'inoculated' cast iron.
Piston rings for aero, automobile and
diesel
engines. Has wear-resistance and long life.
Automobile crankshafts. Hard, strong and
tough.
Good resistance to wear and heat-cracks.
Used for brake drums and clutch plates.
Crankshafts
for diesel and petrol
engines.
Good strength, shock-resistance and
vibration-damping capacity.
Mechanical
properties
Brinell
230
230
300
220
300
Tensile
strength
278
355
371
278
448
Compositon
(%)
Other
elements
Vanadium
0.17
Molybdenum
0.9
Copper
1.25
Molybdenum
0.8
Copper
0.15
Cr
0.32
0.9
0.65
0.10
Ni
1.5
0.5
0.35
1.75
P
0.17
0.50
0.09
0.10
0.10
0.05
0.05
0.08
0.05
0.05
Mn
0.8
0.6
0.8
0.6
0.7
2.1
1.4
2.8
2.1
2.0
2.1
C
3.2
2.8
3.6
3.1
3.4
2.9
Type
of iron
Chromidium
Ni-Tensyl
Wear-resistant
Ni-Cr-Mo
iron
Heat-resistant
Wear-
and shock-
resistant
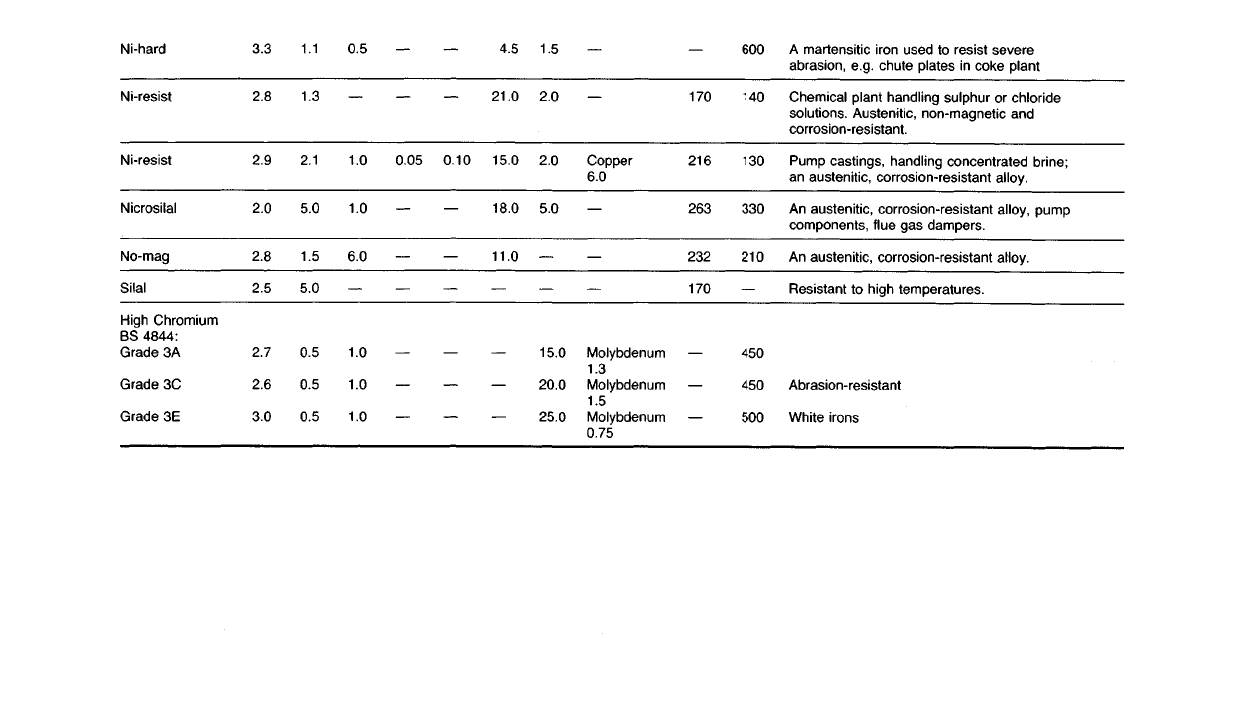
A
martensitic iron used to resist severe
abrasion, e.g. chute plates in coke plant
Chemical
plant handling sulphur or chloride
solutions.
Austenitic, non-magnetic and
corrosion-resistant.
Pump castings, handling concentrated brine;
an austenitic, corrosion-resistant alloy.
An
austenitic, corrosion-resistant alloy, pump
components, flue gas dampers.
An
austenitic, corrosion-resistant alloy.
Resistant to high temperatures.
Abrasion-resistant
White
irons
600
140
130
330
210
450
450
500
170
216
263
232
170
Copper
6.0
Molybdenum
1.3
Molybdenum
1.5
Molybdenum
0.75
1.5
2.0
2.0
5.0
15.0
20.0
25.0
4.5
21.0
15.0
18.0
11.0
0.100.05
0.5
1.0
1.0
6.0
1.0
1.0
1.0
1.1
1.3
2.1
5.0
1.5
5.0
0.5
0.5
0.5
3.3
2.8
2.9
2.0
2.8
2.5
2.7
2.6
3.0
Ni-hard
Ni-resist
Ni-resist
Nicrosilal
No-mag
SiIaI
High
Chromium
BS
4844:
Grade
3A
Grade
3C
Grade
3E
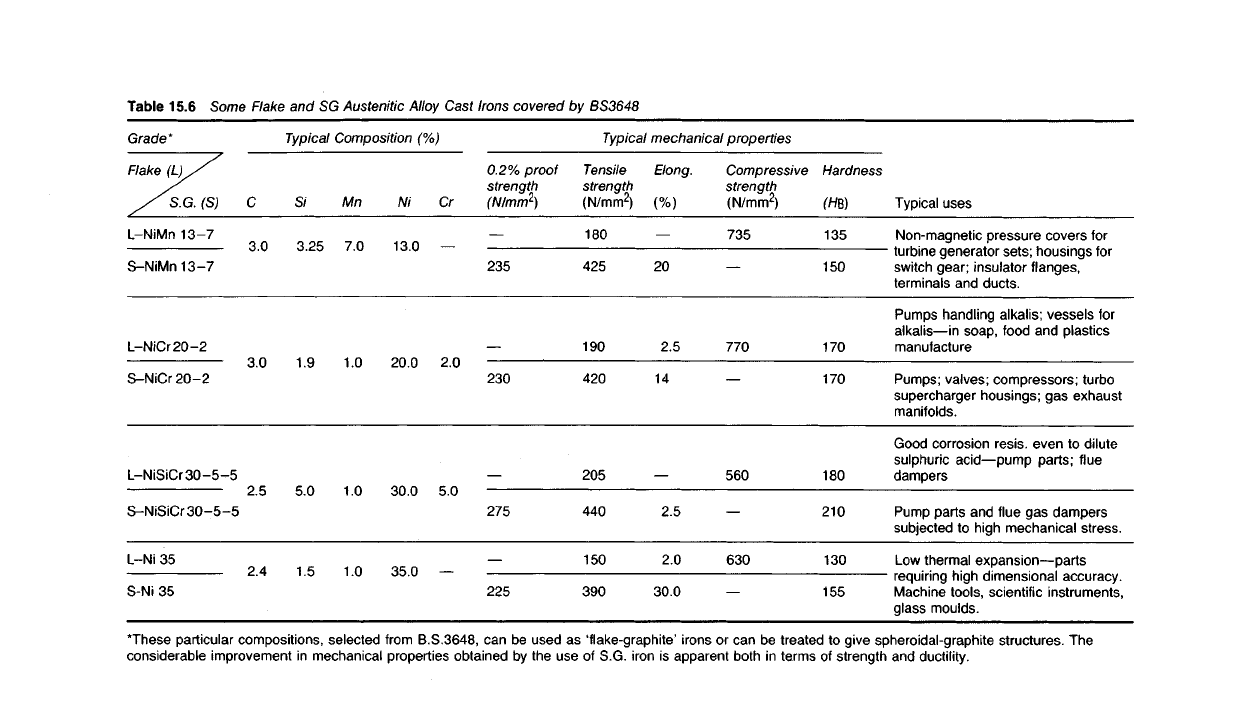
Table 15.6
Some
Flake
and SG Austenitic
Alloy
Cast
Irons
covered by BS3648
Typical uses
Non-magnetic pressure covers for
turbine generator sets; housings for
switch
gear;
insulator
flanges,
terminals and ducts.
Pumps
handling alkalis; vessels for
alkalis—in soap, food and plastics
manufacture
Pumps; valves; compressors; turbo
supercharger housings; gas exhaust
manifolds.
Good corrosion resis. even to
dilute
sulphuric acid—pump parts; flue
dampers
Pump parts and flue gas dampers
subjected
to high mechanical stress.
Low
thermal expansion—parts
requiring high dimensional accuracy.
Machine tools, scientific instruments,
glass moulds.
Typical
mechanical properties
Typical
Composition (%)
Hardness
(H*)
135
150
170
170
180
210
130
155
Compressive
strength
(N/mm
2
)
735
770
560
630
Elong.
(%)
20
2.5
14
2.5
2.0
30.0
Tensile
strength
(N/mm
2
)
180
425
190
420
205
440
150
390
0.2%
proof
strength
(N/mm
2
)
235
230
275
225
Cr
2.0
5.0
Ni
13.0
20.0
30.0
35.0
Mn
7.0
1.0
1.0
1.0
S/
3.25
1.9
5.0
1.5
C
3.0
3.0
2.5
2.4
Grade*
Flake
(L)/'
//SG. (S)
L-NiMn 13-7
S-NiMn
13-7
L-NiCr
20-2
S-NiCr
20-2
L-NiSiCr
30-5-5
S-NiSiCr30-5-5
L-Ni 35
S-Ni
35
"These particular compositions, selected from B.S.3648, can be used as 'flake-graphite' irons or can be treated to give spheroidal-graphite structures. The
considerable improvement in mechanical properties obtained by the use of S.G. iron is apparent both in terms of strength and ductility.

is a better conductor of heat than is the iron matrix, it follows that CG
irons have a thermal conductivity only slightly less than grey iron but better
than nodular graphite irons. All of these factors make CG iron attractive
as a heat-resisting material and it was developed originally for the manufac-
ture of ingot moulds and vehicle brake components. In modern engineering
there are many other applications, eg gear pumps, eccentric gears, fluid
and air cylinders, discharge manifolds, etc.
15.74 The choice between ordinary grey iron, SG iron and malleable
iron for a specific application is, as with most materials problems, dictated
by both economics and technical requirements. Grey iron is, of course,
lowest in cost and also the easiest in which to produce sound castings. With
regard to the relative merits of the two high-strength competitors, SG iron
and malleable iron, the choice may be less easy. Malleable iron is the more
difficult to cast since at this stage it will have a white structure. This also
involves limitations as to cross-section as compared with SG iron. More-
over, the cost of the malleabilising process makes the product a little more
expensive than SG iron. However, it is sometimes necessary to anneal SG
iron in order to improve its machinability, and in this case malleable iron
may prove more attractive cost-wise.
Alloy Cast Irons
15.80 The microstructural effects which alloying elements have on a cast
iron are, in most cases, similar to the effects these elements have on the
structure of a steel. Alloying elements can therefore be used to improve
mechanical properties, to refine grain, to increase the hardness by stabilis-
ing cementite and forming other harder carbides; and to stabilise, when
desirable, martensitic and austenitic structures at ambient temperatures.
Improvements in resistance to atmospheric corrosion and also deterio-
ration at high temperatures are also attainable.
Plate 15.6 15.6A A martensitic alloy cast
iron.
Graphite in a matrix of martensite. x 600. Etched in 4% picral. (Courtesy of BCIRA)
15.6B An austenite alloy cast iron (Ni-resist).
Graphite and carbides in an austenite matrix, x 100. Etched in 4% picral. (Courtesy of
BCIRA).

15.81 Nickel is a common alloying element and, as in steel, it tends
to promote graphitisation. At the same time it has a grain-refining effect,
so that whilst nickel will help to prevent chilling in thin sections, it will
also prevent coarse grain in thick sections. Nickel also reduces the tendency
of thin sections to crack.
15.82 Chromium is a strong carbide stabiliser, and so inhibits the
formation of graphite. Moreover, the carbides formed by chromium are
more stable and less likely to graphitise under the application of heat than
is iron carbide. Irons containing chromium are therefore less susceptible
to growth.
As in the low-alloy steels, the disadvantages attendant on the use of
either nickel or chromium separately are overcome by using them in con-
junction in the ratio of two to three parts of nickel to one part of chromium.
15.83 Molybdenum, when added in small amounts, dissolves in the
ferrite, but in larger amounts forms double carbides. It increases the hard-
ness of thick sections and promotes uniformity of microstructure. Impact
values are also improved by amounts in the region of 0.5% molybdenum.
15.84 Vanadium promotes heat-resistance in cast irons, in so far as
the stable carbides which it forms do not break down on heating. Strength
and hardness are increased, particularly when vanadium is used in conjunc-
tion with other alloying elements.
15.85 Copper is only sparingly soluble in iron, and has a very slight
graphitising effect. It has little influence on the mechanical properties, and
its main value is in improving the resistance to atmospheric corrosion.
15.86 Martensitic irons, which are very useful for resisting abrasion,
usually contain 4-6% nickel and about 1% chromium. Such an alloy is
Nihard, included in Table 15.5. It is martensitic in the cast state, whereas
alloys containing rather less nickel and chromium would need to be oil-
quenched in order to obtain a martensitic structure. Austenitic irons usu-
ally contain between 10 and 30% nickel and up to 5% chromium. These
are corrosion-resistant, heat-resistant, non-magnetic alloys. Some of them
are treated to produce structures containing spheroidal instead of flake
graphite. Table 15.6 indicates the degree of improvement in both strength
and ductility which arise from this additional treatment.
Exercises
1.
What factors control the structure of cast iron? Explain how these principles
are used in the foundry to control the strength of grey-iron castings. (15.20
and 15.30)
2.
Discuss the influence of the following elements on the structure and properties
of cast iron: (i) silicon; (ii) manganese; (in) sulphur; (iv) phosphorus. (15.22
to 15.25)
3.
Describe briefly the methods available to control the size and shape of graphite
in cast iron, and explain how the mode of occurrence of the graphite affects
the properties. (15.22; 15.30;
15.71;
15.73)
4.
Explain fully what is meant by the term growth as applied to cast irons. Discuss
methods which are used to overcome this phenomenon. (15.50)

5.
Cast iron is a cheap, easily produced engineering material, but ordinary grey
cast iron has relatively poor mechanical properties. Discuss, in general terms,
the methods employed to overcome these limitations.
(15.71;
15.72; 15.73)
6. Discuss the effect of chemical composition and cooling rate on the structure
and properties of cast irons. Briefly describe one method for producing (a)
malleable iron and (b) nodular iron. (15.20; 15.30; 15.72; 15.71)
7.
Most cast irons consist of a dispersion of graphite in a steel-like matrix. Illus-
trate this statement by sketches of microstructures and discuss the variations
in properties that can arise, by reference to: (a) pearlitic grey cast iron; (b)
blackheart malleable iron; (c) SG iron; (d) compacted-graphite iron. Indicate
briefly the treatment necessary to produce blackheart malleable iron, SG cast
iron and compacted-graphite cast iron. (15.40; 15.72;
15.71;
15.73)
8. How are malleable castings produced? What are the most important engineer-
ing properties of these materials? (15.72)
9. Discuss the role of microstructure in determining the general properties of cast
iron. (15.20; 15.30; 15.70)
10.
Discuss the uses of nickel, chromium and molybdenum in cast irons. (15.50;
15.81 to 15.83)
Bibliography
Angus, H. T., Cast Iron: Physical and
Engineering
Properties,
Butterworths, 1960.
Copper Development Association, Copper in Cast Iron, 1964.
Hume-Rothery, W., The Structures of Alloys of Iron, Pergamon, 1966.
BS 1452: 1977
Specification
for Grey Iron Castings.
BS 6681: 1986
Specifications
for Malleable Cast Iron.
BS 2789: 1985 Iron Castings with Spheroidal or Nodular Graphite.
BS 1591: 1983
Corrosion-resistant
High Silicon Iron Castings.
BS 3468: 1986 Austenitic Cast Iron.

Copper and the
Copper-Base Alloys
16.10 Copper was undoubtedly the first useful metal to be employed by
Man. In many countries it is found in small quantities in the metallic
state and, being soft, it was readily shaped into ornaments and utensils.
Moreover, many of the ores of copper can easily be reduced to the metal,
and since these ores often contain other minerals, it is very probable that
copper alloys were produced as the direct result of smelting. It is thought
that bronze was produced in Cornwall by acc
;
dentally smelting ores
containing both tin- and copper-bearing minerals in the camp fires of
ancient Britons.
As a schoolboy the author was taught that the very protracted period of
Man's history when progress was extremely slow, namely the 'Stone Age',
was followed by a 'Bronze Age' when primitive technology developed
relatively quickly. It is now fairly certain that the Bronze Age was preceded
by a comparatively short Copper Age but, because copper corrodes more
quickly than bronze, few artefacts of that period have been recovered by
archaeologists. It is fairly certain that the Egyptians used copper com-
pounds for colouring glazes some 15 000 years ago and they may have been
the first to extract the metal. In Europe copper production seems to have
begun in the Balkans some 6000 years ago, and very soon metal workers
were hardening copper by the addition of small amounts of arsenic for the
manufacture of knives and spear heads. This may well have been the first
instance, in Europe at least, of the deliberate manufacture of an alloy.
16.11 At the end of the eighteenth century practically all the world's
requirement of copper was smelted in Swansea, the bulk of the ore coming
from Cornwall, Wales or Spain. Deposits of copper ore were then dis-
covered in the Americas and Australia, and subsequently imported for
smelting in Swansea. Later it was found more economical to set up smelting
plants near to the mines, and Britain ceased to be the centre of the copper
industry.
Climbers and ramblers who ascend Snowdon by the popular Pyg track
16

will pass the ruins of an old copper-mine, perched on the mountain side
below the Crib Goch ridge. This small mine was one of many located in
the mountainous areas of Britain, but all have long since ceased pro-
duction. Their output was insignificant compared with that of a modern
mine like that at Chuquicamata, in Chile. Nevertheless supplies of the
higher grades of copper ore are diminishing and the demand for yet higher
outputs has meant that low-grade, less economical sources have to be
worked. Until a couple of decades ago the world tonnage of copper pro-
duced annually was second only to that of iron but now copper ranks third,
having been overtaken by aluminium.
Although the USA is still the largest producer of copper, as of many
other metals, her output is being closely approached by those of the CIS
and Chile. Canada, Zambia, Zaire and Peru are also leading producers,
whilst in Europe the outputs of Poland and Yugoslavia are significant. The
bulk of Britain's supply of copper comes from Zambia and Canada.
Properties and Uses of Copper
16.20 A very large part of the world's production of metallic copper is
used in the unalloyed form, mainly in the electrical industries. Copper has
a very high specific conductivity, and is, in this respect, second only to
silver, to which it is but little inferior. When relative costs are considered,
copper is naturally the metal used for industrial purposes demanding high
electrical conductivity.
16.21 The Electrical Conductivity of Copper The International
Annealed Copper Standard (IACS) was adopted by the International Elec-
trochemical Commission as long ago as 1913, and specified that an
annealed copper wire 1 m long and of cross-section 1 mm
2
should have a
resistance of no more than 0.017241 ohms at 20
0
C. Such a wire would be
said to have a conductivity of 100%. Since the standard was adopted in
1913 higher purity copper is now commonly produced and this explains
the anomalous situation where electrical conductivities of up to 101.5%
are frequently quoted.
As indicated in Fig. 16.1, the presence of impurities reduces electrical
conductivity. To a lesser degree, cold-work has the same effect. Reduction
in conductivity caused by the presence of some elements in small amounts
is not great, so that up to 1% cadmium, for example, is added to telephone
wires in order to strengthen them. Such an alloy, when hard-drawn, has a
tensile strength of some 460 N/mm
2
as compared with 340 N/mm
2
for
hard-drawn, pure copper, whilst the electrical conductivity is still over 90%
of that for soft pure copper. Other elements have pronounced effects
on conductivity; as little as 0.04% phosphorus will reduce the electrical
conductivity to about 75% of that for pure copper.
16.22 The Commercial Grades of Copper include both furnace-
refined and electrolytically refined metal. High-conductivity copper (usu-
ally referred to as OFHC or oxygen-free high conductivity) is of the highest
purity, and contains at least 99.9% copper. It is used where the highest
