Czichos H., Saito T., Smith L.E. (Eds.) Handbook of Metrology and Testing
Подождите немного. Документ загружается.

698 Part D Materials Performance Testing
Current (μA)
60
50
40
30
20
70
10
400030002500200015001000 3500500
Time (s)
0
1% NaCl, inhibitor-free
Addition NaNO
2
to 2×10
–3
mol/l Addition NaNO
2
to 4.2×10
–2
mol/l
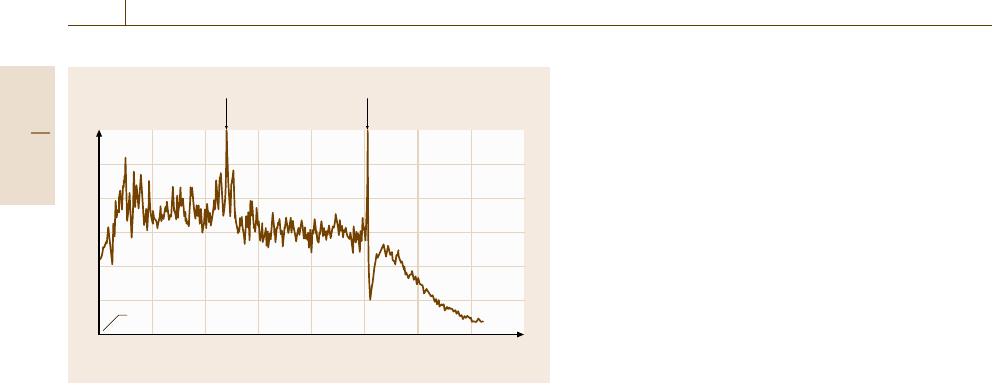
Fig. 12.36 Current noise plot of mild steel in air saturated 1 mass %
NaCl solution. Effect of NaNO
2
concentration
Similar results were obtained with Admirality brass,
which generally showed only localized corrosion but
with a higher initiation rate of pits in cooling water 2.
These and other results reported in the literature
allow one to conclude that ENA can be used for cor-
rosion monitoring with the possibility of distinguishing
between uniform and local corrosion.
However, it has been shown [12.65–67] that mea-
suring only potential noise can be misleading in cases
where small fluctuations in mass-transport control can
produce large changes in the corrosion potential U.
Such is the case for metals and alloys that are immune
to corrosion such as Pt or are passive, such as stain-
less steel in neutral, aerated aqueous solutions; e.g. it
was found that potential noise plots, as analyzed with
the use of power-spectral density (PSD), were simi-
lar for Pt and for an Al/SiC metal matrix composite,
which pits severely in aerated 0.5N NaCl. However,
the current-noise PSD plots were very different for the
two materials. The explanation is that mass transport of
oxygen can play a large role in potential noise.
For corrosion monitoring, specifically large-scale
corrosion monitoring and field testing, the statistical
analysis of noise data resulting in RMS values has many
advantages over spectral analysis by FFT or MEM
which requires expensive equipment and/or compli-
cated analysis programs. It was shown [12.65] that the
noise resistance R
n
and the polarization resistance R
p
have similar values and trends; therefore theoretical
relationships between R
n
and R
p
are currently investi-
gated. Thus for cases where only qualitative results are
needed in a fast, simple and inexpensive way, collection
and evaluation of R
n
data is sufficient. However, a quan-
titative understanding of electrochemical noise and its
relationship to corrosion, specifically to localized corro-
sion phenomena, can only be achieved through spectral
analysis of noise data, leading to the determination of
the spectral noise resistance R
Sn
which shows very
strong relations between R
n
and R
p
[12.66, 67]. The
pitting index (PI)definedas
PI =δI/I
mean
, (12.74)
where δI is the RMS current noise and I
mean
the mean
coupling current [12.63], appeared not to be applicable
for all corrosion systems for the determination of the na-
ture of the corrosion mechanism, specifically localized
corrosion; e.g. for the system steel/aqueous salt solu-
tion the PI gave misleading results [12.66,67]. This can
be understood considering that I
mean
in the above defi-
nition equation should be close to zero for all cases of
uniform corrosion. PI can therefore show large fluctua-
tions independent of the actual corrosion mechanism.
ENA seems to be very useful for investigation
of corrosion-inhibitor performance. The great advan-
tage is that only two metal electrodes are necessary
to measure a current signal, which indicates high or
low general corrosion rates and susceptibility to pit-
ting and other localized corrosion. Figure 12.36 gives
an example how in a very short time the critical con-
centration of a dangerous anodic inhibitor (NaNO
2
)for
steel corrosion in aerated 1% NaCl solution can be
evaluated. In this example two identical mild-steel elec-
trodes were placed in the inhibitor free brine. In the first
20 min considerable current fluctuation indicates typi-
cal oxygen corrosion of the steel electrodes. Addition
of 2 × 10
−3
mol/lNaNO
2
reduces the noise, however,
the noise level indicates insufficient inhibition. After an-
other 20 min more NaNO
2
isaddedtomakeaNaNO
2
concentration of 4.2×10
2
mol/l, which is above the
critical concentration of NaNO
2
. Consequently the cur-
rent noise ceases nearly completely, indicating complete
inhibition. ENA technology today is advanced enough
to be used as a monitoring device for critical inhibitor
concentration and the start of localized corrosion.
ENA can also be applied in media with low conduc-
tivity and also in multiphase media because, in contrast
to other electrochemical methods, it needs no reference
electrode which could give measuring problems due to
high IR drops and – specifically in oil/water mixtures
– plugging or sealing of the diaphragm. In technical
systems the life of the reference electrode is also deter-
mined by the tendency of the test media to contaminate
the electrolyte in the electrode.
Part D 12.3

Corrosion 12.5 Corrosion Without Mechanical Loading 699
12.4 Exposure and On-Site Testing
There is a considerable number of sources of material
testing data that have been accumulated over the years
and are available to process plant designers to assess the
most suitable materials selection for a particular plant
and operating conditions. However, many of the fac-
tors involved in the selection of an optimum choice are
subject to variability, and this makes the choices more
complex.
The ranking of the most valuable corrosion data for
plant designers is generally understood to be as follows.
1. Operating experience on full-scale equipment under
the actual process environment.
2. Operating experience on a pilot plant with similar
feedstock and operating conditions.
3. Sample tests in the field, coupons, electrical resis-
tance (ER) probes, or stressed samples exposed to
the process environment.
4. Laboratory evaluations on actual plant fluids, or
simulated environments.
5. Materials and corrosion database.
It should also be recognized that the economics of the
various material selection choices is not constant. A de-
cision that makes economic sense at the design time, or
at the materials selection time, may not make sense by
the time the plant is actually built or operating. In ad-
dition, the economics of plant shutdowns due to failure
can depend on the market for the product being gen-
erated by the plant, although these costs are generally
always upward.
The balance between choice of a more-expensive
corrosion-resistant alloy versus the cost of subsequent
chemical treatment costs to inhibit corrosion is also
subject to change, due to changes in the raw mater-
ial costs of chromium, nickel, and other constituents in
corrosion-resistant alloys. Sometimes a less-expensive
material that would otherwise be unacceptable due to
low corrosion resistance can still be the best choice
when used with chemical corrosion inhibitors.
Corrosion testing may be conducted in the labora-
tory, pilot plant, or field. Systems that are either in the
design phase or operating benefit from laboratory and
pilot plant testing. In situ testing is useful for pilot plant
testing and for providing information for systems that
are currently operating.
Laboratory tests should simulate the water chem-
istry and operating conditions of the freshwater system.
A sample of water from the system may be used if it is
available. Immersion testing and electrochemical test-
ing (Sect. 12.1) are commonly used for laboratory tests.
In situ testing is commonly conducted in pilot
plants. This testing is useful in evaluating or monitor-
ing a water-treatment system or the effects of varying
operating parameters such as flow rate and temperature.
In addition, the performance of different alloys in a sys-
tem may be studied by in situ testing. In situ testing
provides information on uniform corrosion rate, types
of corrosion, and pitting tendencies (Sect. 12.4).
In situ testing is also performed in the field to de-
termine the effectiveness of a water treatment system,
the performance of difference alloys, and the effects of
varying operating parameters such as flow rate and tem-
perature. Uniform corrosion rates, types of corrosion,
and pitting tendencies are identified with in situ testing.
Another form of testing the corrosion behavior es-
pecially for coatings is atmospheric exposure under
various climatic conditions. This type of testing yields
more reliable information on the corrosion behavior
than testing in climatic chambers. A disadvantage is the
long duration of such tests. For reasons of practicabil-
ity a mixture of laboratory testing and exposure under
natural environmental conditions is recommended.
12.5 Corrosion Without Mechanical Loading
By taking into consideration the different causes of
corrosion and their mechanisms, as well as the ap-
pearance of the attack, one can differentiate between
several types of corrosion. A common approach is
to distinguish between corrosion systems with or
without mechanical loading. In the latter case the re-
sult of corrosion is the initiation and propagation of
cracks. If only corrosion takes place and there is
no conjoint action of mechanical loading the vari-
ous forms of corrosion occurring in such systems can
either be described as local or uniform. Also cor-
rosion testing is different if mechanical loads have
to be included in the test set up. Therefore this
section is specifically dedicated to types of corro-
sion where mechanical loading is unavailable in the
system.
Part D 12.5
700 Part D Materials Performance Testing
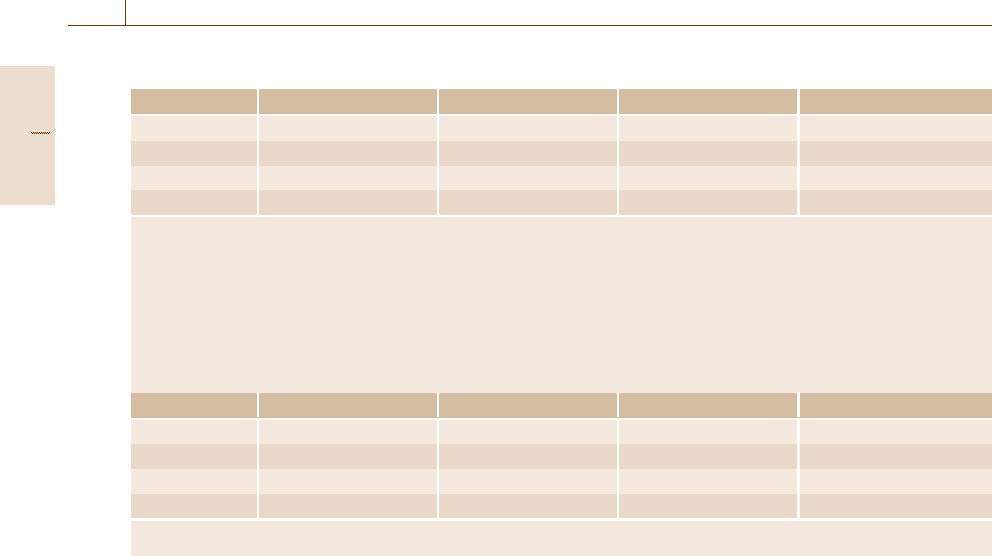
Table 12.2 Corrosion rate conversion
mA/cm
2
mm year
−1
mpy gm
−2
day
−1
mA/cm
2
1 3.28 M/nd 129 M/nd 8.95 M/n
mm year
−1
0.306 nd/M 1 39.4 2.74 d
mpy 0.00777 nd/M 0.0254 1 0.0694 d
gm
−2
day
−1
0.112 n/M 0.365/d 14.4/d 1
where: mpy = milliinch per year
n = number of electrons freed by the corrosion reaction
M = atomic mass
d = density
Note: you should read the table from left to right, i. e.:
1mA/cm
2
=(3.28M/nd)mmy
−1
=(129 M/nd)mpy= (8.95M/n)gm
−2
day
−1
For example, if the metal is steel or iron (Fe), n = 2, M =55.85 g and d =7.88gcm
−3
and the table of conversion becomes
mA/cm
2
mm year
−1
mpy gm
−2
day
−1
mA/cm
2
1 11.6 456 249
mm year
−1
0.0863 1 39.4 21.6
mpy 0.00219 0.0254 1 0.547
gm
−2
day
−1
0.00401 0.0463 1.83 1
Note: you should read the table from left to right, i. e.: 1 mA/cm
2
=11.6mmy
−1
=456 mpy =249gm
−2
day
−1
12.5.1 Uniform Corrosion
Uniform corrosion is given when a metal is corroding
with the same rate over the whole surface of the metal
exposed to the corrosive environment. The extent can
be given by the weight loss per unit area or the aver-
age penetration, which is the average of the corrosion
depth. This can be determined by direct measurement
or by calculation from the weight loss per unit area
when the density of the material is known. Uniform
corrosion takes place as a rule via the effect of cor-
rosion cells without clearly defined anode and cathode
surfaces. A prominent case for uniform corrosion is the
rusting of railways due to atmospheric exposure. From
a technical standpoint uniform corrosion is quite easy to
handle because the design engineer must only include
an additional thickness to the material which equals the
loss due to corrosion over the life time of the structure.
Uniform corrosion rates may be determined by
weight loss or electrochemical methods. Both methods
average a specimen’s corrosion rate over its sur-
face area. Corrosion rates calculated from weight-loss
data also average the loss over the exposure period.
Electrochemical methods may yield instantaneous or
time-averaged corrosion rates. Weight-loss testing is
performed by immersing a coupon in a test solution
either in the laboratory or in situ. Another method is
exposing a metal or a coated specimen to a specific
environment (atmosphere) or a climatic chamber and
measure the corrosion loss over a certain period of time.
It should be noted that measurements carried out in cli-
matic chambers very rarely correspond to the behavior
of a material under practical conditions. Therefore in-
vestigations in the laboratory are mostly advisable for
comparing different materials and give a ranking on
their sensitivity to corrosion in a laboratory environment
than to get reliable information on their performance in
service.
There is a correlation between the electrical param-
eters and the weight loss of the material. Table 12.2
yields information how the values correspond. The fol-
lowing charts provide a simple way to convert data
between the most common corrosion units in usage, i. e.
corrosion current (mA/cm
2
), mass loss (g/(m
2
day))
and penetration rates (mm/y or mpy) for all metals or
for steel.
Another important factor for determining uniform
corrosion and its rate of corrosion is the removal of
corrosion products from the surface to measure the cor-
rosion loss in weight units.
There are a large number of chemical solutions
available for the different materials used in practice to
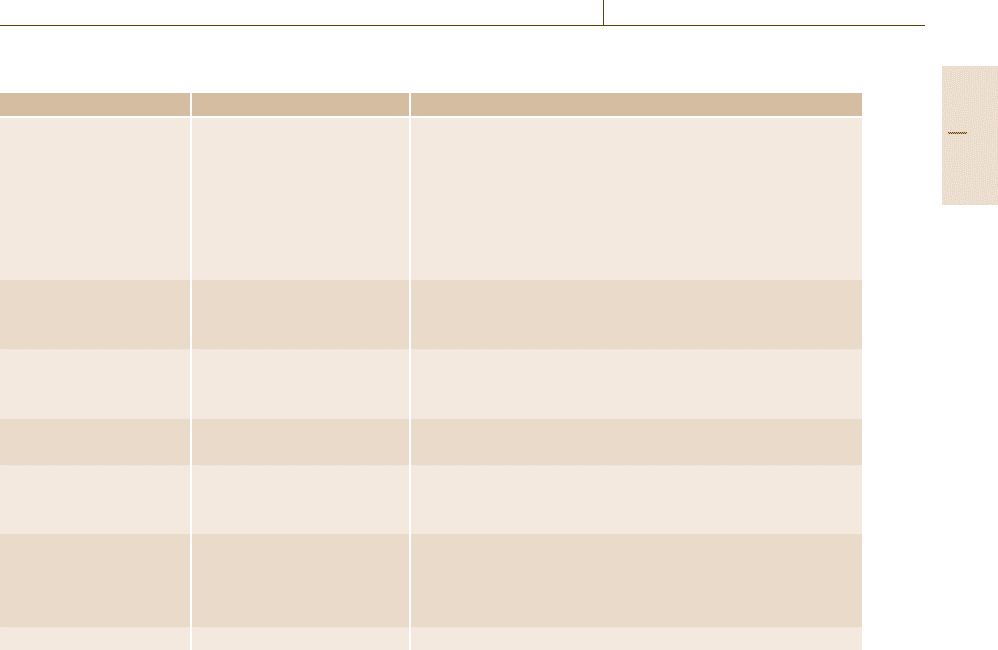
remove oxide layers from surfaces. Table 12.3 yields
information on typical solutions for various materials.
Part D 12.5
Corrosion 12.5 Corrosion Without Mechanical Loading 701
Table 12.3 Some solutions used to remove corrosion products from metals
Metal Corrosion products Reagents and temperature
Iron and steel FeO; Fe
2
O
3
×H
2
O; 1. HCl (20–25%) +0.8–1% PB-5 inhibitor
Fe
3
O
4
×H
2
O 2. 0.15 l H
2
SO
4
(specific gravity 1.84)+0.85 lH
2
O+1% PB-5 or
PB-8 inhibitor
3. 10% ammonium tartrate + NH
4
OH, 25–70
◦
C
4. 10% ammonium citrate +NH
4
OH, 25–70
◦
C
5. 10% H
2
SO
4
+ 1% As
2
O
3
,25
◦
C
6. 5% NaOH + Zn (granulated or turnings), 80–90
◦
C, 30–40 min
Zinc-coated iron, Zn(OH)
2
;ZnCO
3
; 1. 10% ammonium persulfate
zinc ZnO; ZnCO
3
×2ZnO×3H
2
O; 2. Saturated CH
3
COO(NH
4
)
ZnCO
3
× 3Zn(OH)
2
Lead PbO; PbSO
4
; 1. Saturated CH
3
COO(NH
4
)
Pb(OH)
2
2. 1% CH
3
COOH, boiling
2PbCO
3
× Pb(OH)
2
3. 80 g/lNaOH+ 50 g/l mannite + 0.62 g/l hydrazine sulfate
Tin SnO
2
;SnO 1. 5% HCl
2. 15% solution of neutral sodium phosphate
Copper and its alloys Cu
2
O; CuO; Cu(OH)
2
1. 5% solution of H
2
SO
4
CuSO
4
× 3Cu(OH)
2
; 2. 18% HCl
CuCO
3
× Cu(OH)
2
Aluminum and its alloys Al
2
O
3
; Al(OH)
3
1. 5% HNO
3
2. 5% HNO
3
(specific gravity 1.4) +1% H
2
Cr
2
O
7
3. 65% HNO
3
4. 20% orthophosphoric acid +8% CrO
3
Magnesium MgO; MgCO
3
1. 20% CrO
3
+ 1% AgNO
3
, boiling solution, 1 min
12.5.2 Nonuniform and Localized Corrosion
In contrast to uniform corrosion nonuniform or local-
ized corrosion is corrosion attack that is preferentially
concentrated on discrete sites of the metal surface ex-
posed to the corrosive environment. Localized corrosion
can result in, for example, pits, cracks, grooves or scars.
In any case it is the occurrence of a local nonuniform
attack due to the build up of corrosion cells over the
metal, which means a local and uneven distribution of
anodic and cathodic sites over the metal surface. What
makes the case worse is the comparatively high corro-
sion rate at the anodic sites and, especially in pitting
or crevice corrosion, self-accelerating processes occur
in the course of the corrosion process. Therefore pit-
ting and crevice corrosion are the most prominent and
dangerous forms of corrosion attack as well as inter-
crystalline corrosion which can be encountered along
or in the vicinity of grain boundaries.
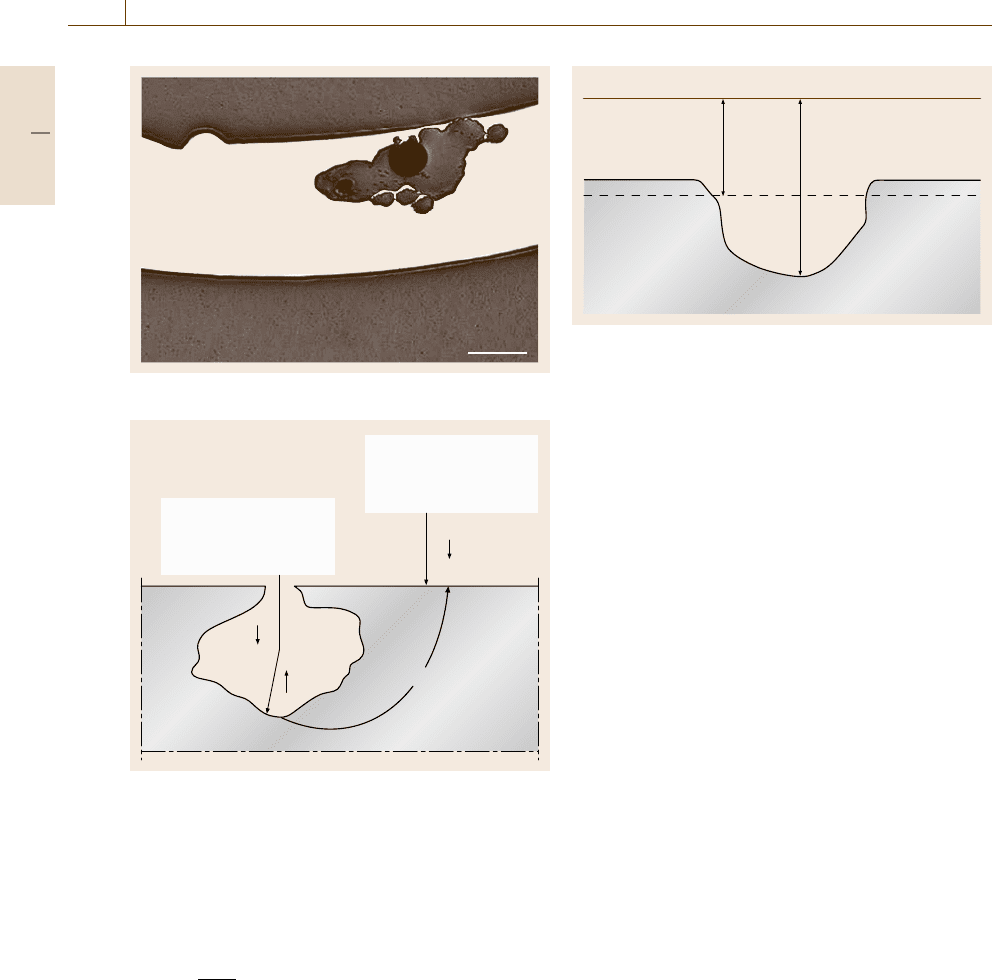
Pitting
Pitting is localized corrosion which results in pits in the
metal surface (Fig. 12.37). This type of corrosion gener-
ally takes place in corrosion cells with clearly separated
anode and cathode surfaces. The anode is situated in the
pit and the cathode usually on the surrounding surface
(Fig. 12.38). Pitting usually results in worse damage
than uniform corrosion because it can lead to perfora-
tion in a very short period of time.
When evaluating attack caused by pitting the fol-
lowing should be taken into account
•
the number of pits per unit area,
•
the diameter of the pits, and
•
the depth of the pits.
The number of pits per unit area and the pit diameter are
easily determined by comparison with a pictorial stan-
dard. In the case of pit depth it is usually the maximum
depth that is determined. In some cases this means the
depth of the deepest pit observed on the sample exam-
ined, but in others the mean value of, for example, the
five deepest pits. The depth of the pits can be measured
with a microscope by focusing first on the bottom of the
pit and then on the surface of the uncorroded metal. In
this way the distance between the two focusing levels
is obtained. The depth of pitting can also be determined
Part D 12.5
702 Part D Materials Performance Testing
500 μm
Fig. 12.37 Pitting in stainless steel
Pit: Anode
• high Cl
–
concentration
• low pH
Oxidation
Free surface: Cathode
• high pH
• high O
2
concentration
Reduction
O
2
OH
–
H
+
Me
+
Cl
–
Fig. 12.38 Principle of pitting corrosion
with a micrometer or by cutting a cross section through
the pit followed by direct measurement – possibly with
the aid of a microscope.
The ratio of the maximum pit depth (P
max
)andthe
average penetration (P
aver
) is called the pitting factor
(F)(Fig.12.39)
F =
P
max
P
aver
. (12.75)
Pitting can occur in most metals. Amongst passivated
metals this type of corrosion is initiated only above
a certain electrode potential, the pitting potential.
Especially stainless steels where the protective ef-
fect results from passivation of the steel are prone to
pitting under certain environmental conditions. Stain-
less steels are iron-based alloys in which chromium is
the main alloying additive at a concentration of at least
Initial surface
P
aver
P
max
Fig. 12.39 Pitting factor F = P
max
/P
aver
(after [12.10])
12%. Because of the chromium content, stainless steels
are easily passivated and hence have good corrosion re-
sistance in many of the more common environments. In
unfavorable conditions, however, even stainless steels
can be subject to, for example, uniform corrosion, pit-
ting, crevice corrosion, intergranular corrosion or stress
corrosion cracking.
One can distinguish between different types of
stainless steels depending on their structure, e.g. ferritic,
austenitic and ferritic–austenitic steels. Differences in
structure convey differences in corrosion characteristics
and even differences in weldability, hardening capacity
and magnetic properties. Ferritic and ferritic–austenitic
steels are magnetic in contrast to pure austenitic steels.
TheActiveandPassiveStates
The conditions for passivation are shown in the an-
odic polarization curves of the steels. If, in the case
of a stainless steel in sulphuric acid solution, the elec-
trode potential is increased then the current density rises
to a maximum, with dissolution of the metal taking
place in the active state; the current density is an ex-
pression of the dissolution rate. At a certain potential,
the passivation potential, the corrosion current density
is drastically reduced and the metal surface becomes
passivated. Passivation is associated with the forma-
tion of a thin, protective coating which largely consists
of a mixed iron–chromium oxide and hydroxide. If the
potential is further increased to very high values the cur-
rent density will increase again as a result of, so-called,
transpassive corrosion.
Mechanism of Pitting and Crevice Corrosion
When using stainless steel in an environment with
a high chloride content, such as sea water or the bleach-
ing liquors used in the pulp industry, localized corrosion
will often occur in the form of pitting – which can some-
Part D 12.5

Corrosion 12.5 Corrosion Without Mechanical Loading 703
times result in perforation of pipe walls – or in the form
of crevice corrosion, e.g. in flanged joints. These two
corrosion types are related.
Stainless steels are sensitive to localized corrosion,
especially in the presence of halogen ions. Amongst
these the chloride ion is the most corrosive and also of
greatest practical importance.
Addition of sulphide considerably increases the cor-
rosiveness of the environment and may take place, e.g.
due to the dissolution of sulphide inclusions in the steel
surface.
Pitting in stainless steel also affects the shape of the
anodic polarization curve. Thus, if the potential is in-
creased above a certain critical value, referred to as the
breakdown potential, the current density will begin to
increase and the curve often shows thereafter a series
of current peaks. Since this rise marks the beginning of
pitting the breakdown potential is in this case called the
pitting potential. If the potential is then decreased, pas-
sivation is achieved again, but only when a protection
potential, which is a little below the pitting potential,
is reached. A similar development occurs with corro-
sion in crevices or under surface deposits. The existence
and value of the pitting potential can be demonstrated
by using an auxiliary electrode and an applied volt-
age. In practice, the presence of an oxidizing agent,
e.g., oxygen, chlorine or peroxide, in the solution is of-
ten sufficient to raise the potential to the pitting value
with consequent attack. The breakdown potential is not
a well-defined constant but depends to a large extent on
conditions such as chloride concentration, temperature
and the method of measurement.
Localized corrosion is observed only after a cer-
tain incubation time during which the initiation of the
attack takes place. This is followed by the propaga-
tion stage and the growth of the pit. Both initiation and
propagation take place by a mechanism which involves
electrochemical corrosion cells.
Many mechanisms have been proposed for the initi-
ation stage. In some the initiation can consist of a new,
unpassivated metal surface being created as a result of
the dissolution of sulphide or sulphide/oxide inclusions
in the surface. This could then lead to an acidic solu-
tion containing a high sulphide content developing in
the incipient cavities; these conditions would not al-
low passivation of the stainless-steel surface to occur
at these sites. In other cases, initiation can be associated
with depletion of oxygen in a crevice or under a de-
posit. This would give rise to an oxygen concentration
cell with the anode in the crevice or under the deposit,
and the cathode outside these regions. Hydrolysis of an-
odically dissolved metal ions in the various situations
gives rise to acidic, unpassivating conditions at anodes.
When localized attack has been initiated and the
growth has reached steady-state conditions then the pro-
cess can be said to have reached the propagation stage.
Certain characteristic conditions now prevail in the pit,
as detailed below.
The pH value is lower than in the bulk of the so-
lution because anodically dissolved metal ions, such as
Fe
z+
and Cr
3+
have been hydrolyzed with the formation
of oxides, hydroxides or hydroxide salts, thus releasing
hydrogen ions. The actual pH value will depend on the
composition of the steel and will be lower the more cor-
rosion resistant the steel. Often a pH value of 0–1 can
arise.
The solution in the pit has a higher chloride con-
centration than the bulk of the solution. This is because
chloride ions migrate against the electric current in
through the mouth of the pit. In practice a chloride
concentration as high as 5 M can occur in the pit.
The resistance of stainless steels towards localized
corrosion can be evaluated by
•
determination of the breakdown potential, i. e. the
pitting potential or crevice corrosion potential; this
can be done by recording the anodic polarization
curve in a solution of chloride (American Society
for Testing and Materials (ASTM) G 61),
•
determination of the critical pitting temperature
(cpt) and the critical crevice corrosion tempera-
ture (cct); the cpt or cct, is the lowest temperature
at which attack takes place, while maintaining the
stainless steel at a constant potential.
•
total immersion testing in a suitable corrosive agent,
e.g. ferric chloride solution (ASTM G 48), followed
by measurement of the maximum pit depth; in this
type of test an elastic string or plastic disc is pressed
against the test piece and the depth of attack is
measured at the points of contact after the exposure
period.
Crevice Corrosion
Corrosion which is associated with a crevice and which
takes place in or immediately around the crevice, is
called crevice corrosion. In some cases crevice corro-
sion can simply be caused by corrosive liquid being held
in the crevice, while surrounding surfaces dry out. If the
crevice and the surrounding metal surfaces are in a solu-
tion, the liquid in the crevice can be almost stagnant. As
a result of corrosion in the crevice the conditions there
can be changed; for example, the pH value can decrease
Part D 12.5
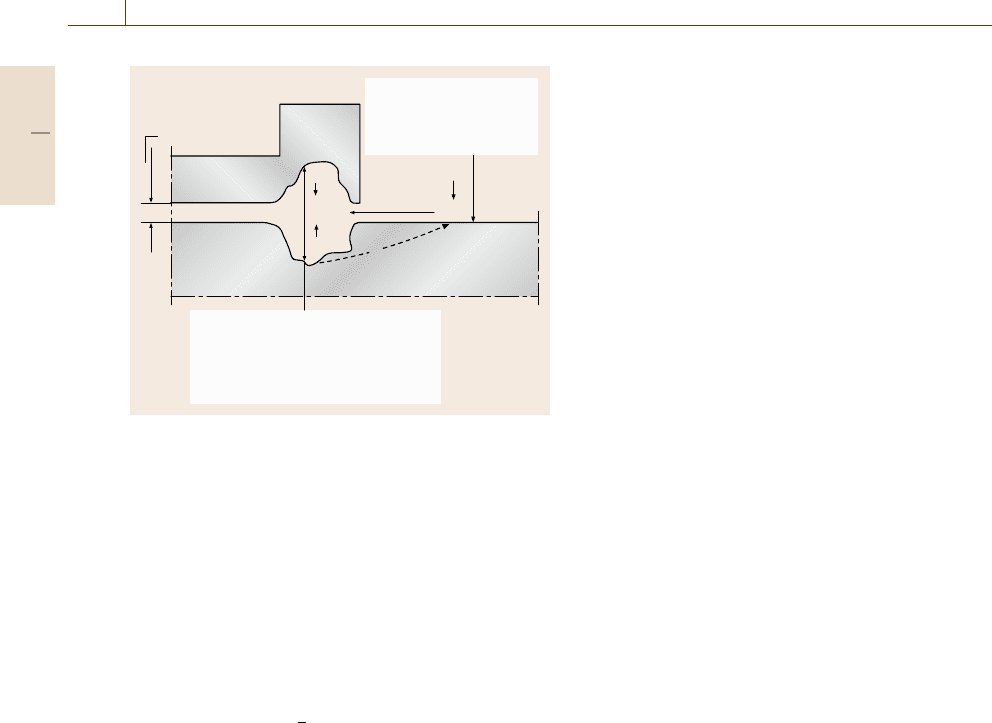
704 Part D Materials Performance Testing
e
Crevice: Anode
Interstice
• low pH
• low O
2
concentration
• high concentration of Cl
–
, Me
+
, H
+
Oxidation
Free surface: Cathode
• high pH
• high O
2
concentration
Reduction
O
2
OH
–
H
+
H
+
Me
+
Me
+
Cl
–
Cl
–
Cl
–
Fig. 12.40 Principle of crevice corrosion
and the concentration of chloride increase, and as a con-
sequence the corrosiveness can be higher in the crevice
than outside (Fig. 12.40).
When stationary conditions have been established,
anodic attack of the metal usually takes place near the
mouth of the crevice, while cathodic reduction of oxy-
gen from the surroundings takes place on the metal
surfaces outside
Anode reaction: Me →Me
+
+n e
−
, (12.76)
Cathode reaction:
1
2
O
2
+H
2
O+2e
−
−2OH
−
.
(12.77)
Crevice corrosion can take place in most metals. The
risk of crevice corrosion should be especially heeded
with passive metals, e.g. stainless steel.
Crevice corrosion not only takes place in the
crevices between surfaces of the same metal, but also
when the metal is touching a nonmetallic material.
A combination of crevice corrosion and bimetallic cor-
rosion can take place when two different metals form
a crevice. The presence of Cl
−
,Br
−
or I
−
ions generally
accelerates this type of corrosion. As in the case of pit-
ting, crevice corrosion is initiated only above a certain
electrode potential.
Deposit Corrosion
Corrosion which is associated with a deposit of corro-
sion products or other substances and which takes place
under or immediately around the deposit is called de-
posit corrosion.
This type of corrosion is caused by moisture being
held in and under the deposit. Because the movement of
water is poor, corrosive conditions can be created under
the deposit in a similar way to that described in crevice
corrosion. The result is that a corrosion cell is formed
with the anode under the deposit and the cathode at, or
just outside, the edge.
Deposit corrosion is found, for example
•
under the road-mud in the wheel arch of a car,
•
under leaves which have collected in guttering, and
•
under fouling on ships’ hulls and in sea-water-
cooled condensers.
Selective Corrosion
Selective corrosion can be found in alloys and results
from the fact that the components of the alloy corrode
at different rates.
The most well-known example of selective corro-
sion is the dezincification of brass. During dezincifica-
tion the zinc is dissolved selectively, while the copper
is left as a porous mass having poor structural strength.
Similar corrosion processes are the dealuminization of
aluminum bronze and the selective dissolution of tin in
phosphor bronze.
Graphitic corrosion in grey cast iron provides an-
other example of selective corrosion where the metallic
constituents of the iron are removed. The remain-
ing graphite allows the object concerned to maintain
its shape but its strength and weight are severely
reduced.
Intergranular Corrosion
Intergranular corrosion means corrosion in or adjacent
to the grain boundaries of the metal. Metals are usu-
ally built of crystal grains. When a metal solidifies or is
heat treated, several processes take place which cause
the grain-boundary region to take on other corrosion
characteristics than the main mass of the grain.
Intergranular corrosion can occur in most metals
under unfavorable conditions. Most well known is in-
tergranular corrosion in stainless steel (Fig. 12.41)as
a consequence of chromium carbide formation when the
carbon concentration is too high and unfavorable heat
treatment has occurred, e.g. in the heat-affected zone
along a weld.
Intergranular corrosion can occur in certain types
of stainless steel which have a high carbon content
(0.05–0.15% C). This can take place if the stainless steel
is heat treated so that chromium carbide precipitates in
the grain boundaries and the material is subsequently
Part D 12.5

Corrosion 12.6 Corrosion with Mechanical Loading 705
exposed to an acidic solution or sea water. The pre-
cipitation of chromium carbide takes place only under
certain conditions, for austenitic steel this is in the
temperature range 550–850
◦
C. The steel is then said
to be sensitized. The carbide precipitation results in
a narrow zone near the grain boundaries becoming so
depleted in chromium that the steel loses its stainless
character.
Sensitization is not restricted to manufacturing heat-
treatment processes. It can also occur as a result of
welding, since a region of the metal near the weld will
pass through the above temperature range. On expo-
sure to a corrosive environment the chromium-depleted
zones, together with the remaining parts of the grains,
form corrosion cells. In these the chromium-depleted
metal acts as the anode and is attacked, resulting in
intergranular corrosion.
Intergranular corrosion does not usually influence
the shape of the object but the strength characteristics
can be catastrophically reduced. In a chloride environ-
ment, e.g. sea water, the attack can be visible as pitting.
Intergranular corrosion also results in the loss of metal-
lic sound when the affected component is struck.
Countermeasures are designed so as to counteract
the precipitation of chromium carbide, which is the
cause of the sensitization. The following possibilities
exist.
•
The choice of stainless steel with a low carbon con-
tent (< 0.05% or preferably < 0.03%),
•
The choice of steel which has been stabilized by the
addition of titanium or niobium, which bind the car-
bon as carbides so that the formation of chromium
carbide is prevented,
•
The shortest possible time at 550–850
◦
Cand
•
Solution heat treatment; the material is heated to
about 1050
◦
C to dissolve precipitates of chromium
carbide that have formed, followed by subsequent
rapid cooling so that new chromium carbide precip-
itates have no time to form.
The resistance of stainless steel to intergranular corro-
sion can be evaluated through
Fig. 12.41 Intercrystalline corrosion
•
Strauss test (ASTM A 262 practice E); the test piece
is exposed to a boiling copper sulphate solution
containing sulphuric acid and metallic copper; af-
ter exposure the test piece is deformed by bending,
and the surface is then examined for cracks,
•
Streicher test (ASTM A 262 practice B); the
test piece is exposed to a boiling ferric sulphate-
sulphuric acid solution for 120 h; after the exposure
the weight loss of the test piece is determined, and
•
Huey test (ASTM A 262 practice C); the test piece is
exposed to a boiling 65% nitric acid solution; eval-
uation consists of determining weight loss; Huey
testing is particularly significant for material that is
to be used in strongly oxidizing media.
Layer Corrosion
With layer corrosion the attack is localized to internal
layers in wrought metal. As a rule the layer is parallel
to the direction of processing and usually to the surface.
The attack can result in the unattacked layers becom-
ing detached and looking like the pages in a book. But
the result can also be the formation of blisters swelling
the metal surface, because of the voluminous corrosion
products. Layer corrosion is rather unusual. It is best
known amongst certain aluminum alloys.
12.6 Corrosion with Mechanical Loading
When investigating corrosion systems it is advisable to
distinguish between corrosion systems where no exter-
nal mechanical load is applied or residual stresses are
present and systems which include influences of me-
chanical stresses. The distinction is useful because the
forms of corrosion involved are different from their ap-
pearance as well as their mechanistic background. The
same considerations apply for the test methods. Testing
Part D 12.6

706 Part D Materials Performance Testing
of corrosion systems where mechanical stress is part of
the environmental conditions the metal is subjected to
means to study the influence of additional parameters,
e.g. mechanical stress or strain, which may be supplied
to the material in a static or dynamic regime. In sum-
mary this requires the study of the interaction of all
parameters involved and very often the mechanical pa-
rameters are of higher importance with respect to the
corrosion behavior than the electrolytic conditions.
There is also completely different equipment and
evaluation of test data. The introduction of mechani-
cal stresses into the test system needs an apparatus to
supply stresses or strains to the system in a static or dy-
namic manner and the type of specimen may be smooth,
notched or precracked, depending on the specific pa-
rameter to be studied (crack initiation or propagation).
12.6.1 Stress Corrosion
General
Stress corrosion cracking means crack initiation and
propagation in the presence of certain corrosive me-
dia under static tensile stresses. In some cases residual
stresses within the material are sufficient to initiate this
form of corrosion. Contrary to most other manifesta-
tions of corrosion, the failure, due to a sudden brittle
fracture, often occurs without formation of detectable
corrosion products and is not necessarily characterized
by any previously occurring visible damage.
For assessing the corrosion, parameters of the
medium as well as of the material are important. In
practice, where complex corrosion conditions often ex-
ist, adequate knowledge of the corrosion process is
therefore required in the evaluation of failures. Stress
corrosion cracking can be subdivided into an initiation
and a propagation process. In theory, such a differentia-
tion is possible, but experimentally it is very difficult to
detect as the two processes overlap during the stepwise
progress of the cracking [12.68]. Furthermore, metal
surfaces often have incipient cracks and crevices al-
ready present in the as-delivered state which could act
as crack nuclei, which means that crack initiation in
such cases is not necessary at all.
Considerable differences can be found when com-
paring experimentally determined crack-growth rates of
various metal/environment systems. This immediately
indicates that stress corrosion cracking cannot be ex-
plained satisfactorily by only one theoretical model.
The spectra of the proposed mechanisms range from
models where crack growth is regarded as a very fast
local metal dissolution at the crack tip to models that
assume that the adsorption of certain species of the
medium at the metal surface weakens the binding forces
between the metal atoms and favors brittle fracture
along the cleavage planes. A comparison of both mech-
anisms shows that in the first case hydrogen-induced
and in the second case brittle fracture is the predomi-
nant factor. Between both cases various transitions are
possible which means that the degree of the corrosion
effect is also different [12.68].
When discussing stress corrosion cracking a dif-
ferentiation has to be made between an anodic and
a cathodic mechanism. Stress corrosion cracking is usu-
ally described as anodic when the propagation rate is
equivalent to the anodic metal dissolution rate at the
crack tip. Cathodic stress corrosion cracking (HISCC)
results from the embrittlement of the material due to
hydrogen, which is generated by cathodic hydrogen
evolution at the metal surface. Although the external ap-
pearance of the damage in both cases shows similarities,
the mechanisms responsible for the failure are different.
Anodic Stress Corrosion Cracking
Anodic stress corrosion cracking requires several pre-
conditions: (i) the electrolyte must have a specific
effect on the material, (ii) the material itself has to be
susceptible to stress corrosion cracking, and (iii) the
tensile stresses have to be sufficiently high. The stress
corrosion cracking susceptibility of a material is not
a material property comparable to the tensile strength
but a manifestation which the material shows under
specific conditions. It is based on a three-component
system consisting of the material (strength, microstruc-
ture, surface condition), mechanical stresses (external
stresses, residual stresses) and a specific electrolyte.
Furthermore the existence of protecting layers on the
metal surface is a necessary but not sufficient prereq-
uisite for initiation of stress corrosion cracking. Local
damage of this surface layer – which could result from
chemical as well as mechanical effects – provides start-
ing points for crack formation. The mechanisms of
crack propagation can be subdivided into two groups.
The first group considers stress corrosion cracking as
selective electrochemical metal dissolution at the crack
tip while the other group supposes that the reduction
of binding forces of the atoms at the crack tip in
the presence of specific ions or molecules is responsi-
ble [12.37].
Test Methods for Stress Corrosion Cracking
Corrosion systems with simultaneous corrosion load
and mechanical stresses are very complex as the condi-
Part D 12.6

Corrosion 12.6 Corrosion with Mechanical Loading 707
tions change with time and the changes resulting from
the parameter interactions are often difficult to inter-
pret. It is therefore necessary to set up stress corrosion
cracking tests in such a way that the main parame-
ters are clearly defined and measured during the test.
The existing literature provides standards [12.69, 70]
and summaries [12.71] in which all relevant aspects are
presented and discussed. In the next section the main
parameters will be discussed on the basis of these refer-
ences as these provide a basis for a practice-related test
method for assessing the susceptibility of prestressing
steels. Nearly all mechanical types of stress have been
used for investigating prestressed steels.
Aim of Stress Corrosion Test
From the definition of stress corrosion it is clear that
stress corrosion cracking is a special case of stress cor-
rosion and that under certain circumstances corrosion
will not lead to the formation of cracks.
Although there is an agreement that crack formation
is the normal test result, other manifestations have also
been found like intergranular corrosion or elongated fine
cracks which are intensified in the presence of stresses.
There is a large variety of methods to assess the stress
corrosion cracking properties of metals. Each of them
has its specific advantages and disadvantages.
It is important to recall that the term test in connec-
tion with the resistance or susceptibility to stress cor-
rosion cracking has a special meaning. Whether stress
corrosion cracking occurs in a certain case or not de-
pends on the environmental and mechanical conditions
as well as the material properties of the material. The
word susceptibility does not describe a material property
or quality which can be related to an overall valid scale
as the ranking of a series of alloys can be very different
depending on the exposure conditions.
In an ideal case for a given application the likeli-
hood of occurrence of stress corrosion cracking can only
be determined by carrying out simulation tests under all
possible exposure conditions. In practice this is difficult
and sometimes even impossible. Therefore, on the basis
of practical experience, a certain number of standard test
methods have been developed which give useful guid-
ance for the likely practical behavior in specific cases
of application. These laboratory standard test methods
are only suitable for such practical conditions if they are
based on experience or where relevant relations exist,
even if they are only empirical.
The fact that a given material may or may not pass
a test which was previously useful for another mater-
ial may not be significant and, similarly, a test which
correctly distinguishes between two materials for one
application does not necessarily provide reliable evi-
dence when exposure conditions are different. Therefore
the use of a standard test for conditions outside the scope
of the test requires validation.
Selection of the Test Method
Prior to starting a programme for stress corrosion crack-
ing testing it is necessary to decide which kind of test is
suitable. Such a decision mainly depends on the purpose
of the test and the type of information to be obtained.
While some test methods try to simulate practical con-
ditions as far as possible, this being of great value to
plant engineers, other tests are directed towards eval-
uating certain mechanistic effects of failure. In the first
case limitations with respect to e.g. material, space, time
etc. can lead to relatively simple test methods while un-
der different conditions refined methods are necessary.
For example, investigations on the crack growth rate can
require the use of cracked specimens, but these are un-
suitable for evaluating the effects of surface treatments.
Although improved techniques are available the use of
a simple test can be valuable for conditions where the
more complicated techniques cannot be used.
In selecting a test method which only gives a fail-
ure/nonfailure result it is important to ensure that it is
not so severe that it leads to rejection of a material which
has already proven its suitability in a special practical
application. On the other hand it must not be so mild
that it encourages the use of a material under conditions
which could lead to rapid failure.
In general the aim of a stress corrosion cracking
test is to obtain quicker information on the prediction
of practical behavior than is possible from practical ex-
perience. There are various possibilities to achieve this,
such as using higher stresses, continuous slow straining,
cracked specimens, higher concentration of certain sub-
stances in the test environment and higher temperatures,
i. e. compared to service conditions, and also electro-
chemical stimulation. However, it is very important that
the methods used and parameters selected do not change
the details of the failure mechanism.
Loading Systems
General. The methods for loading the specimens
(smooth, notched or cracked) are usually classified as
follows
•
constant strain,
•
constant load,
•
slow strain rate.
Part D 12.6
