Ghodssi R., Lin P., MEMS Materials and Processes Handbook
Подождите немного. Документ загружается.

424 S. Tadigadapa and F. Lärmer
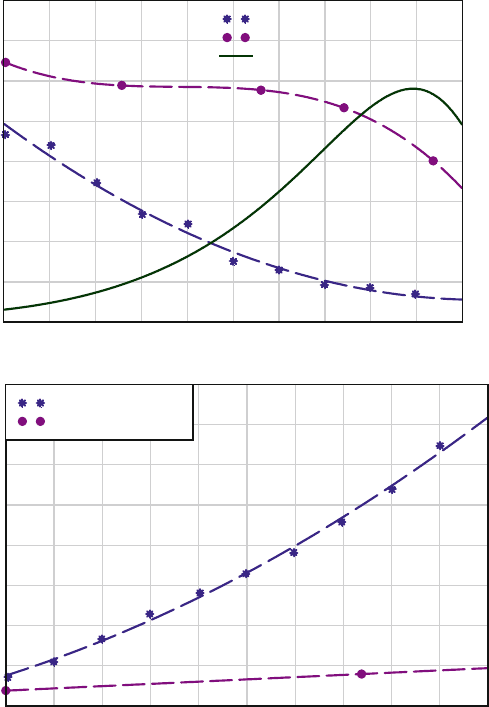
Figure 7.9 shows the polymer film thickness and etch rates of silicon and silicon
dioxide upon 0–50% H
2
addition to CF
4
plasma [43]. An increasing thickness of the
fluorocarbon with increasing hydrogen concentration and a proportional decrease in
the etch rate of silicon is observed, whereas the SiO
2
shows neither an enhanced
formation of the fluoropolymer film nor any appreciable dependence of the resulting
etch rate. Using this technique SiO
2
: Si selectivity of up to 20:1 has been reported.
CF
4
− H
2
plasma provides high selectivity between photoresist and SiO
2
films as
shown in Fig. 7.10.
Percentage of H
2
in CF
4
/H
2
Fluorocarbon Film Thickness (nm)
0 5 10 15 20 25 30 35 40 45 50
0
0.5
1
1.5
2
2.5
3
3.5
4
For Si Surface
For SiO
2
Surface
Percentage of H
2
in CF
4
/H
2
Etch Rate (nm/min)
SiO
2
/Si Etch Rate Ratio
0 5 10 15 20 25 30 35 40 45 50
1
0
2
5
3
10
4
15
5
20
6
25
7
30
8
35
9
40
Silicon Etch Rate
Silicon Dioxide Etch Rate
SiO
2
/Si Etch Rate Ratio
Fig. 7.9 Fluorocarbon film thickness on Si and SiO
2
measured after 5 min RIE under identical
condition (bottom). Formation of the relatively thick layer of fluorocarbon effectively suppresses
the etch rate of silicon in CF
4
/H
2
(top) plasma providing high selectivity conditions suitable for
etching SiO
2
[43] (Used with permission, copyright 1989, The Electrochemical Society)
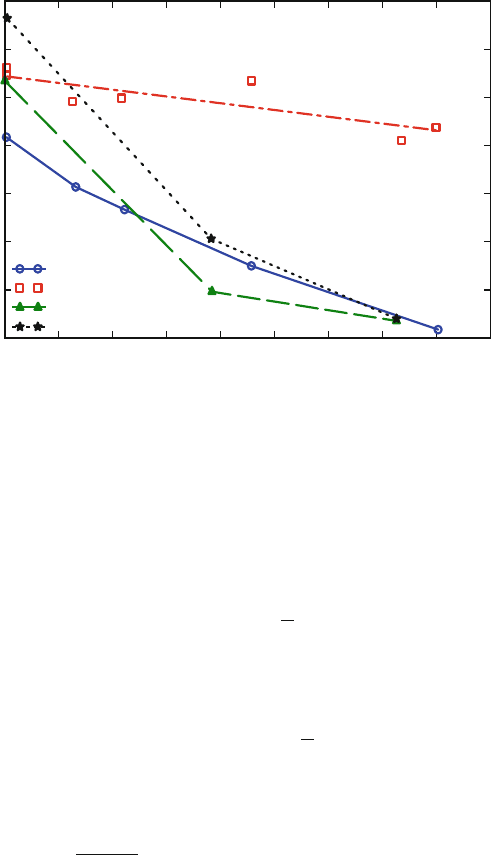
7 Dry Etching for Micromachining Applications 425
% H
2
in CF
4
Etch Rate (nm/min)
0 5 10 15 20 25 30 35 40 45
0
10
20
30
40
50
60
70
RF Power = 0.26 W/cm
2
Pressure = 0.47 Pa
Flow Rate = 28 sccm
Silicon
Silicon Dioxide
AZ1350B Resist
PMMA
Fig. 7.10 Etch rate of photoresists, silicon, and silicon dioxide in H
2
/CF
4
plasma. At high hydro-
gen percentage, a large value of selectivity between SiO
2
and these materials is obtained [42](Used
with permission, copyright 1979, The Electrochemical Society)
Silicon to Silicon Dioxide Etch Selectivity: Fluorine plasma naturally provides an
inherent selectivity for etching silicon with respect to silicon dioxide. Flamm et al.
measured the luminescence during silicon etching in fluorine plasma and concluded
that fluorine atoms etch silicon at a rate (R
F(Si)
)given by [44]
R
F(Si)
= 2.91 ± 0.20 × 10
−12
√
Tn
F
e
−0.108 eV/k
B
T
, (7.4)
where n
F
is the atom concentration of fluorine given in #/cm
−3
. The etch rate for
silicon dioxide (R
F(SiO
2
)
) in fluorine plasma is given by the expression
(R
F(SiO
2
)
) = 6.14 ±0.49 × 10
−13
√
Tn
F
e
−0.163 eV/k
B
T
(7.5)
resulting in a relative Si to SiO
2
etch ratio of
R
F(Si)
R
F(SiO
2
)
= (4.74 ± 0.49)e
−0.055 eV/k
B
T
(7.6)
and ranges from a value of 41 at room temperature to 26.2 at 100
◦
C. Etching of
silicon in chlorine plasma has been presented by Schwartz et al. [7]. In general
silicon etching in chlorine plasma is an ion-beam induced process and therefore
results in poorer selectivity.
Addition of Inert Gases: In addition to oxidants and radical scavengers, inert
gases are often added to the plasma. This is done for several reasons: (i) inert gases
such as Ar stabilize the plasma because they readily donate electrons to the plasma
426 S. Tadigadapa and F. Lärmer
in contrast to the chemical species which, owing to their high electronegativity, cap-
ture available electrons needed to maintain the glow discharge; (ii) the high sputter
yield of Ar
+
ions improves the ion-assisted etch rate; (iii) the directional nature
of ion bombardment through the sheath improves the anisotropy of the etch; and
(iv) the high thermal conductivity of inert gases such as He when added to the
plasma can improve the heat transfer from the substrate wafer to the supporting
chuck and plasma chamber. Of course large quantities of inert gas addition will
dilute the reactive gas component and the etch process will shift towards physical
sputter etching.
Recently, Yamakawa et al. [45] have demonstrated very high speed etching of
silicon dioxide (BPSG) film using microwave excited nonequilibrium atmospheric
pressure plasma. The authors were able to demonstrate an ultrahigh etch rate of
SiO
2
(14 μm/min) and an unprecedented selectivity of 200 with respect to silicon
using NF
3
/He along with addition of H
2
O as the etching gas. The fast etch rate can
be attributed to the presence of NF
x
radicals arising from the breakup of NF
3
into
NF
∗
x
+F
∗
−radicals. NF
x
−radicals (with x > 0) are extremely aggressive towards
SiO
2
and other dielectric (and polymers) materials and etch them at extremely high
speed. Addition of water vapor (H
2
O) consumes the fluorine radicals by HF forma-
tion (2F
∗
+H
2
O → 2HF+ < O >) whereas NF
x
radicals are much less scavenged.
Water vapor therefore acts as a selective fluorine radical scavenger and consequently
reduces any undesirable Si-attack. However, if NF
3
is completely broken down, for
example, as in high-density, high-power plasma, then the plasma primarily consists
only of fluorine and nitrogen radicals. Under these conditions, a complete quenching
of the fast etch rate of SiO
2
and other dielectrics has been observed. The complete
breakup of NF
3
molecules under appropriate plasma conditions is also confirmed
by the plasma color which changes from a deep red to a bluish color, indicating a
change in preponderance from NF
x
to fluorine radicals in the plasma.
Silicon Nitride Etching: Silicon nitride is readily etched in fluorine plasma and
has intrinsic etch characteristics between silicon and silicon dioxide. Because sili-
con nitride is typically used in the LOCOS (local oxidation of silicon) process, there
is often a need to etch silicon nitride over silicon dioxide and silicon with high selec-
tivity. The intrinsic rate of etching for Si
3
N
4
is about 5–10 times higher than that
of SiO
2
depending upon temperature. In a CF
4
/O
2
plasma the obtained Si
3
N
4
:SiO
2
selectivity is ∼2[46]. In general adding nitrogen to CF
4
/O
2
plasma has been found
to improve the selectivity of silicon nitride over silicon dioxide to 10:1.
Sanders et al. reported achieving a high selectivity of >10:1 for etching silicon
nitride over silicon dioxide by the addition of CF
3
Br to CF
4
/O
2
plasma [46]. It
is considered that this chemistry converts some of the F atoms into BrF and BrF
3
which selectively etch silicon nitride whereas silicon dioxide is not affected by the
interhalogen gas species. Furthermore, Br and Cl radicals in the plasma are thought
to convert the atomic oxygen (radicals) into more inert oxygen molecules. This
quenching of oxygen radicals, which would otherwise oxidize the Si
x
N
y
surface
explains the enhancement of Si
x
N
y
etching in comparison to SiO
2
in interhalogen
gases. The highest selectivity in etching silicon nitride over silicon dioxide has been
reported when using interhalogen fluoride gases such as ClF
3
and BrF
3
[47].
7 Dry Etching for Micromachining Applications 427
In summary, due to the spontaneous formation of SiF
4
, silicon is more easily
etched than SiO
2
which requires the etching process to be induced via ion bom-
bardment. Addition of oxygen in RIE etch processes increases the etch rate of both
Si and SiO
2
. Addition of hydrogen can be used to selectively etch silicon dioxide
over silicon through the formation of fluorocarbon films. Silicon nitride etches at a
rate that is in between that of silicon and silicon dioxide. Highly selective etching
of silicon nitride over silicon dioxide layer can be performed in 1:2 ratio NF
3
/Cl
2
downstream etchers.
7.5 Case Study: High-Aspect-Ratio Silicon Etch Process
Fluorine-based plasma etching processes for silicon yield high etch rate, etch depth,
and mask selectivity, and in particular enable photoresist masking with good selec-
tivity between silicon and photomask. This is mainly due to the high chemical
reactivity and spontaneous etching nature of the fluorine radicals towards silicon,
and the high volatility of the silicon fluorides as reaction products. As a conse-
quence, the etch is intrinsically isotropic. Fluorine radicals do not need ion activation
to initiate or enhance their reaction with silicon, or to remove the reaction products
from the silicon surface; anisotropy can only be achieved by the inclusion of side-
wall passivation schemes to the process. The existing approaches to deep reactive
ion etching (DRIE) of silicon are distinguished by the way sidewall passivation
is achieved, the key to anisotropy and overall performance of the etch process.
Cryogenic etching and the so-called “Bosch process” with alternating etch and pas-
sivation cycles are the two most well-known high-aspect-ratio silicon etch processes
and are discussed in this section.
A high-density plasma tool with so-called remote or decoupled plasma is ben-
eficial or even a must for all silicon DRIE approaches, be it cryogenic or Bosch
processing. Silicon DRIE is based on the chemical activity of the plasma. Chemical
activity requires both a sufficiently high concentration of passivating r adicals, and a
sufficiently high concentration of fluorine radicals to obtain the high etch rates. For
a high density of chemically active species, a process pressure regime of typically
between 1 and 10 Pa is most appropriate. Energy of the ion flux bombarding the
wafer surface must be controlled independently from the plasma excitation, through
substrate electrode biasing by a radio frequency or low frequency bias generator.
For a good control of ion acceleration independent of plasma excitation, the plasma
potential should be kept as low as possible near ground potential. The latter involves
decoupling of the plasma excitation zone from the substrate region.
Traditional plasma sources for RIE fail in one or more of these require-
ments. Diode and triode reactors show insufficient plasma excitation density, below
10
16
m
−3
. Electron cyclotron resonance (ECR) sources are inappropriate for the
very low pressure regime (<0.1 Pa) tolerated by the cyclotron resonance mode.
ECR is a typical ion-current type of source and lacking sufficient chemical activ-
ity. Microwave surfatron sources (a tube or horn made from dielectric material,
to guide the microwave field along the interface between the dielectrics and the
428 S. Tadigadapa and F. Lärmer
plasma burning inside the tube or horn, down to the process chamber, (e.g., see
[48]), helicon sources, and inductively coupled plasma (ICP) sources open the pres-
sure window up to 10 Pa and to even higher pressures. Amongst these alternatives,
the ICP has emerged as the most appropriate and widespread source technology for
DRIE and as the industry standard.
7.5.1 Cryogenic Dry Etching
As explained, sidewall passivation is necessary to obtain anisotropic silicon etches.
One approach is to use fluorine radicals created from sulfur hexafluoride gas (SF
6
)in
the plasma discharge as the main etching species which would readily and isotropi-
cally remove silicon wherever it is unprotected and accessible. Oxygen radicals are
created in the plasma from oxygen gas (O
2
) and serve for silicon surface oxidation.
Oxidation saturates dangling bonds on the silicon surface and builds up a passivat-
ing silicon oxide film, which inhibits fluorine attack of the silicon. After finding the
appropriate SF
6
to O
2
flow ratio, an oxide scavenging gas such as trifluoromethane
CHF
3
may be added to improve results and widen the process window to some
degree (so-called “Black Silicon Method”, see [49]). At room temperature, the per-
centage of oxygen needed to achieve anisotropic profiles is larger than 50%, which
reduces both etching speed and mask selectivities considerably.
Cryogenic dry etching is a variation of the sidewall passivation technique based
on surface oxidation. Lowering the wafer temperature into the cryogenic regime
typically around 173 K (–100
◦
C) by liquid nitrogen (LN
2
) cooling of the substrate
electrode, brings down the required oxygen amount for anisotropic etching to a few
percent of the total gas flow, thus pushing up etching speed and mask selectivi-
ties drastically. In addition, the process window for achieving anisotropic etching is
widened strongly.
This results from an enhancement of the chemical stability of the inhibiting sil-
icon oxide film in a fluorine-based plasma, mainly because desorption of reaction
products of silicon oxyfluoride-type from unbombarded surfaces is frozen by the
low temperature. Ion bombardment of the etch floor removes the passivating film
there by sputtering off most of the chemical compounds. Perfectly vertical etch-
ing in silicon can be achieved with a strong overweight of etching fluorine radicals,
compared to the passivating oxygen radicals, yielding high etch rates and a relatively
wide process window, in comparison to the room temperature situation.
Impressive results are achieved with cryogenic dry etching in inductively cou-
pled plasma tools such as the Alcatel reactor, with silicon etch rates reaching
4–6 μm/min, and mask selectivities of several 100:1 for SiO
2
-hardmasks [50, 51].
Use of photomasks is difficult due to cracking and delamination reasons at the
low substrate temperatures, however, not impossible if special resist treatment for
stress adjustment is performed before the etch. The main drawback of the cryogenic
etching method is related to the low substrate temperatures needed and the criti-
cal dependence of process properties on the substrate temperature, given the strong
heat impact from the plasma to the substrate and the exothermic etching reaction
7 Dry Etching for Micromachining Applications 429
between silicon and the fluorine radicals. In addition, the substrate as the coldest
part in the whole reactor configuration acts as a cryopump, freezing out compounds
from the plasma which act as hard-to-remove micromasks on the wafer surface.
Micromasking is responsible for the formation of microneedles and micrograss on
the silicon which is often observed in deep cryogenic etches as a disturbing and
often unacceptable factor. Nevertheless, cryogenic dry etching is a very important
silicon microstructuring technique, for example, in microoptical applications, where
smooth sidewalls on a nanometer scale are of key importance.
7.5.2 Bosch Process
The etching technique described before makes use of relatively hard to remove com-
pounds as passivating layers, in the form of silicon oxides or oxyfluorides resulting
from silicon surface oxidation. Their complete removal from the etch floor without
leaving micromasking residues requires energetic ion impact, eventually in com-
bination with added scavenging agents. Excessive ion sputtering and the use of
scavengers reduce the selectivity towards the masking materials. This is especially
true in the case of photoresist masking. There is a tradeoff between a clean trench
bottom and high mask selectivity.
A technique that avoids the formation of such hard to remove compounds is the
deposition of smooth polytetrafluoroethylene (PTFE) or Teflon
R
-like films on the
silicon surface during etching. Early published work describes the importance of
the balance between etching and polymerizing species, and effects of changing this
balance for both Si and SiO
2
etching [52].
Plasma polymerization can be achieved by the generation of (CF
2
)
n
-type radi-
cals in the plasma, starting from precursor gases such as hexafluoropropene (C
3
F
6
)
or octafluorocyclobutane (RC318
R
,C
4
F
8
), the latter being a nontoxic and stable
dimer of tetrafluoroethylene (TFE, C
2
F
4
). The deposited smooth passivating film
consists of a network of long linear (CF
2
)
n
-chains with only little cross-linking,
which can be easily removed from the etch floor by low-energetic ion bombard-
ment without leaving behind any residues. A mixture of sulfur hexafluoride gas for
the fluorine radical supply and octafluorocyclobutane gas for the supply of polymer-
forming radicals can be used in the plasma to achieve both passivation at the sidewall
and etching at the etch floor, yielding anisotropic profiles in silicon. In addition, hav-
ing fluorocarbons in the process to some extent scavenges undesired oxygen in the
background gas or oxygen precipitates in Si and thus prevents the formation of hard
passivation (silicon oxides) from the etched surface (floor).
However, the presence of fluorine radicals as etching species and of Teflon
R
film-forming monomer species together in the plasma, at the same time, leads
to recombination and mutual extinction of both kinds of species. This makes the
“mixed process” difficult to control for deeper etches and lowers process perfor-
mance mainly with respect to etch-rate. Although, these drawbacks are somewhat
mitigated using high-density ICP plasma, still the potential of the “mixed process
approach” remains limited to shallow trenches with a depth on the order of 10 μm.
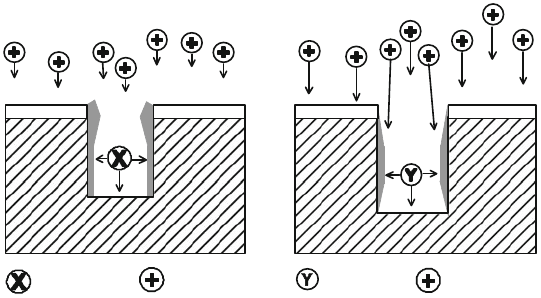
430 S. Tadigadapa and F. Lärmer
The recombination problem was overcome by the patented Bosch-process tech-
nology [48], which is a variation of the Teflon
R
-based sidewall passivation
technique. Figure 7.11 illustrates the process mechanism: passivation and etching
gases are introduced separately and alternately into the process chamber and the
substrate to be etched is exposed to a high-density plasma, during the so-called pas-
sivation and etch cycles, respectively. In each passivation cycle, thin Teflon
R
-like
film is deposited onto the walls of etched structures from C
4
F
8
-precursor species.
Some scavenging of oxides from the silicon etch floor may also happen during
or after the Teflon
R
deposition step. During the subsequent etching cycle, part of
this film is removed from the coated sidewall by off-vertical ion i mpact and driven
deeper into the trench. At the same time the trench bottom is cleared of fluorocarbon
polymer and etched by fluorine radicals released from SF
6
in the plasma. Switching
times between steps normally range from a few seconds up to 1 min, depending on
sidewall roughness that can be tolerated. Because the passivating polymer film can
be easily removed by small ion impact sputtering, mask selectivity reaches very high
values, for example, 150:1 for photoresist and »300:1 for SiO
2
hard masks. If harder
passivating polymers were used, more aggressive ion impact would be required and
as a consequence, the selectivity towards the masking material would be reduced.
CF2-species
Passivation
F-species
Etching
positive ions
positive ions
Fig. 7.11 Illustration of the basic mechanism of the Bosch process. The protecting Teflon
R
-like
film deposited onto the structures during the passivation step is cleared from the trench floor and
driven deeper into the trenches during the (intrinsically isotropic) fluorine-based etching step
Transport of the sidewall film (i.e., removal and redeposition of Teflon
R
-
material) yields some local anisotropy of the etching steps that would otherwise be
completely isotropic, in the vicinity of the sidewall, but does not adversely affect the
main etching reaction away from the sidewall. Sidewall roughness is thus reduced
despite the discontinuous process. Plasma polymerization is nonconformal i n nar-
row trenches and film formation preferentially takes place at the trench opening,
leaving deeper sidewall regions with less deposited passivating film, therefore the
flow of polymer material along the sidewalls towards the trench floor also provides a
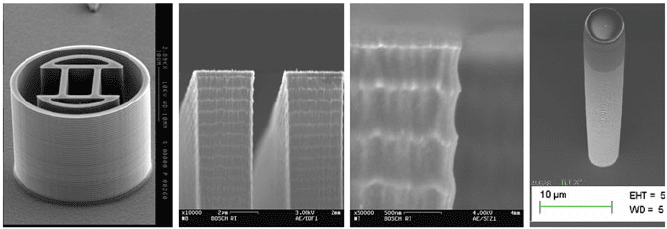
7 Dry Etching for Micromachining Applications 431
Fig. 7.12 Trench profiles and sidewall structure resulting from a typical Bosch DRIE process
recipe
more uniform sidewall passivation over the trench depth. Figure 7.12 shows typical
structures, profile forms, and characteristic sidewall scalloping arising from standard
Bosch DRIE process recipes.
The discontinuous nature of the process overcomes a number of general problems
involved with anisotropic etching.
Firstly, recombination loss of reactive species, by gas phase reactions causing
their pairwise extinction, is excluded by separating them in the time domain. This
is most important for the volume of the plasma source itself, where the species are
generated at high concentration levels and where recombination would be strong.
Outside the high-density source area, in a more diluted state, coexistence of species
is easier to achieve and some mixing there, in the space between the source and
the substrate, can be tolerated. For a high-density plasma source, sufficiently small
in volume, switching times to below 1 s, for example, down to 100 ms are pos-
sible. Passivating and etching species remain separate in the limited volume of
high-density plasma excitation, but may overlap on their way from the source to
the substrate. This so-called “ultrafast gas-switching condition” yields very smooth
sidewalls with hardly any remaining sidewall scalloping at all, as is depicted in the
SEM pictures of Fig. 7.13.
Secondly, a vertical etching process having high mask selectivity bears an inher-
ent risk for micromasking the trench floor. Even minor contamination on the etch
surface may lead to formation of residues, microroughness, and even microneedles.
Having a sidewall with a periodic nanostructure or ripple on the order of 10 nm
in size is an advantage in this respect: micromasks smaller than the characteristic
dimension of the scallops are undercut and extinguished from the surface, leav-
ing back no etch residues. Only if the size of a micromask exceeds the critical
dimension limit that the process can tolerate by undercutting will it cause a potential
residue problem. This has to be considered in connection with the before-mentioned
approach where the sidewall scalloping is practically gone: the latter process is more
critical with respect to formation of microroughness, resulting from micromasking
effects starting from a random distribution of incidental contaminants on the etched
surface.
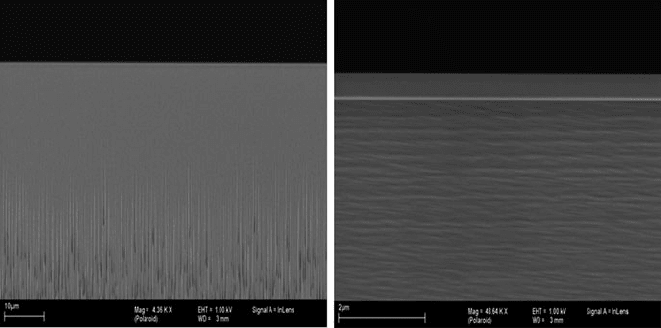
432 S. Tadigadapa and F. Lärmer
Fig. 7.13 SEM pictures of the sidewall resulting from a Bosch process using an ultrafast switch-
ing recipe: hardly any horizontal scalloping is visible at the sidewall any more. Some vertical
striations are visible in the lower part of the left-hand side SEM picture, indicating the beginning
of microroughness formation at the trench bottom, which also affects the sidewall
7.5.3 Understanding Trends for DRIE Recipe Development
High-Aspect-Ratio Etches: As a general observation in plasma etching processes,
net etch rate decreases with rising aspect ratio of the trenches. In addition, reduced
ion impact reaching the trench floor shifts the etching process towards more effective
passivation with higher aspect ratios. As a consequence, profiles tend to develop
positive slopes and typically trenches end up tipping when their aspect ratios begin
to approach values of greater than 20:1.
Given the condition of having all trenches within a similar range of widths, adap-
tation of the process recipe stepwise or continuously [53, 54] helps to reduce the
amount of passivation in the process and keeps the profiles straight. For example,
passivation cycle time or passivation gas flow can be ramped down steadily. As
an alternative, etching cycle time or etching gas flow can be ramped up steadily.
Although other options including source power, bias power, or pressure ramping do
exist; changing cycle times is the most obvious parameter ramping s trategy, to affect
the etch : deposition balance in a predictable and straightforward manner. With the
use of parameter ramping, trenches of >50:1 aspect ratio can be fabricated, while
keeping the sidewalls straight and vertical.
Aspect Ratio Dependent Etching – RIE Lag: Etching speed and profile evolu-
tion depend strongly on the depth : width ratio of the trench, the so-called aspect
ratio. In general, the etch in narrower trenches progresses at a lower speed than in
wider trenches; that is, higher-aspect-ratio trenches lag behind the lower-aspect-ratio
trenches. This is partly due to the fact that the amount of ions reaching the trench
floor progressively diminishes for reasons of aperture effects at the trench opening,
in combination with the angular distribution of the ions. Another important aspect
is transport limitations of gaseous species – radicals – in high-aspect-ratio trenches.
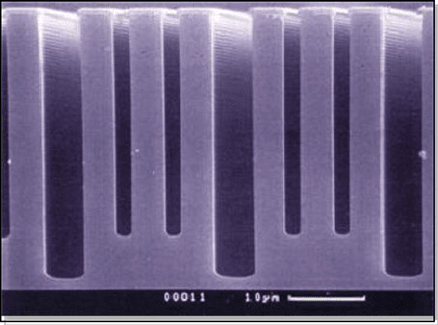
7 Dry Etching for Micromachining Applications 433
The amount of the lag effect is determined mainly by the mean free path of the
gaseous species, that is, gas pressure.
Control of RIE Lag: RIE lag is a common and well-understood transport phe-
nomenon in plasma etching [55]. The amount of the lag effect depends mainly
on the mean free path of the gaseous species which determines their transport in
etched trenches. The dependence of etch rate on the aspect ratio of the feature has
been elucidated based upon a simple vacuum conductance model [56]. In addition,
interaction and change of balance between etching and passivating species during
transport in etched trenches is important, which is again related to mean free path
and process pressure. Changes in the balance of etching and passivating species with
increasing aspect ratio also affect the anisotropy and the profile. Figure 7.14 shows
a characteristic example of RIE lag in high-aspect-ratio Bosch-process etches.
Fig. 7.14 Illustration of RIE
lag effect: etch depth
decreases with narrower gap
sizes corresponding to higher
aspect ratios
In a discontinuous process with separate etching and passivating cycles that can
be controlled independently of each other, there is an individual lag effect both for
the etching and for the passivating part of the overall process. For the etch step, the
higher the aspect ratio of the trench the fewer fluorine radicals per time unit can
reach the trench floor and etch the silicon, the size of the effect depending mainly
on the etch step pressure. For the deposition step, the higher the aspect ratio of the
trench the fewer polymer formers per time unit can reach the trench floor and build
up an inhibiting film on the floor, the size of the effect depending again mainly
on the deposition step pressure. Because a thinner inhibiting film on the etch floor
boosts the rate for the subsequent etch step, this involves some compensation of
the rate decrease with the aspect ratio resulting from the reduced fluorine radical
supply. Deposition step lag and etching step lag can compensate each other to reach
a lagfree overall process over a wide range of aspect ratios. This is achievable by
individual control of the etch step and deposition step parameters; mainly by the
pressure values set.
Setting the pressure higher for the individual step increases its lag effect; setting
the pressure lower decreases its lag effect. Increasing deposition step pressure with
