Ghodssi R., Lin P., MEMS Materials and Processes Handbook
Подождите немного. Документ загружается.

404 S. Tadigadapa and F. Lärmer
7.1 Dry Etching
Etching is the process of controlled removal of a desired material from a substrate
via physicochemical methods. Etching can be performed either using chemicals in
liquid state – defining a process commonly referred to as wet etching – or can be
performed using chemicals in gaseous or plasma phase – defining the process of
dry etching. Dry etching processes include plasma etching, reactive ion etching,
chemically assisted ion beam etching, ion milling, and gas phase chemical etching.
The chemical species used for the controlled removal of the material of interest is
called an etchant. When the process of etching uses chemical species that can spon-
taneously react with the material to be etched and form volatile or gaseous products,
the etching method is a purely chemical process governed essentially by the ener-
getics of the reaction. However, most etching processes require additional energy
to speed up the kinetics of the reaction and to engineer reactions onto pathways
resulting in the formation of volatile products. This is often achieved by imparting
considerable physical energy to the etchant molecules, radicals, and ions thereby
resulting in a physicochemical reaction.
In the limiting case where a particular material cannot be removed easily by
chemical reactions, a purely physical etching process known as ion beam etching
or ion milling is used. Most dry etching processes lie in between the two extreme
regions of pure chemical and pure physical etching processes. Excellent references
on plasma etching for micromachining applications are available and the interested
reader is referred to these for more detailed information [1–5]. A general overview
on reactive ion etching can be found in [6], a review on silicon RIE is given in [7].
The capability of reactive ion etching (RIE), namely crystal orientation indepen-
dent etching to realize arbitrarily shaped high-aspect-ratio microstructures, makes
it an invaluable micromachining technology suitable for the micro- and nanoscale
systems. Most of the present-day dry etching techniques have their origins in the
classical RIE approaches developed for semiconductor manufacturing in the 1970s
and 1980s [8–11]. This chapter focuses on presenting dry etching techniques from
a process development perspective for a broad range of commonly encountered
materials in microsystems applications.
7.1.1 Etch Metrics
Etching can be characterized by several important metrics. These metrics include
etch rate, anisotropy, selectivity, etch stop, loading effects, uniformity, and so on.
Figure 7.1 schematically depicts some of the important etch metrics. Briefly, the
definitions can be given as follows.
Etch Rate: Defines the rate at which the exposed material is removed under given
etch conditions. Etch rate is typically expressed in terms of thickness of the material
removed per unit time or in the units of nm/min or μm/min. Fast etch rates are often
desirable.
7 Dry Etching for Micromachining Applications 405
Etch Rate (R
v
) (nm/min)
Anisotropy (A)
Selectivity (a:1)
Selectivity is given as a ratio: a:1,where a is the ratio of the etch rate
of substrate to the etch rate of the mask.Typical selectivities in the
range of 10: 1 to 100:1 are desirable.
Etch Rate Uniformity (%)
The uniformity of etch rate measured across a wafer or from wafer to
wafer is given by:
Notching
Microtrenching
R
L
R
v
Perfectly Anisotropic Etch
A=1
Perfectly Isotropic Etch
A=0
Partially Anisotropic Etch
v
L
R
R
A −= 1
%100(%) ×
+
−
=
MinMax
MinMax
RateEtchRateEtch
RateEtchRateEtch
UniformityRateEtch
Notching arises due to transient charging of
exposed insulator underneath theetched layer
which in turn severely affects the trajectories of
energetic ions impinging on the substrate.
Microtrenching describes the appearance of
narrow grooves at the feet of the sidewalls in the
direction of ion-bombardement. The etch profile
irregularity is attributed to the forward scattering
of the ions at sidewalls.
+ ++ ++ +
Insulator
Silicon
Photoresist
+
+
+
+
+
+
Time (min)
Etch Depth
l
etch
(nm)
dt
dl
etch
Etch Rate =
+
+
Photoresist
Silicon
+
+
Fig. 7.1 A brief summary of various etching metrics and parameters
Anisotropy: The degree of anisotropy (A) depends upon the etch rates in the ver-
tical (perpendicular to the substrate surface) and the lateral (in-plane) directions. It
can be mathematically expressed as
A = 1 −
R
L
R
V
, (7.1)
where R
V
is the vertical etch rate and R
L
is the lateral etch rate. An ideal anisotropic
etch has a value of 1 where the lateral etch rate is insignificantly small in relation
to the vertical etch rate and a completely isotropic etch has an anisotropy value of 0
when the two etch rates are equal.
Selectivity: Selectivity refers to the relative etch rate of the material of interest
with respect to the masking material which is typically photoresist. A high selec-
tivity is required to achieve good masking and high fidelity pattern transfer through
the process.
Etch Stop: Often etch processes need to be terminated after a defined depth is
attained. Such a control on the etch depth can be achieved via timed etches or by
the use of a layer of material upon which the etching process terminates. Etch stop
layers allow for greater process control and compensation for nonuniformities in

406 S. Tadigadapa and F. Lärmer
etch rate across the wafer arising due to loading effects and/or equipment. A good
etch stop layer must exhibit high inertness in the etch process especially in relation
to the material to be etched. Typically etches are terminated on the etch stop layer
using spectroscopic methods, residual gas analysis of the volatile etch products,
laser interferometry, or laser reflectometry.
Etch Nonuniformity: Etch nonuniformity refers to the variation in the etch depth
of features across a wafer. To ensure meaningful measurement of the nonuniformity
across a wafer or from wafer to wafer, the etch depth must be measured on identical
features. It is well known that the etch rate in plasma etching varies depending upon
the feature width, an effect more commonly known as aspect-ratio-dependent etch-
ing (ARDE). In this effect, narrow features are found to be etched less than wider
features under same plasma conditions. Compensation f or the resulting nonunifor-
mity in etch depth typically requires 20–30% overetching of wider features and the
availability of an etch stop layer is vital to the success of achieving high uniformity.
Etch uniformity across a wafer or from wafer to wafer is defined as:
Etch Rate Non − Uniformity(%) =
Etch Rate
Max
− Etch Rate
Min
2 ∗ Average
× 100%
(7.2)
Loading Effects: The etch rate is not only a function of the gas chemistry and
physical settings of the process, but also depends upon the total material (area) to
be etched. In general etch rates are found to decrease as the area of material to be
etched increases, especially in chemically dominant processes and is a direct con-
sequence of the increased consumption of the etchant (reactant) species or gases.
Loading effects manifest macroscopically at the wafer level when the total area of
the material to be etched is changed by either increasing the number of wafers or
via new mask designs. Loading effects also manifest at the microscopic level espe-
cially when etching high-aspect-ratio structures due to the local depletion of etchant
molecules in etched features. Reactive ion etching lag or aspect-ratio-dependent
etching describes the difference in the etch rate between large and small width fea-
tures within the same wafer. These effects can also manifest across a wafer where,
for example, an aluminum substrate holder is used to etch silicon in fluorine plasma.
Aluminum does not react with fluorine radicals, therefore there is reduced consump-
tion of the radicals around the periphery of the wafer from those in the middle of the
wafer. This results in the well-known bullseye effect whereby features at the edges
of the wafer are etched more rapidly than in the middle of the wafer. Such nonuni-
formity is typically compensated by overetching the substrate material onto a high
selectivity etch stop layer. In general the etch rate R
n
, when etching n-wafers each
with an etch area A, can be written as [12, 13]
R
n
=
R
0
1 + nkA
, (7.3)
where R
0
is the etch rate in empty chamber, and k is a constant related to the ratio
of the amount of etchant molecules consumed in etching a wafer to the amount
7 Dry Etching for Micromachining Applications 407
of etchant lost via recombination in the chamber volume/surface. As long as the
volume or surface recombination of etchant molecules dominates, the loss arising
due to consumption during etching can be neglected and thus loading effects are
minimal. This is practically realized in large etch chambers and large flow rates.
Microscopic loading is typically mitigated by performing t he etches at reduced pres-
sure and when ion bombardment or physical etch mechanisms dominate the overall
character of the etch process.
Notching: The notching effect manifests as a narrow lateral opening (wedge)
at the interface of the conductive material being etched and an insulating under
layer. Typically, notching occurs during the overetch phase in the regions where the
insulating underlayer is first exposed. In the case of silicon etching on a layer of
silicon dioxide, the origin of notching is found in the local electric-field-induced
trajectory bending of energetic ions that directly bombard and etch the foot of the
Si sidewall. The process continues through the subsequent forward scattering or
electrostatic deflection of energetic ions at the newly exposed SiO
2
surface under
the notch [14].
Microtrenching: Microtrenching manifests as the appearance of rapidly etched
narrow grooves at the bottom corners of etched vertical sidewalls. This enhance-
ment in the etch rate in these corner regions is typically attributed to the preferential
shallow angle forward scattering of energetic ions by the mask and etched substrate
sidewall. Microtrenching is dominant in high-energy ion-bombardment-driven etch-
ing processes including ion beam etching. Although the phenomenon is observed in
all kinds of substrates, charging effect in insulating substrates can also enhance the
microtrenching processes. A detailed exposition on this subject can be found in [15].
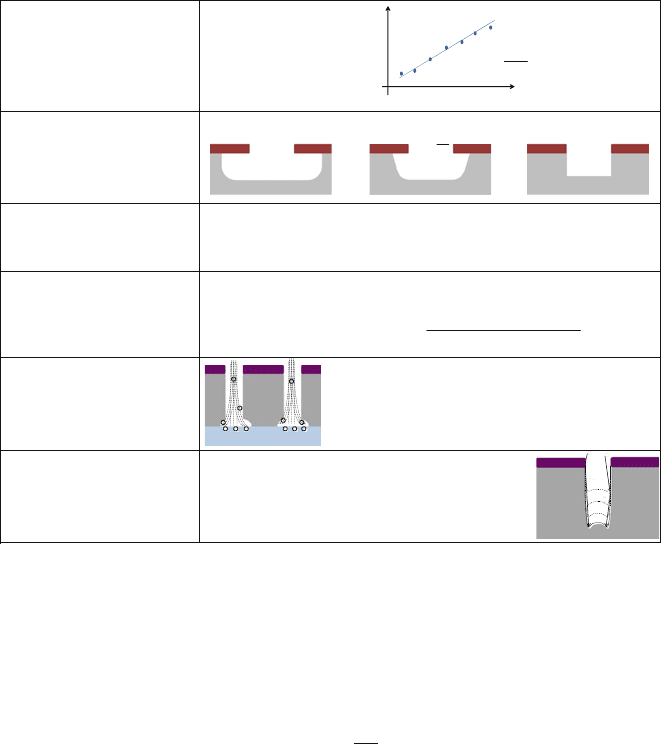
7.2 Plasma Etching
Plasma etching (PE) and/or reactive ion etching (RIE) are well established process
technologies in the semiconductor industry [3, 6, 7]. The classical tool is the diode
reactor, as shown in Fig. 7.2. The scheme depicts the key elements of a typical
plasma etching system: a number of reaction gases provided to the chamber by
adjustable mass flow controllers, a grounded showerhead electrode as a gas inlet, a
substrate electrode carrying the wafer, and a power supply to the substrate electrode
by radio frequency (RF) via a coupling capacitor (which is mostly part of a more
complex matching-unit to adapt RF impedances). Detailed descriptions of different
plasma reactors and processes can be found in [16].
In RIE configuration, ions from the plasma are accelerated to high energy lev-
els (several 100 eVs) and perpendicularly directed towards the powered substrate.
In the plasma etching (PE) configuration, the showerhead is powered and the sub-
strate electrode grounded. In this case, acceleration of ions from the plasma to the
substrate is restricted to minor energy levels of only a few eVs and the directional-
ity of the i ons is lower than in RIE configuration. In the triode configuration, both
the substrate electrode and the showerhead electrode are powered simultaneously,
408 S. Tadigadapa and F. Lärmer
Platen
Reaction Gases
Pumping
Helium
Backside
Cooling
Electrostatic
Chuck
Gas Inlet
Wafer
U
RF
Showerhead
Gas1
Gas2
Mass Flow Controllers
Fig. 7.2 Schematic
illustration of a diode plasma
or reactive ion etcher. The
reactor is pumped to
low-pressure conditions.
Reaction gases are supplied
to the chamber via t he upper
showerhead electrode. The
lower electrode is powered by
RF to drive the plasma
discharge and accelerate the
ions towards the substrate
(RIE configuration)
either at the same or at different frequencies, see below. The goal is to generate
the plasma at the desired plasma density controlled by the power level supplied to
the upper electrode, and to accelerate the ions towards the substrate at the desired
energy value controlled by the power level supplied to the substrate electrode, more
or less independently of each other.
Radio frequencies generally used are in the low-frequency (380 kHz), mid-
frequency (2 MHz), or high-frequency (13.56 MHz) regime. Typically, 13.56 MHz
is used as a standard for plasma generation.
Pumping provides the low-pressure conditions in the reactor chamber needed for
the electrical discharge to form between the powered and the grounded electrode,
which builds up the plasma in an atmosphere of one or more compound gases, at a
pressure ranging from 0.1 to 100 Pa.
The coupling capacitor allows the powered electrode to float at a DC-potential
generated from the applied RF voltage and arising due to the difference in the mobil-
ities of negative charges (electrons) and positive charges (ions) in the plasma. This
is the so-called DC-bias voltage, which is responsible for energizing the ions.
7.2.1 Types of Etching
Isotropic Plasma Etching: With regard to etching, a reactive plasma consists of
two relevant kinds of species: ions and radicals. The plasma is by far dominated
by chemically active neutral species, the so-called radicals (∼10
19
− 10
22
m
−3
)in
comparison to the electron and ion densities (10
15
− 10
20
m
−3
). Active radicals
are created by the decomposition of gas molecules into fractional parts after colli-
sions between gas molecules and energetic electrons in the plasma. In most cases, an
electron-neutral collision breaks up the neutral molecule into neutral radicals. Being
electrically uncharged, radicals reach the substrate with no or only little direction-
ality, bond to the surface, and react readily with the material to etch, to more or less
volatile compounds. If the reaction takes place instantaneously and the volatility
7 Dry Etching for Micromachining Applications 409
of the reaction products is high enough, the volatile compounds leave the etched
surface quickly yielding isotropic etching behavior. This so-called chemical etch
depends mainly on the properties of the materials involved, provides high selectiv-
ity, and makes it, in general, easier to find appropriate etching masks (e.g., SiO
2
for
masking a Si-etch).
Fluorine radicals delivered in a plasma discharge from fluorine-rich gaseous
compounds such as sulfur hexafluoride SF
6
, carbon tetrafluoride CF
4
, or nitrogen
trifluoride NF
3
, exhibit isotropic etching behavior towards silicon. Once adsorbed to
the silicon surface, fluorine radicals react spontaneously with silicon atoms to form
silicon fluorides SiF
x
(x = 1,2,3,4). The reaction main products, SiF
2
and SiF
4
,are
volatile enough to leave the surface without a need for physical assistance, and in
the process remove silicon atoms from the etched surface. Because there is no direc-
tionality contained in the mechanism, silicon etching proceeds with no preferential
orientation, and the profiles are isotropic. This is an ideal behavior for underetch-
ing micromechanical structures in a so-called sacrificial etching or release-etching
step, for the fabrication of free-standing and movable elements on top of a wafer.
In contrast to that, etching masks such as SiO
2
or photoresist are not or only slowly
attacked by fluorine radicals, without physical assistance by the energetic ions. A
number of advanced micromachining technologies make use of combinations of
anisotropic and subsequent isotropic silicon etching: first etch deep in the vertical
direction, then protect the structures and underetch in the lateral direction [17, 18].
A well-known process example is single-crystal reactive etching and metallization
(SCREAM) [19].
Plasmaless Isotropic Etching: For the purpose of silicon sacrificial etching, it is
not even necessary to use a plasma source of fluorine radicals. A number of gaseous
compounds are well known for the ability to etch silicon spontaneously, without a
need for plasma excitation. XeF
2
[20, 21], BrF
3
[22], and ClF
3
[23] deliver fluorine
radicals which once adsorbed onto the surface, lead to the spontaneous etching of
the silicon. For example, XeF
2
is a white solid at room temperature and atmospheric
pressure. However at ∼395 Pa and room temperature the solid sublimates and in this
form is able to etch silicon. Gas phase xenon difluoride isotropically etches silicon
according to the chemical reaction:
2XeF
2
+ Si → 2Xe + SiF
4
.
The reaction is exothermic and may result in an increase in the local tempera-
ture, especially in thermally isolated structures. This effect is mitigated by the
use of pulsed rather than continuous etch systems [24]. The absence of any liq-
uid phase eliminates stiction-related problems. Typical etch rates greater than
1 μm/min have been reported which can be reduced by diluting XeF
2
with nitrogen.
Furthermore, the etch process is a purely chemical isotropic process and requires
no other form of physical energy input for it to occur. XeF
2
has very high etch-
selectivity between silicon and all other known materials used in the semiconductor
processes [25]. Selectivities may well exceed values of 1000 for most metals or
dielectrics.
410 S. Tadigadapa and F. Lärmer
A typical XeF
2
etching process in a pulsed mode is as follows: The cycle begins
by evacuating the expansion and etching chambers to a pressure of ∼13.2 Pa. This
allows the XeF
2
crystals to sublimate and fill the expansion chamber to a selected
pressure. The etching and expansion chambers are then connected and the pressure
is allowed to equilibrate to 130–400 Pa. Typically etching begins immediately and
the pressure begins to rise due to an increase in the total number of moles of the
gases. After a selected etch-time has passed, both chambers are again evacuated
to a 13.2 Pa, held for 10 s, and the process is repeated. The advantage of pulsed
systems over continuous systems is that the quantity of etchant delivered each cycle
is known and can be controlled. However, it is important to note that the etch rates
are dependent on the quantity of silicon present in the chamber. The presence of
water vapor can also have a deleterious effect on the etch performance inasmuch
as water vapor can react with XeF
2
to form HF molecules which are ineffective in
etching silicon. Furthermore, the etch rate in the first few cycles can be lower than
later cycles, due to the presence of native oxide on the silicon. Silicon dioxide is
found to offer a good selectivity whereas nonstoichiometric silicon nitride (Si
x
N
y
)
is found to be etched in XeF
2
. Table 7.1 shows the etching rates obtained for single-
crystal silicon and polysilicon for the conditions used [26].
Table 7.1 Measured etch rates in XeF
2
gas phase etching system [26]
Material
XeF
2
pressure (Pa)
N
2
pressure
(Pa)
Etch time
(s/cycle)
Cycle time
(s)
Etch rate
(nm/cycle)
Si (SC) 395 0 45 90 100
Polysilicon 395 0 45 90 2500
SiO
2
395 395–790 45 90 0.1
Si
x
N
y
395 395–790 45 90 10
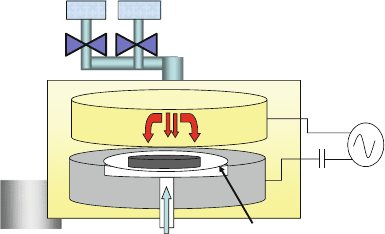
Figure 7.3 shows the typical silicon surface obtained after etching in XeF
2
.The
uniform and randomly nucleated spontaneous etching process causes the surface to
roughen the longer the etching is performed. Typical RMS surface roughness val-
ues range from 100 nm to 1 μm. The process can also be controlled and slowed
down by the addition of nitrogen to provide controlled thinning of silicon structures
at the nanometer scale. In a recently published paper, high selectivity was demon-
strated for the first time for selectively etching a SiGe sacrificial layer with respect
to Si functional structures in chlorine trifluoride gas [27]. This makes any need for
passivation and protection of the functional silicon during the release step obsolete.
XeF
2
,BrF
3
, and ClF
3
react with silicon via a physisorbed surface layer.
However, in comparison to XeF
2
and BrF
3
,ClF
3
shows slower reaction kinetics
due to the formation of a fluorosilyl skin [28]. The hampering effect of the fluo-
rosilyl layer is explained via a diffusion like transport of ClF
3
molecules through
a fluorosilyl layer [29]. This is the basis for anisotropic etching of Si at low tem-
peratures and the reason for the extremely high selectivity of Si over SiGe, where
Ge atoms act as accelerators of the ClF
3
decomposition reaction. ClF
3
shows crystal

7 Dry Etching for Micromachining Applications 411
Fig. 7.3 Typical texture of
the single-crystal silicon
surface resulting after etching
in XeF
2
gas phase etching
orientation-dependent “anisotropic” etching of silicon below 0
◦
C. The <110> direc-
tion shows a maximum whereas <100> direction shows a minimum of the angular
etch-rate distribution, with a ratio of <110>/<100> ≈ 1.5.
Anisotropic Etching: The second major species of importance in plasma etch-
ing of substrates is the positively charged ions also known as cations. Although less
numerous than radicals, ions are, however, entirely responsible for any directionality
and anisotropic nature of the etch. They are created during collisions of neutral gas
molecules with energetic electrons in the plasma in which an electron is knocked off
from the molecule and leaves behind a positive charge. Being electrically charged,
ions can be accelerated towards the substrate by negatively biasing the latter with
respect to the plasma potential using DC or RF voltage. This results in the i ons bom-
barding the substrate surface in a more or less perpendicular orientation. The ions
represent the directional contribution provided by the plasma, and all approaches to
create anisotropic plasma etching rely on a perpendicular ion flux to the substrate.
Owing to their directionality, ions do preferentially hit the bottom of a structure
and only to a lesser extent their sidewalls. The incoming ions transfer their kinetic
energy to the local area of impact. Although the number of ions is small compared
to the number of chemically active neutral species, their energetic impact can sig-
nificantly affect or drive the progress of the etching reaction locally. Ion-assisted
directional etching can be caused by one or several of the following mechanisms.
(i) Induction of chemical reactions between adsorbed radicals from the plasma
and the material to etch, by providing the activation energy through ion impact,
especially if the reactivity of etching species with the material is low or
hindered by an energy barrier.
(ii) Desorption of reaction products from the surface by the sputter action of
incoming ions, especially if the volatility of the reaction products is low and the
412 S. Tadigadapa and F. Lärmer
latter stick to the material surface, forming an inhibiting film there. In this case,
ion impact removes the inhibiting film of reaction products from the surface.
(iii) Desorption of other inhibiting films by the sputter action of incoming ions,
for example, polymer films formed from polymerizing additives to the plasma,
or films formed from nonvolatile reaction products between the material to be
etched and other additives to the plasma.
Etching based on the halogens of lower reactivity such as chlorine and bromine is
strongly ion-driven, which gives anisotropy to the process, however, limits achiev-
able etch rates (typically to below 1 μm/min), etch depths (a few μms) and mask
selectivities (e.g., 20–30:1 for SiO
2
-hardmask). Because physical impact from the
ion flow to the substrate plays a key role in the etch, selectivity as a result of individ-
ual chemical properties of the involved materials becomes less significant. Typically,
there is a fundamental tradeoff between increasing the anisotropy by stronger ion
bombardment on the one side, and maintaining mask selectivity sufficiently high on
the other side.
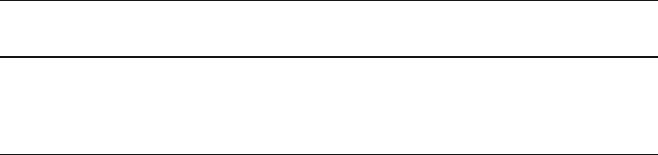
7.2.2 Plasma Sources
Reactive ion etching, except in the case of gas phase chemical etching involves the
use of various plasma sources into which the substrates can be introduced for etch-
ing. Depending upon the exact configuration of the electrodes and how the RF power
is coupled into the chamber, plasma reactors can be classified as: (i) capacitive,
(ii) inductive, and (iii) wave etchers. Furthermore, depending upon the location of
the plasma with respect to the substrate, etchers can be classified as (i) plasma con-
tact or (ii) downstream etchers. Figure 7.4 lists the major RIE systems that are briefly
described next. Detailed information on plasma sources and etcher configurations
can be found in [30].
Barrel Etchers: These systems are mainly used for surface treatment and were
the plasma system configuration in early designs. Plasma i s typically generated
with RF-powered electrodes (capacitive coupling) or with a coil wrapped around
the dielectric tube (inductive coupling). These systems can be used to remove
photoresist residues from wafers using oxygen glow discharge. Initial experiments
demonstrating CF
4
-based silicon etching were performed in these systems, which
resulted predominantly in isotropic etching due to the lack of directionality of the
ions (See Fig. 7.4).
Capacitively Coupled RF Diode System was the work horse of the 1980s for
reactive ion etching applications. The wafer is placed on the powered electrode and
the best results are usually obtained at low pressures (<13.2 Pa). This approach
does not allow independent control of the ion energy and the ion current at a fixed
pressure and RF frequency. Typically ion energies of hundreds of eV are required to
achieve the desired etch rate. A particularly effective implementation of the diode
approach was the “hexode” machine developed at Bell Labs and developed to allow
7 Dry Etching for Micromachining Applications 413
Gas Inlet
Gas Inlet
Plasma
rf
rf
rf
rf
RF
RF
Pump
Pump
Pump
Downstream Etcher Triode Etchers Barrel Etcher
Etch Gas
Fig. 7.4 Schematic
illustration of major plasma
sources and etcher
configurations
etching of several wafers simultaneously. This plasma etcher configuration was a
dominant batch-processing machine in the 1980s [31].
Capacitively Coupled Planar Diode and Triode Systems: The planar diode
plasma configuration has been already discussed in the previous section. The tri-
ode geometry shown in Fig. 7.4 was well known in sputter deposition technology
and introduced in plasma etching technology to allow independent control of ion
energy and ion flux. This approach allows the ion energy to be reduced while keep-
ing the etch rate constant by increasing the ion flux density. The wafer is placed
on the lower electrode; the lower electrode power (bias power) is used to control
the ion energy and the upper electrode power (source power) is used to control the
ion flux. It is well known that the RF voltage needed to deliver a fixed power to
the plasma decreases strongly as the frequency is increased. Thus large power can
be delivered to the plasma through the high frequency (typically 13.56 MHz) with-
out high-energy bombardment of the electrode. The ion bombardment energy at the
wafer surface (lower electrode) can be controlled by a lower frequency RF power
applied at the lower electrode. The term “triode” is used for this two-electrode
system to recognize the fact that the grounded wall serves as a third (reference)
electrode.
Chemical Downstream Etching: Figure 7.4 shows the schematic illustration of
a chemical downstream etching system. In these systems, the wafer chamber is
separated from the plasma source by a tube with no line-of-sight connection to
the plasma source. Few ions and electrons reach the surface of the substrate and
etching is carried out by the energetic neutral radicals. The process is specifically
designed to minimize damage onto the surface but the etching process is isotropic
