Ghodssi R., Lin P., MEMS Materials and Processes Handbook
Подождите немного. Документ загружается.

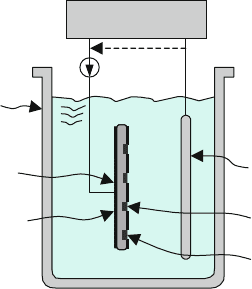
624 D.W. Burns
from the front or more commonly the back of a wafer can enhance and even mod-
ulate the pore diameter [507]. An etch bath that accommodates one or more wafers
and a working or counterelectrode along with an external power supply or poten-
tiostat allows the electrochemical etching of silicon, and under select conditions,
porous silicon, as illustrated in Fig. 8.18. External light illumination (not shown)
may be applied through one of the walls of the etch tank and into a fixture that holds
the wafer. Alternatively, a side or bottom of the etch tank may be modified to secure
the wafer in a leak-tight manner and allow light to be applied externally while the
etchant acts on the inner surface.
Etch
Bath
Etchant
Patterned
Wafer
(Anode)
Ohmic
Contact
Voltage Supply
Counter
Electrode
Protective
Mask
V+
Porous
Silicon
(+)
(–)
Fig. 8.18 Porous silicon
formation is dependent
primarily on dopant type, HF
concentration, applied
voltage, current density, and
illumination levels. The
silicon wafer can be masked
to form porous silicon or
completely remove the silicon
at selective locations.
Selective doping and biasing
of the substrate can produce
controlled 3-D structures
At large anodic (positive) bias, the p-type and n-type material are electro-
chemically etched and disappear. At lower anodic bias, p-type material can be
selectively etched over the n-type. Combinations of n-and p-type diffusions, epi
layers with various bias levels, and control of incident light can result in selec-
tive etching or selective porous silicon formation with subsequent removal to form
three-dimensional microstructures. Masking with metal masks such as gold with
an adhesion layer, unbiased polysilicon layers, silicon carbide, and silicon nitride
allow localized etching or porous silicon formation. Silicon dioxide and even pho-
toresist may be used for short times in a dilute HF solution. Many excellent reviews
and summaries of porous silicon formation that emphasize micromachining aspects
are available to the interested reader [557, 559, 591–595]. Materials such as GaAs
and other III-V compounds also exhibit porous structures with wet electrochemical
etching [596].
8.8.1 Nanoporous, Mesoporous, and Macroporous Silicon
Formation
P-type silicon can be made porous with anodic biasing at nearly every doping level.
N-type silicon requires relatively heavy doping in excess of about 1 × 10
18
cm
−3
with anodic biasing, and exhibits a diminished formation rate between 1 ×10
16
and
1 ×10
18
cm
−3
[556]. Visible light may be applied to transform n-type material into
8 MEMS Wet-Etch Processes and Procedures 625
porous silicon for low doping levels, which generally has little effect on p-type or
degenerate n-type material.
Macroporous silicon is obtained from low-doped n-type silicon with anodic bias
and light in an HF solution, with pores having geometries larger than ∼500 Å that
generally grow perpendicular to the surface that can be initiated with prepatterned
surface pits [595]. Micron-diameter pore sizes are controlled in part by the doping,
the HF concentration and the applied current density [597]. Mesoporous silicon is
formed from heavily doped degenerate n-type or p-type silicon, resulting in large
inner surface areas and pore sizes between 20 and 500 Å with smooth surfaces for
subsequent processing. Nanoporous silicon, also referred to as microporous silicon,
is formed from low-doped p-type or low-doped n-type (with light assistance) silicon,
resulting in geometries less than 20 Å with very high inner surface area and possible
luminescence.
The conditions for porous silicon formation change for p-type and n-type sili-
con when illuminated [559]. Without light and without bias, there are essentially no
reactions at either the p-type or n-type silicon surfaces when exposed to the etchant.
Without light and with low applied anodic bias (positive on the silicon with respect
to a counter- or working electrode), porous silicon forms in the p-type material and
no reactions occur at the n-type surface. For cathodically biased (negative) silicon
and no light, p-type silicon shows no reaction whereas the n-type surface evolves
hydrogen. With light and without bias, the p-type material weakly evolves hydro-
gen and porous silicon is formed in the n-type material. With light and applied
anodic bias, porous silicon forms in t he p-type material and the n-type material. For
cathodically biased silicon with light, hydrogen is evolved at both the p-type and
n-type silicon surfaces. With high applied anodic bias, electrochemical etching (i.e.,
electropolishing) occurs for both p-type and n-type silicon [598, 599]. Organic elec-
trolytes may also be effective in the formation of porous silicon [592]. Some porous
silicon etch rates and etchant details are provided in Table 8.35.
8.8.2 Selective Porous Silicon Removal
As porous silicon can have pore sizes less than 20 Å and more than 500 Å (up to
10 µm for macroporous silicon) with porosities of 50% or higher, large amounts of
surface area occur that enable etching or oxidizing at effective rates much higher
than the bulk, allowing for the selective removal of the porous silicon even with
nonselective etchants. A list of some approaches to the selective removal of porous
silicon is found in Table 8.36
8.8.3 Examples: Porous Silicon Formation
8.8.3.1 Example 1: Chemical Porous Silicon Formation
A 1–3 cm p-type boron doped wafer is inserted into a polypropylene beaker with a
mixture of HF(49%):HNO
3
(70%):H
2
O 4:1:5 that is primed with several fragments
of a silicon wafer prior to adding the DI water. The wafer is s tain-etched between
626 D.W. Burns
Table 8.35 Porous silicon formation rates and processes
Material
Formation
rate
(Å/s) Etchant Remarks and references
1 Silicon (Si), n-type
(100)
HF(49%):H
2
O
1:9
15
◦
C; DHF etchant (9:1); 5 wt% HF; n-type (2-6 cm) silicon with
n
+
implanted backside transparent contact; +2 V applied to silicon
anode; light intensity modulation; pre-structured with oxide mask
and shallow TMAH etch pits; macroporous silicon [507, 508]
2 Silicon (Si), p-type
(100)
30 HF(49%):H
2
O
2:3
Room temperature; DHF etchant (3:2); 20 wt% HF; p-layer with
1 × 10
18
cm
−3
boron between two n-layers with 1 × 10
15
cm
−3
dopant; lateral formation rate; no external bias; 100 mW/cm
2
light
intensity from topside; porous silicon [503]
3 Silicon (Si),
n
–
/p
+
/n
–
/p
+
/p
–
(substrate), (100)
HF(49%):H
2
O
2:3
Room temperature; DHF etchant (3:2); 20 wt% HF; p
+
porous layers
∼ 1 × 10
18
cm
−3
each, n
–
barrier layers ∼ 5 × 10
15
cm
−2
,
p-substrate ∼2 × 10
15
cm
–2
(8–16 cm); multi-level porous
silicon formation; two masking steps [600]
4 Silicon (Si) 65 HF(49%)
undiluted
Room temperature; HF etchant (49 wt%); 5 mA/cm
2
(65 Å/s) then
100 mA/cm
2
for dual-rate anodization; subsequent epi deposition,
local patterning and etching of epi, then rapid (>100x) wet
oxidation of porous silicon layers at 950
◦
C; silicon-on-oxidized
porous silicon (SOPS) process for isolated silicon islands [601]
5 Silicon (Si), n-type
(100)
625 HF(49%):Ethanol
1:2
Room temperature; 1.4–2.3 cm; anodic bias; 25 mA/cm
2
;fine
pores; augmented with thin gold upper electrode; emits visible light
[602]
6 Silicon (Si), p-type
(100)
500 HF(49%):Ethanol
1:1
Room temperature; Hf etchant (25%) with ethanol; p-type (1 cm)
substrate with implanted backside contact; anodic biasing;
230 mA/cm
2
(formation rate reduces to 50 Å/s for 20 mA/cm
2
);
mask with oxide, n-doped poly-Si, NiCr/Au and PR; a lcohol added
to improve bubble release [558]
7 Silicon (Si), p
–
type
(100)
4000 HF(49%):Ethanol
1:1
Elevated temperature; boron-doped 1.0 cm substrate;
1000 mA/cm
2
; dark; microporous silicon [603]

8 MEMS Wet-Etch Processes and Procedures 627
Table 8.35 (continued)
Material
Formation
rate (Å/s) Etchant Remarks and references
8 Silicon (Si), n
+
type
(100)
7500 HF(49%):Ethanol
1:1
Elevated temperature; n-doped 0.067 cm substrate; 400 mA/cm
2
;
dark; mesoporous silicon [603]
9 Silicon (Si), n
–
type
(100)
15000 HF(49%):Ethanol
1:1
Elevated temperature; phosphorus-doped 5.0 cm substrate;
1000 mA/cm
2
; backside illumination; macroporous silicon;
pre-structured surface [603]
10 Silicon (Si), p
+
type
(100)
HF(49%):Ethanol
2:1
Room temperature; boron-doped 0.01–0.02 cm substrate;
7mA/cm
2
;12µm-thick porous silicon with 15% porosity;
subsequent low-temperature thin oxidation, brief HF dip, epi
deposition, 1000 Å oxidation and bonded to oxidized Si handle
wafer, then handle wafer is ground to expose porous silicon which
is then stripped in HF:H
2
O
2
1:5; ELTRAN process [590]
11 Silicon (Si), p-type,
(100)
HF(49%):
HNO
3
(70%):H
2
O
1:5:10
Room temperature (no intentional heating); HNW etchant; 0.05 cm;
stain etching; no bias; no impact by room light while etching;
generates visible stains; visibly luminescent with applied UV light;
prime solution with silicon fragments prior to adding water; 30-s to
10-min etches [604]
12 Silicon (Si), p-type,
(111)
HF(49%):
HNO
3
(70%):H
2
O
1:5:10
Room temperature; HNW etchant; 30–40 cm; stain etching; no bias;
luminescent with UV light [605]
13 Silicon (Si), p-type,
(100)
HF(49%):
HNO
3
(70%):H
2
O
4:1:5
Room temperature (no intentional heating); HNW etchant; 1–3 cm;
stain etching; no bias; generates visible stains; visibly luminescent
with applied UV light; also (111) material and n-type material [604]
14 Silicon (Si), p
–
type,
(100)
450 HF(49%):H
2
O
4:1
Room temperature; DHF etchant (1:4); 40 wt% HF; FIPOS process;
1.5 cm; proton-implanted silicon n-type islands; 50 mA/cm
2
;
dual chamber for anodization; oxidize porous silicon (10–20x faster
than thermal) and restore islands to p-type [606]

628 D.W. Burns
Table 8.36 Selective porous silicon removal rates and processes
Material
Etch rate
(Å/s) Etchant Remarks and references
1 Silicon
(Si),
porous
400 HF(49%):
H
2
O
2
(30%)
1:5
Room temperature; p
+
porous silicon
with 15% porosity; doesn’t
significantly etch Si (<0.01 Å/s)
[590]
2 Silicon
(Si),
porous
HF(49%):
HNO
3
(70%):
Acetic 3:16:1
Room temperature; HNA etchant; 5-s
dip may be adequate [509]
3 Silicon
(Si),
porous
KOH:H
2
O
10 g:1000 mL
Room temperature; KOH etchant
(1 wt%); 0.1 wt% KOH for
nanoporous silicon to 10 wt% KOH
for mesoporous silicon; also TMAH
etchant or TMAH-based developer
[557, 559, 595]
4 Silicon
(Si),
porous
KOH:H
2
O
250 g:750 mL
25
◦
C; KOH etchant (25 wt%); 30-s
dip may be adequate [509]
5 Silicon
(Si),
porous
Oxidize then HF Room temperature; HF, B HF, or DHF
etchant; oxidize at 1000
◦
C(wet)
then etch [503]
30 s and 10 min to produce a porous silicon layer about 0.5 µm thick. After rinsing
and drying, the resulting stains are visually luminescent with a reddish-orange color
when exposed to UV light at 365 nm. Exposure to light during stain-etching does
not affect the result [604].
8.8.3.2 Example 2: Nanoporous Silicon Formation
A 525 µm-thick p-type double-side polished silicon wafer with a resistivity of 1–
10 cm is implanted with boron to a dosage of 1 × 10
16
cm
−2
, annealed and then
coated with a 0.5 µm-thick layer of aluminum layer on one side. The wafer is placed
in a fixture that allows electrical contact to the aluminum layer via a metal spring
while keeping the metalicized side dry. The fixture is inserted into a 1:1 mixture
of HF(49%) and ethanol with the metal spring connected to an adjustable voltage
supply. A platinum mesh serving as a counterelectrode is placed in the etchant and
also connected to the power supply. The etch tank is covered to keep the wafer dark.
The voltage is adjusted to supply 10 mA/cm
2
. After 20 min, nanoporous silicon has
formed to a depth of 10 µm. The wafer is rinsed in DI water, dried, and removed
from the fixture [603].
8.8.3.3 Example 3: Mesoporous Silicon Formation
A lightly doped n-type wafer with a 10 µm-thick phosphorus-doped epi layer having
a resistivity of 0.07 cm and an ohmic backside contact is inserted into a fixture
8 MEMS Wet-Etch Processes and Procedures 629
and placed in a 1:1 mixture of HF(49%):ethanol at room temperature. A power
supply provides 15 mA/cm
2
of electrical current between the wafer and a platinum
electrode in the etchant for 5 min to form a mesoporous silicon layer that is 10 µm
thick and stops on the lightly doped substrate [603].
8.8.3.4 Example 4: Macroporous Silicon Formation
An n-type (100) silicon wafer with a resistivity of 2–6 cm and a single 150 µm-
thick s ilicon diaphragm has a patterned frontside oxide and a cleared backside that
is implanted to form an n
+
contact. While in a fixture, the frontside is etched in
TMAH(25 wt%) etchant for 3
1
/
2
min at 80
◦
C to form pyramidal pits that are 2 µm
wide on each side and periodically spaced in the x- and y-directions with a center-
to-center spacing of 4 µm. The wafer is placed in a second fixture. The fixture and a
platinum counterelectrode are inserted into an etch bath with HF(5 wt%) and some
surfactant at 15
◦
C, and the wafer is biased at +2 V. Light from an external halogen
lamp illuminates the wafer backside, and the n-silicon is anodically etched from the
frontside starting at the bottoms of the pyramidal pits. As the pores are formed, the
light is modulated to vary the diameter of the pore holes with a 4 µm period until
a depth of about 130 µm is reached. After opening the pores from the backside by
removing the remaining silicon, the membranes are immersed in TMAH(25 wt%)
for 60 min at 5
◦
C to encourage (110) etching over (100) etching throughout the
length of the pores, resulting in the periodic interconnecting of adjacent pores in a
3-D structure with bandgap properties as a photonic crystal [507].
8.9 Layer Delineation and Defect Determination
with Wet Etchants
Wet chemical etching can be used to delineate thin films and substrate layers for vali-
dating designs and processes, and to detect and locate defects that cause yield losses
or long-term reliability concerns. These etchants are often applied to devices that
have been cross-sectioned to allow direct observation of structure details. Absolute
doping levels may be intractable, however, dopant-selective etchants can reveal the
type and relative doping levels in a substrate and delineate p–n junctions by decorat-
ing and making visible variations and transitions in the doped regions for verification
of implant and epi profiles, thermal diffusion coefficients, and the thickness of
various layers.
Although difficult to locate and observe on a molecular scale, crystalline defects
such as dislocations and stacking faults can be detected with selective etchants.
Short and sometimes extended etch times are required to generate sizeable etch
pits in a substrate that indicates the presence of one or a series of defects, benefiting
from the property of some etchants to attack defects preferentially. Etchants may be
preferential to dislocations and defects that surround individual crystalline grains,
630 D.W. Burns
and grain boundaries in deposited films such as polysilicon and aluminum can be
selectively etched to reveal the grain size.
Cross-sectioning of devices for process characterization, verification, qualifi-
cation, or failure analysis may involve the delineation of layers to enhance their
visibility in microscope or SEM micrographs. The micrographs can be enhanced
by selective decoration or the generation of preferentially etched steps in a cross-
sectioned stack of materials. Selective etchants for delineating different oxides,
dielectrics, metals, and semiconducting regions in a device are described below.
Other etchants are suitable for locating and determining the density of defects such
as pinholes in deposited layers. This section ends with several examples of junction
location and layer delineation. Many of the etchants and techniques presented in this
section require special care and expertise to use well, as many are light-sensitive or
can etch portions of the sample very rapidly. Several excellent references can be
consulted for further details and discussions [607–610].
8.9.1 Dopant Level and Defect Determination with Wet Etchants
Selective etchants, such as hi–low etchants or p–n junction delineation etchants,
can be used to determine junction depths for single p–n junctions or for complex
configurations such as twin-well BiCMOS processes with n-type and p-type buried
layers and multiple levels of interconnect. These etchants may stain or discolor one
dopant type over another, or etch one type faster than the other to allow the user to
locate metallurgical junctions in cross-sectioned samples. The hi–low etchants will
etch highly doped material preferentially over lightly doped material. For increased
accuracy in junction depth measurements, lapping and polishing a sample with an
angled mounting fixture as low as 1
◦
may be used. SEM micrographs often use
90
◦
mounts for accurate dimensioning between layers. A series of dopant-sensitive
etchants including hi–low etchants, p–n junction etchants and staining etchants for
silicon are listed in Table 8.37.
Defects in crystalline or polycrystalline samples may be determined by the for-
mation of etch pits or other visible features with orientation-sensitive etchants that
etch more rapidly in the presence of dislocations and stacking faults in the substrate.
Some of the etchants are more suitable for quantifying defect densities in boules or
wafers of silicon, as they can etch the material quite rapidly. Table 8.38 shows a
series of etchants for decorating defects in silicon with emphasis placed on etchants
containing common cleanroom chemicals. Table 8.39 presents additional etchants
containing metal compounds for decorating defects in silicon that may be more suit-
able for a failure analysis laboratory. Table 8.40 includes etchants that preferentially
etch grain boundaries in certain materials of interest. Compilations of dislocation
etchants for other materials such as III-V and II-VI compounds can be found in
several references [76, 611, 612].
8 MEMS Wet-Etch Processes and Procedures 631
Table 8.37 Silicon dopant-sensitive etchants and etch processes
Material
Etch rate
(Å/s) Etchant Remarks and references
1 Silicon (Si), n
+
/n
Silicon (Si), n
+
/p
Silicon (Si), p
+
/n
Silicon (Si), p
+
/p
0.8–400 HF(49%):
HNO
3
(70%):Acetic
1:3:10
25
◦
C; HNA etchant; also Dash etchant; hi–low etchant; bevel and polish sample; strong
illumination; swab, squirt or drip etchant; 1–15 s; look for discoloration; etches lightly
doped Si(100) 22 Å/s, Si(111) 0.8 Å/s; etches heavily doped n-type or p-type Si faster
(∼400 Å/s f or < 5 × 10
18
cm
−3
); 10–15 s for cross-sectioning; stain will appear and
steps may be generated [45, 76, 441, 613–615]
2 Silicon (Si), n
+
/n
Silicon (Si), n
+
/p
Silicon (Si), p
+
/n
Silicon (Si), p
+
/p
HF(49%):
HNO
3
(70%)
50 mL: 6 drops
Room temperature; hi–low etchant; bevel and polish sample; strong illumination; swab,
squirt or drip etchant; 1–15 s; look for discoloration on p-type side [613, 616, 617]
3 Silicon (Si),
n
+
/p, alloyed
with Al
Silicon (Si),
p
+
/n, alloyed
with Al
HF(49%):
HNO
3
(70%):Acetic
1:3:6
Room temperature; HNA etchant; p–n junction etchant; bevel and polish sample ∼3
◦
;no
illumination; swab, squirt, or drip etchant; 10–60 s; generates step [613]
4 Silicon (Si), n/p
Silicon (Si), p/n
HF(49%)
undiluted
Room temperature; HF etchant (49 wt%); p–n junction etchant; strong illumination;
n-type turns dark [618]
5 Silicon (Si), n/p
Silicon (Si), p/n
HF(49%):
HNO
3
(70%)
200:1
Room temperature; p–n junction e tchant; 5–10 s; illumination amplifies effect; p-type is
stained [45]
632 D.W. Burns
Table 8.37 (continued)
Material
Etch rate
(Å/s) Etchant Remarks and references
6 Silicon (Si), n/p
Silicon (Si), p/n
HF(49%):
HNO
3
(70%)
3:97
Room temperature; p–n junction e tchant; also 97:3 etchant; 3–5 s; n-type is stained [45]
7 Silicon (Si), n/p
Silicon (Si), p/n
HF(49%):
CuSO
4
.
5H
2
O:H
2
O
10 mL:8 g:980 mL
Room temperature; p–n junction e tchant; copper sulphate etchant; stains dopant levels
down to 1 ×10
14
cm
−3
; bevel lap and polish prior; 5–60 s with illumination; rinse and
dry; n-type is stained [45, 619]
8 Silicon (Si), n/p
Silicon (Si), p/n
HF(49%):H
5
IO
6
:KI:
H
2
O
2 mL:5 g:5 mg: 50
mL
Room temperature; Sponheimer–Mills etchant; p–n junction etchant; add KI last; bevel
lap and polish; add one part acetone to 30 parts staining solution before use; ∼25 s in
ultrasonic; simultaneous delineation of active devices including junctions, implants,
oxides, nitrides, aluminum, tungsten, and polysilicon; p-type preferentially etched [45,
620]

8 MEMS Wet-Etch Processes and Procedures 633
Table 8.38 Silicon dislocation delineation etchants and etch processes: I
Material
Etch rate
(Å/s) Etchant Remarks and references
1 Silicon (Si) 830 HF(49%):HNO
3
(70%):Acetic
8:75:17
25
◦
C; HNA etchant; planar etchant; all planes [441]
2 Silicon (Si) HF(49%):
HNO
3
(70%):Acetic
2:3:2
Room temperature; HNA etchant; silicon polishing etchant; 2–3 min
[45]
3 Silicon (Si),
(100), (110),
(111)
22 HF(49%):
HNO
3
(70%):Acetic
1:3:10
Room temperature; HNA etchant; also Dash etchant; reveals
dislocations and stacking faults; 15–20 min for (100) and (110)
surfaces; ∼4 h for (111) surfaces; etch rate for n
–
or p
–
(100)
material; faster for n
+
and p
+
(∼400 Å/s) [441, 611, 614, 615]
4 Silicon (Si) HF(49%):
HNO
3
(70%):Acetic
3:5:3
Room temperature; HNA etchant; also CP-4A etchant; highlights
twinning defects in silicon [609]
5 Silicon (Si), poly HF(49%):
HNO
3
(70%):Acetic
1:3:6
Room temperature; HNA etchant; delineates polysilicon grain
boundaries; ∼6min[609]
6 Silicon (Si), poly 1000 HF(49%):
HNO
3
(70%):Acetic
36:1:20
Room temperature; HNA etchant; also Sopori etchant; defect etchant
for polysilicon; dislocations and stacking faults; 5–30 s; cool to
10
◦
C for slower etch rates; HNO
3
component may be doubled [621]
