Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.


Fig.
15.2 A three-dimensional impression of graphite flakes in a eutectic
cell.
hyper-eutectic in composition and so deposit primary graphite (instead of
cementite) before the eutectic cells start to form. This primary graphite is
generally coarse and was known as 'kish'.
Rapid cooling, which produces a greater degree of undercooling,
initiates the formation of a greater number of eutectic cells and also more
frequent branching in the eutectic graphite 'leaves', giving much finer
graphite flakes. The smaller the eutectic cells, the finer the graphite flakes
and hence the better the mechanical properties.
The presence of graphite gives a softer iron which machines well because
of the effect of the graphite flakes in forming chip cracks in advance of the
edge of the cutting tool (6.61). Moreover, since graphite occupies a greater
volume in the solid structure than does carbon in solution in the liquid
iron, it tends to counteract the effects of shrinkage during solidification.
15.22 Silicon dissolves in the ferrite of a cast iron and is the element
which has the predominant effect on the relative amounts of graphite and
cementite which are present. Silicon tends to increase the instability of
cementite (13.12) so that it decomposes, producing graphite and hence,
a grey iron. The higher the silicon content, the greater the degree of
decomposition of the cementite, and the coarser the flakes of graphite
produced.
Thus,
whilst silicon actually strengthens the ferrite by dissolving in it, at
the same time it produces softness by causing the cementite to break down
to graphite. When, however, silicon is present in amounts in excess of that
necessary to complete the decomposition of all the cementite, it will again
cause hardness and brittleness to increase. Both the direct and indirect
effects of silicon must therefore be considered.
The presence of silicon in a cast iron is beneficial in so far as it increases
the fluidity of the molten iron, and so improves its casting properties.
15.23 Sulphur The possible effect of the silicon present in an iron
cannot be completely estimated without some reference to the sulphur
content, since sulphur has the opposite effect, in that it tends to stabilise
cementite. Sulphur, then, inhibits graphitisation and so helps to produce
a hard, brittle, white iron. Moreover, its presence as the sulphide FeS in
cast iron will also increase the tendency to brittleness.
During the melting of cast iron in the cupola there is a tendency for
some silicon to be oxidised and lost in the slag. At the same time some
sulphur will always be absorbed from the coke in the cupola. Both of these
changes in composition tend to make the iron more 'white' as they are
opposed to the formation of graphite. The foundryman therefore makes
allowances for these changes during melting in the cupola.
15.24 Manganese The effect of sulphur is governed, in turn, by the
amount of manganese present. Manganese combines with sulphur to form
manganese sulphide, MnS, which, unlike iron(II) sulphide, is insoluble in
the molten iron and floats to the top to join the slag. The indirect effect
of manganese, therefore, is to promote graphitisation because of the re-
duction of the sulphur content which it causes. Manganese has a stabilising
effect on carbides, however, so that this offsets the effect of sulphur
reduction in promoting graphitisation. The more direct effects of mangan-
ese include the hardening of the iron, the refinement of grain and an
increase in strength.
15.25 Phosphorus is present in cast iron as the phosphide Fe
3
P, which
forms a eutectic with the ferrite in grey irons, and with ferrite and cementite
in white irons. These eutectics melt at about 950
0
C, so that high-
phosphorus irons have great fluidity. Cast irons containing 1% phosphorus
are,
therefore, very suitable for the production of castings of thin section.
High phosphorus contents should be avoided in heavy sections however,
since Fe
3
P is brittle and lowers strength considerably. Its presence tends
to promote increased shrinkage.
Phosphorus has a negligible effect on the stability of cementite, but its
direct effect is to promote hardness and brittleness due to the large volume
of phosphide eutectic which a comparatively small amount of phosphorus
will produce. Phosphorus must therefore be kept low in castings where
shock-resistance is important.
The Effect of Rate of Cooling on the Structure
of Cast Iron
15.30 A high rate of cooling during solidification tends to favour the
formation of cementite rather than graphite. That is, the higher the rate
of cooling for any given cast-iron composition the 'whiter' and more brittle
the casting is likely to be. This effect is important in connection with the
choice of a suitable iron for the production of castings of thin section.
Supposing an iron which, when cooled slowly, had a fine grey structure
containing small eutectic cells were chosen for such a purpose. In thin
sections it would cool so rapidly that cementite would form in preference
to graphite and a thin section of completely white iron would result. Such
a section would be brittle and useless.
15.31 This effect is illustrated by casting a 'stepped bar' of iron of a
suitable composition (Fig. 15.3 and Pl. 15.2). Here, the thin sections have
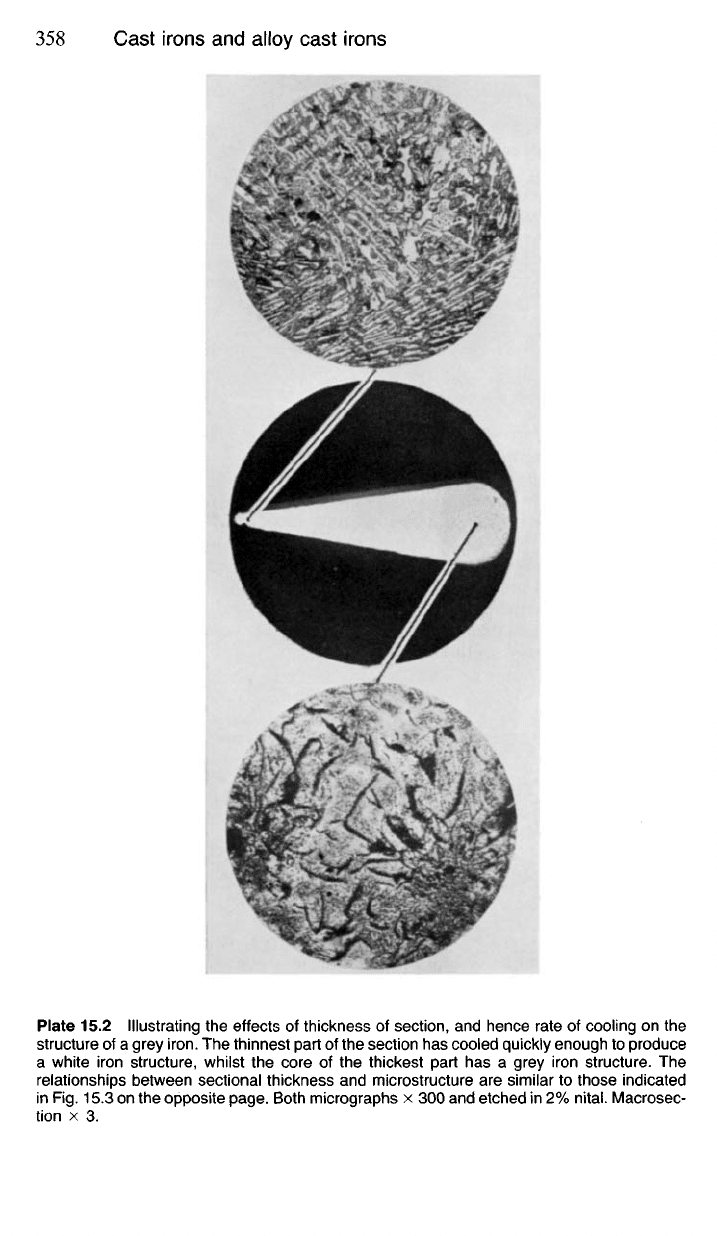
Plate 15.2 Illustrating the effects of thickness of section, and hence rate of cooling on the
structure of a grey
iron.
The thinnest part of the section has cooled quickly enough to produce
a white iron structure, whilst the core of the thickest part has a grey iron structure. The
relationships between sectional thickness and microstructure are similar to those indicated
in Rg. 15.3 on the opposite page. Both micrographs x 300 and etched in 2%
nital.
Macrosec-
tion x 3.
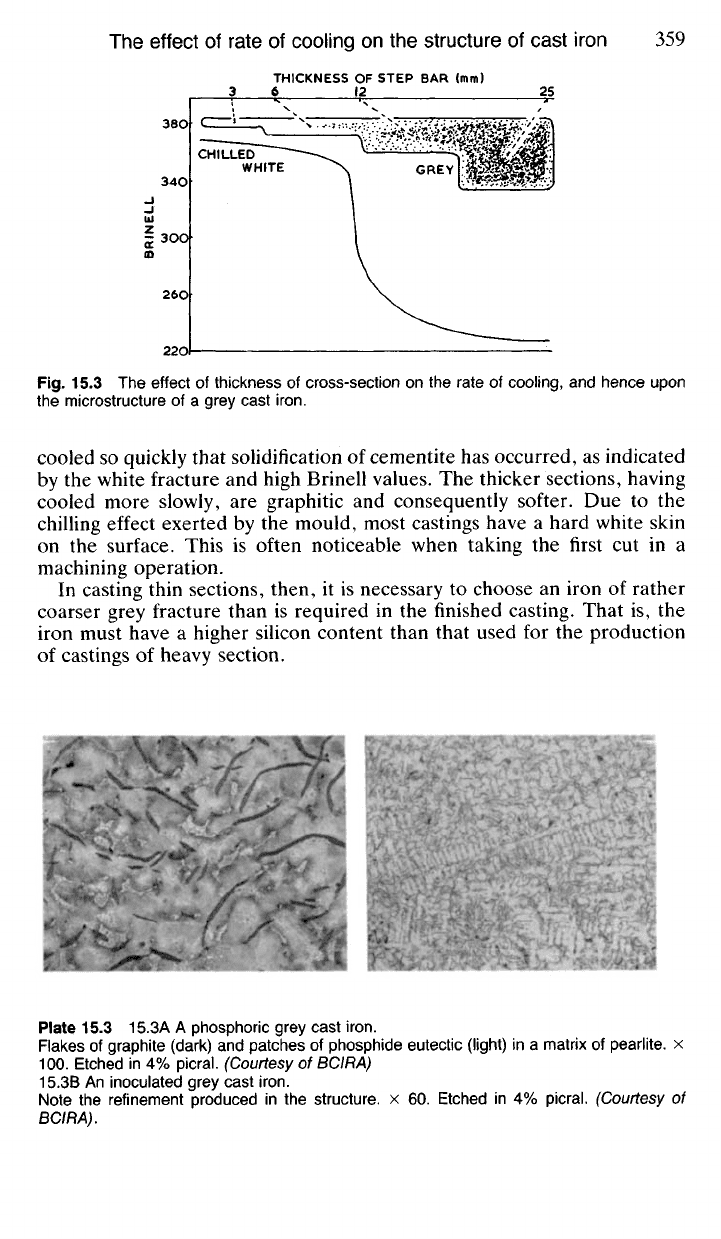
Fig.
15.3 The effect of thickness of cross-section on the rate of cooling, and hence upon
the microstructure of a grey cast
iron.
cooled so quickly that solidification of cementite has occurred, as indicated
by the white fracture and high Brinell values. The thicker sections, having
cooled more slowly, are graphitic and consequently softer. Due to the
chilling effect exerted by the mould, most castings have a hard white skin
on the surface. This is often noticeable when taking the first cut in a
machining operation.
In casting thin sections, then, it is necessary to choose an iron of rather
coarser grey fracture than is required in the finished casting. That is, the
iron must have a higher silicon content than that used for the production
of castings of heavy section.
BRINELL
Plate 15.3 15.3A A phosphoric grey cast
iron.
Flakes of graphite (dark) and patches of phosphide eutectic (light) in a matrix of pearlite. x
100.
Etched in 4% picral. (Courtesy of BCIRA)
15.3B An inoculated grey cast
iron.
Note the refinement produced in the structure, x 60. Etched in 4% picral. (Courtesy of
BCIRA).
THICKNESS OF STEP BAR (mm)
GREY
CHILLED
WHITE

The Microstructure of Cast Iron
15.40 Neglecting any patches of phosphide eutectic already mentioned,
the following structures are possible for cast irons of different compositions
and treatments:
(a) Primary Cementite and Pearlite. This structure is typical of the
hard, white, low-silicon (and possibly high-sulphur) irons and is found
also in other types of iron which have been chilled. A cementite-
austenite eutectic forms at 1131°C and as the alloy cools to 723°C the
austenite transforms to pearlite.
(b) Primary Cementite, Graphite and Pearlite. These are 'mottled'
irons of such compositions that localised small changes in either compo-
sition or cooling rate will favour the formation of either cementite or
graphite. The remaining austenite present at 723°C transforms to
pearlite.
(c) Graphite and
Pearlite.
This structure is typical of a high-duty grey
iron which has solidified as a graphite-austenite eutectic and in which
the austenite has then transformed to pearlite at 723°C.
(d) Graphite, Pearlite and
Ferrite.
This will generally be a coarser grey
iron which will be weaker and softer. The silicon content will be high.
Here the graphite-austenite eutectic has cooled slowly enough through
the eutectoid temperature (723°C) for some of the carbon which was
dissolved in the austenite to deposit on to the existing graphite flakes
leaving a matrix of ferrite. Some of the austenite has also transformed
to pearlite.
(e) Graphite and Ferrite. This variety of iron usually has a very high
silicon content. The graphite-austenite eutectic has cooled slowly enough
for the whole of the carbon dissolved in the austenite to diffuse on to
existing graphite flakes leaving a matrix entirely of ferrite. Such a cast
iron is very soft and easily machined. The ferrite present will contain
dissolved silicon and manganese.
In addition to the phases enumerated above, iron phosphide, Fe
3
P, as
previously mentioned, may be present in the microstructure. In white or
mottled iron it will be present as a ternary eutectic with cementite and
ferrite, whilst in coarse grey irons of the type (e) above, a binary eutectic
with ferrite will be formed. This binary eutectic contains only 10% phos-
phorus, so that an overall 1% phosphorus in the iron may produce suf-
ficient phosphide eutectic to account for 10% by volume of the resulting
structure. The embrittling effect of the phosphide eutectic network will
then be evident.
'Growth'
in Cast Irons
15.50 If an engineering cast iron is heated for prolonged periods above
700
0
C the pearlitic cementite tends to break down to give a mixture of

ferrite and graphite. This leads to an increase in volume of the iron. Inevi-
tably some warping will follow and surface cracks will form. As hot gases
penetrate these cracks internal oxidation will occur and lead to the pro-
gressive deterioration of the casting. Cast iron moulds used for the casting
of non-ferrous ingots develop surface cracks in this way, giving a 'crazy
paving' like surface. Trouble will then arise due to dressing oil collecting
in these cracks. Fire bars in ordinary domestic grates often break up due
to the combined action of 'growth' and internal oxidation.
Certain alloy cast irons have been developed to resist 'growth' at high
temperatures. Silal is relatively cheap and contains about 5% silicon with
low carbon. This very high silicon content will favour the formation of
graphite rather than cementite during solidification so that its structure
consists entirely of ferrite and graphite, and no cementite is present which
can cause growth. Unfortunately Silal is rather brittle, so that, where the
higher cost is justified, the alloy Nicrosilal can be used with advantage.
This is an austenitic nickel-chromium cast iron (see Table 15.5).
Varieties
and
Uses
of
Ordinary Cast Iron
15.60 Foundry irons can be classified in the following groups according
to their properties and uses:
15.61 Fluid Irons which can be used where mechanical strength is of
secondary importance, and in which high fluidity is obtained by means of
high silicon (2.5-3.5%) and high phosphorus (up to 1.5%) contents. Both
silicon and phosphorus improve fluidity, and a high silicon content will
further ensure that thin sections will have a reasonably tough grey struc-
ture,
since silicon prevents deep chilling. Such irons were used extensively
at one time for the manufacture of lamp-posts, fireplaces and railings, but
they have now been replaced by ceramic materials for uses such as these.
15.62 Engineering Irons must have mechanical strength and the best
type of microstructure is one with a small eutectic cell size (small graphite
flakes in a pearlite matrix). An iron of this type will possess the best
all-round mechanical properties and also good machinability. The silicon
content will depend on the thickness of section to be cast, but in general
it will not exceed 2.5% for castings of thin section, and may be as low as
1.2% for castings of heavy section.
The phosphorus content must be kept low where a shock-resistant cast-
ing is necessary, though up to 0.8% phosphorus may be present in some
cases in the interests of fluidity. Sulphur must also be kept low (below
0.1%), as it leads to segregation, hard spots and general brittleness.
Irons of this type can have hard, wear-resistant, chilled surfaces intro-
duced at various parts of the casting if desired. This is done by inserting
chilling fillets at appropriate points in the sand mould. These fillets cause
rapid cooling, and hence a layer of white iron on the surface of the casting
at these points. At the same time the core of the casting remains grey and
tough.
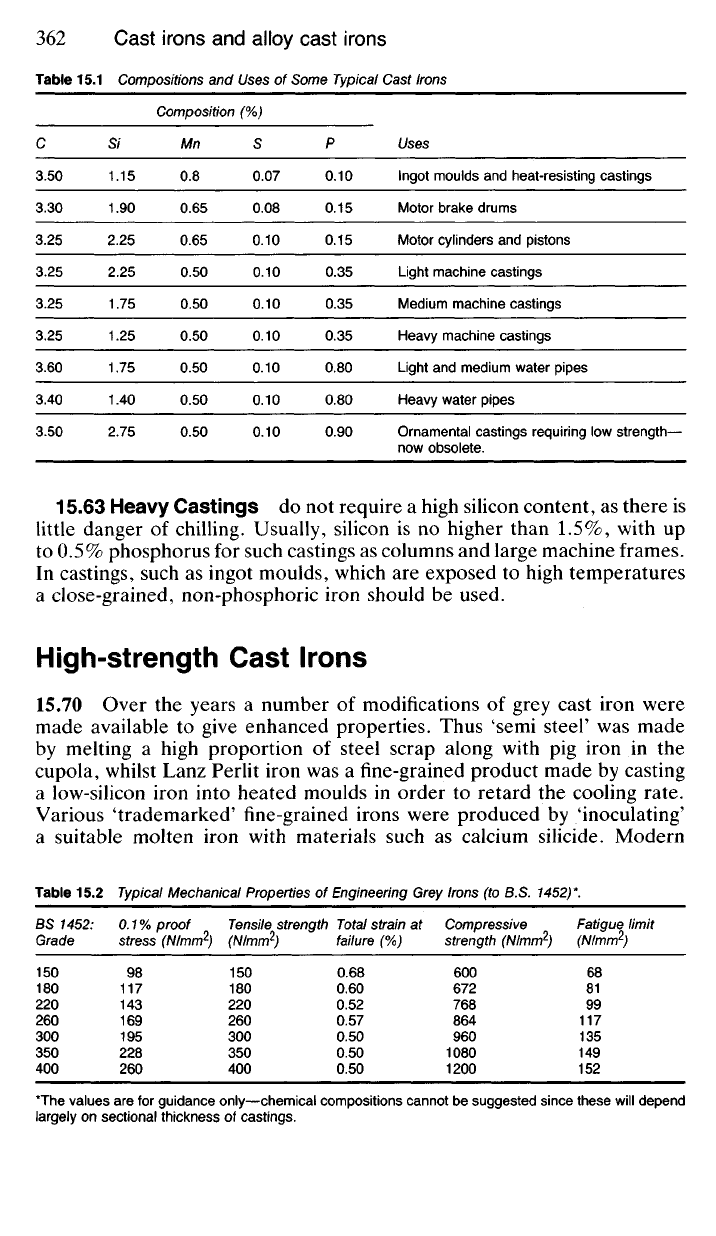
Table 15.1 Compositions and Uses of Some Typical Cast Irons
Composition (%)
C Si Mn S P Uses
3.50 1.15 0.8 0.07 0.10 Ingot moulds and heat-resisting castings
3.30 1.90 0.65 0.08 0.15 Motor brake drums
3.25 2.25 0.65 0.10 0.15 Motor cylinders and pistons
3.25 2.25 0.50 0.10 0.35 Light machine castings
3.25 1.75 0.50 0.10 0.35 Medium machine castings
3.25 1.25 0.50 0.10 0.35 Heavy machine castings
3.60 1.75 0.50 0.10 0.80 Light and medium water pipes
3.40 1.40 0.50 0.10 0.80 Heavy water pipes
3.50 2.75 0.50 0.10 0.90 Ornamental castings requiring low strength—
now obsolete.
15.63 Heavy Castings do not require a high silicon content, as there is
little danger of chilling. Usually, silicon is no higher than 1.5%, with up
to 0.5% phosphorus for such castings as columns and large machine frames.
In castings, such as ingot moulds, which are exposed to high temperatures
a close-grained, non-phosphoric iron should be used.
High-strength Cast Irons
15.70 Over the years a number of modifications of grey cast iron were
made available to give enhanced properties. Thus 'semi steel' was made
by melting a high proportion of steel scrap along with pig iron in the
cupola, whilst Lanz Perlit iron was a fine-grained product made by casting
a low-silicon iron into heated moulds in order to retard the cooling rate.
Various 'trademarked' fine-grained irons were produced by 'inoculating'
a suitable molten iron with materials such as calcium silicide. Modern
Table 15.2 Typical Mechanical Properties of Engineering Grey Irons (to B.S. 1452)*.
BS 1452: 0.1% proof Tensile strength Total strain at Compressive Fatigue limit
Grade stress (N/mm
2
) (N/mm
2
) failure (%) strength (N/mm
2
) (N/mm
2
)
150 98 150 0.68 600 68
180 117 180 0.60 672 81
220 143 220 0.52 768 99
260 169 260 0.57 864 117
300 195 300 0.50 960 135
350 228 350 0.50 1080 149
400 260 400 0.50 1200 152
*The values are for guidance only—chemical compositions cannot be suggested since these will depend
largely on sectional thickness of castings.
high-strength irons rely almost entirely on treatments which replace the
flake-graphite of ordinary grey iron with graphite in the form of either
spherical particles or 'compacted' flakes.
15.71 Spheroidal-graphite (SG) Cast Iron (also known as Nodular
Iron or Ductile Iron). The production of ordinary cast iron has fallen
considerably in recent years due largely to such influences as the develop-
ment of welding fabrication methods and the use of other materials like
concrete and plastics. Nevertheless the introduction of SG iron in 1946 was
without doubt the most important event during this century in the iron
trade since in SG iron the engineer finds a worthy competitor to cast steel
but often at much lower cost.
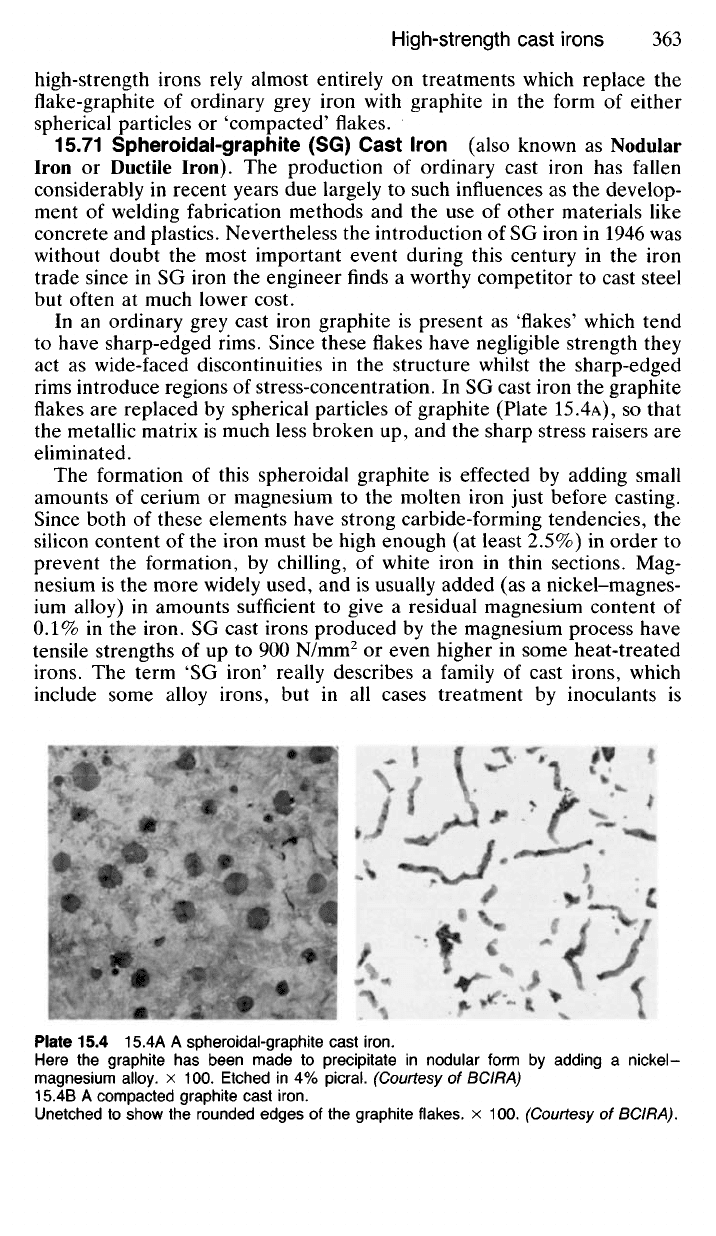
In an ordinary grey cast iron graphite is present as 'flakes' which tend
to have sharp-edged rims. Since these flakes have negligible strength they
act as wide-faced discontinuities in the structure whilst the sharp-edged
rims introduce regions of stress-concentration. In SG cast iron the graphite
flakes are replaced by spherical particles of graphite (Plate 15.4A), SO that
the metallic matrix is much less broken up, and the sharp stress raisers are
eliminated.
The formation of this spheroidal graphite is effected by adding small
amounts of cerium or magnesium to the molten iron just before casting.
Since both of these elements have strong carbide-forming tendencies, the
silicon content of the iron must be high enough (at least 2.5%) in order to
prevent the formation, by chilling, of white iron in thin sections. Mag-
nesium is the more widely used, and is usually added (as a nickel-magnes-
ium alloy) in amounts sufficient to give a residual magnesium content of
0.1%
in the iron. SG cast irons produced by the magnesium process have
tensile strengths of up to 900 N/mm
2
or even higher in some heat-treated
irons.
The term
4
SG iron' really describes a family of cast irons, which
include some alloy irons, but in all cases treatment by inoculants is
Plate
15.4 15.4A A spheroidal-graphite cast
iron.
Here
the graphite has been made to precipitate in nodular form by adding a nickel -
magnesium
alloy, x 100. Etched in 4%
picral.
(Courtesy of BCIRA)
15.4B
A compacted graphite cast
iron.
Unetched
to show the rounded edges of the graphite
flakes,
x 100. (Courtesy of
BCIRA).

*No 'typical compositions' are included since composition, related to the structure in the last column,
will vary with sectional thickness. It will be noted that the 'Grade' designation indicates both tensile
strength and % elongation.
employed to produce spheroidal-graphite particles. Some patents claim the
production of SG iron using the following substances instead of cerium or
magnesium: calcium, calcium carbide, calcium fluoride, lithium, strontium,
barium and argon. In all cases high-quality raw material, as free as possible
from carbide-stabilising elements is required. BS 2789 grades SG irons
according to their mechanical properties and some of these are shown in
Table 15.3.
Those irons consisting of graphite nodules in a ferrite matrix will have
high ductility and toughness whilst those consisting of graphite nodules in
a pearlite matrix will be characterised by high strength. Some of these
irons are heat-treated to give even better mechanical properties. Thus,
sections of the American motor industry harden some of their SG iron
gears by the use of 'interrupted austempering'. This involves austenitising
the gears at 900
0
C for 3.5 h in a nitrogen atmosphere followed by quenching
to 235°C and holding at that temperature for 2 h. Since transformation
from austenite occurs isothermally at 235°C there is little distortion in
shape. It is claimed that SG iron hypoid ring and pinion gears are compar-
able with those of steel in terms of fatigue and also have a greater torsional
strength.
Tensile strengths of the order of 1600 N/mm
2
(with an elongation of. 1%)
can be obtained by austempering SG iron at 250
0
C, following an initial
austenitising at 900
0
C; whilst higher austempering temperatures up to
450
0
C will yield bainitic structures of lower strengths (900-1200 N/mm
2
)
but elongations up to 14%. SG iron crankshafts cast to near final shape
are less expensive and some 10% lighter than equivalent forged com-
ponents. They are heat-treated in a similar way to the gears mentioned
above.
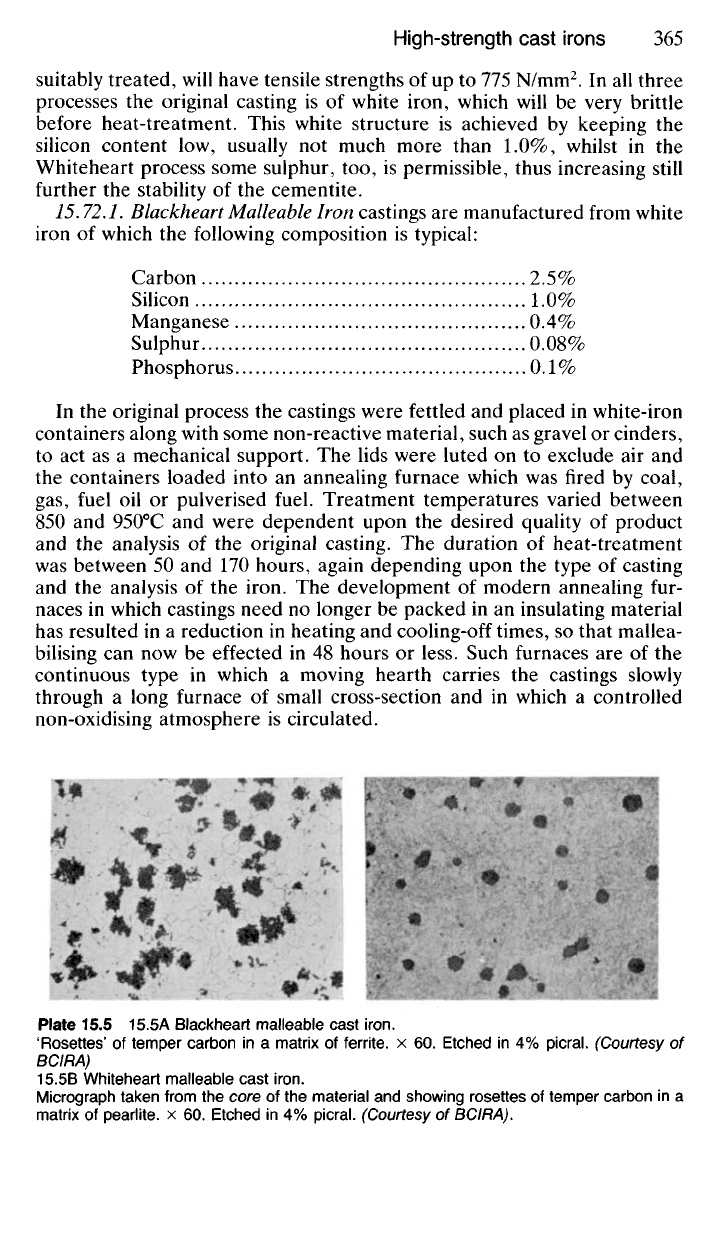
15.72 Malleable Cast Irons are irons which have been cast to shape in
the ordinary way, but in which ductility and malleability have been increased
from almost zero to a considerable amount by subsequent heat-treatment.
The names of the two original malleabilising processes, the Blackheart and
the Whiteheart, refer to the colour of a fractured section after heat-
treatment has been completed. A more recent process for the manufacture
of pearlitic malleable iron aims at the production of castings which, when
Table 15.3 Grades of Spheroidal-graphite Iron to BS 2789*
Grade
370/17
420/12
500/7
600/3
700/2
800/2
0.2% proof
stress (N/mm
2
)
230
250
310
350
400
460
Tensile strength
(N/mm
2
)
370
420
500
600
700
800
Elongation
(%)
17
12
7
3
2
2
Typical hardness
(Hb)
175
200
205
230
265
300
Structure—spheroidal
graphite in a matrix of:
Ferrite
Ferrite
Ferrite-pearlite
Ferrite-pearlite
Pearlite
Pearlite, or a heat-treated
structure.
suitably treated, will have tensile strengths of up to 775 N/mm
2
. In all three
processes the original casting is of white iron, which will be very brittle
before heat-treatment. This white structure is achieved by keeping the
silicon content low, usually not much more than 1.0%, whilst in the
Whiteheart process some sulphur, too, is permissible, thus increasing still
further the stability of the cementite.
75.72.7.
Blackheart
Malleable
Iron castings are manufactured from white
iron of which the following composition is typical:
Carbon 2.5%
Silicon 1.0%
Manganese 0.4%
Sulphur 0.08%
Phosphorus 0.1%
In the original process the castings were fettled and placed in white-iron
containers along with some non-reactive material, such as gravel or cinders,
to act as a mechanical support. The lids were luted on to exclude air and
the containers loaded into an annealing furnace which was fired by coal,
gas,
fuel oil or pulverised fuel. Treatment temperatures varied between
850 and 950
0
C and were dependent upon the desired quality of product
and the analysis of the original casting. The duration of heat-treatment
was between 50 and 170 hours, again depending upon the type of casting
and the analysis of the iron. The development of modern annealing fur-
naces in which castings need no longer be packed in an insulating material
has resulted in a reduction in heating and cooling-off times, so that mallea-
bilising can now be effected in 48 hours or less. Such furnaces are of the
continuous type in which a moving hearth carries the castings slowly
through a long furnace of small cross-section and in which a controlled
non-oxidising atmosphere is circulated.
Plate 15.5 15.5A Blackheart malleable cast
iron.
'Rosettes' of temper carbon in a matrix of ferrite. x 60. Etched in 4% picral (Courtesy of
BCIRA)
15.5B Whiteheart malleable cast
iron.
Micrograph taken from the core of the material and showing rosettes of temper carbon in a
matrix of pearlite. x 60. Etched in 4% picral. (Courtesy of BCIRA).
