Czichos H., Saito T., Smith L.E. (Eds.) Handbook of Metrology and Testing
Подождите немного. Документ загружается.

688 Part D Materials Performance Testing
[Fe(CN)
6
]
3−
at the ring or vice versa
N =
n
Di
I
R
I
Di
n
R
. (12.58)
RRDE with different metal discs and even brittle semi-
conductor electrodes have been formed. Usually the
disc is embedded in a thin layer of resin with a cylin-
der of Au or Pt glued by resin to its surface. The
total set is then surrounded by resin within a cylin-
der of a polymer and the electrodes are contacted from
the rear side. This exchangeable RRDE may then be
attached to a disc rotator with rotating contacts for
a reliable connection of the rotating electrodes to a po-
tentiostat (Fig. 12.22) [12.43]. Frequently two isolated
half cylinders are used instead of a full cylinder, which
I
Di
(μA)
I
R
(μA)
I
Di
I
R1
E
R1
= 0.6V
E
R2
= –0.23V
I
R2
A1
A2
Cu
0.1 M KOH
dE/dt = 10mV/s
50
40
30
20
10
0
60
–10
0
0 200 t (s)15010050
–0.5
–1
–1.5
–2
–2.5
–3
–3.5
0.5
1
1.5
–1.0 –0.6 –0.2 0.80.60.2
E
Di
(V) (SHE)
Fig. 12.23 Current of a copper disc I
Di
andringcurrents
I
R1
and I
R2
of a split RRDE in 0.1 M KOH for a potentio-
dynamic scan of the disc potential dE
Di
/dt = 10 mV/s
i (A/cm
2
)
E (V)
i
A
E
RB
E
RA
E'
RA
ΔU
Ω
E'
RB
i
B
Fig. 12.24 Current densities i
A
and i
B
of two surface sites
A and B with an ohmic drop ΔU
Ω
within the electrolyte in
front and related rest potentials E
RA
and E
RB
opens the possibility to measure the formation of two
products simultaneously at the split ring. The RRDE
requires a bipotentiostat to set the potential of two
working electrodes independently from each other. An
RRDE with a split ring needs a tripotentiostat. The re-
lated block diagram is depicted in Fig. 12.22. The three
working electrodes WE
1
,WE
2
and WE
3
(disc, ring 1
and ring 2) are connected via a differential amplifier
to avoid grounding problems. The three circuits have
one common reference electrode, RE, and one counter-
electrode, CE, which is grounded. Further details are
similar to those discussed for the block diagram of
A
E (V) (1) (2)
(3)
x
BA AB
a)
b)
Fig. 12.25 (a) Current lines (arrows) and curves of equal
potential in the presence of local elements with surface
sites A and B. (b) potential profile with very low (1), low
(2), and very high conductivity (3) of the electrolyte
Part D 12.2
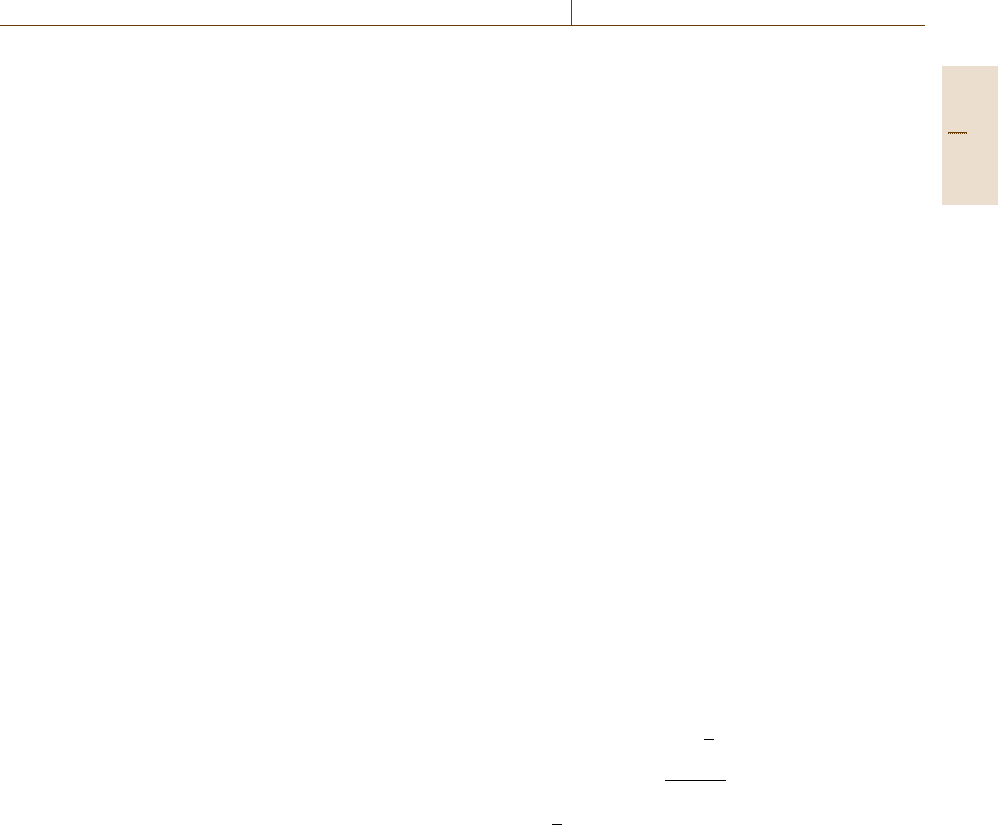
Corrosion 12.2 Conventional Electrochemical Test Methods 689
Fig. 12.4. Figure 12.23 presents as an example the I
D
of
the polarization curve of a Cu disc in 0.1 M KOH. Cu
forms a passivating oxide layer at sufficiently positive
potentials at the anodic peaks A1 and A2. Figure 12.23
depicts I
R1
and I
R2
of the two analytical ring electrodes,
which are set to appropriate potentials E
R1
= 0.6V
and E
R2
=−0.23 V, respectively. These potentials are
suited to oxidize soluble Cu(I) ions to Cu(II) ions and to
reduce Cu(II) to Cu(I) ions respectively. Thus the meas-
ured analytical ring currents permit the determination of
the kind and the amount of soluble corrosion products
from the disc. The calculation of the related disc cur-
rents I
Di
(CuI) and I
Di
(CuII) of soluble products with
(12.58) and their comparison with the measured total
current I
Di
allow the determination of the charge which
is stored as a passivating oxide film on the copper elec-
trode. Thus these measurements with RRDE allow the
determination of the efficiency of layer formation and
dissolution. Figure 12.23 shows the close relation of the
current peaks A1 and A2 of I
Di
for Cu(I) oxide and
Cu(II) oxide formation and the dissolution of the related
ions. Even the dissolution of weakly soluble Cu(I) ions
may be detected by I
R1
in 0.1 M KOH.
A further development of the RRDE is its hydrody-
namical modulation of the rotation speed ω. Here the
thickness δ of the Nernst diffusion layer is modulated
according to (12.57) and thus the diffusion of corrosion
products and their transfer to the rings. With a hy-
drodynamical square-wave modulation one may even
distinguish quantitatively between species of the same
kind from the bulk electrolyte and those formed at the
disc. A detailed description of this method and its appli-
cation to corrosion is beyond the scope of this chapter
and the reader is referred to the literature. An example
of its application to the dissolution and film forma-
tion has been described for Fe in 1 M NaOH [12.44].
The agreement of experiments and calculation is shown
in [12.45].
12.2.12 Ohmic Drops
High current densities cause ohmic drops within the
electrolyte. For a planar electrode with a current density
i the voltage drop ΔU
Ω
changes according to (12.59)
with the electrolyte resistance R
Ω
, the specific con-
ductivity κ of the electrolyte, and the distance d from
the electrode surface. The ohmic drop ΔU
Ω
in front
of a hemispherical electrode of radius a at distance r
isgivenby(12.60). For r →∞, ΔU
Ω
obtains a max-
imum value ΔU
Ω,max
related to the maximum ohmic
resistance R
Ω,max
. Similar to the concentration gradient
related to diffusion in front of a hemispherical electrode
a small radius a reduces R
Ω,max
and ΔU
Ω,max
to very
small values. For i = 1A/cm
2
a distance of d = 5mm,
and κ =22 Ω
−1
cm
−1
for an electrolyte with good con-
ductivity such as 0.5MH
2
SO
4
, one obtains for a planar
electrode at a distance d = 0.5 cm, a value of ΔU
Ω
=
0.023 V =23 mV (12.59). ΔU
Ω
will be much larger for
less-conducting electrolytes and higher current densi-
ties. These values are characteristic of the ohmic drop
between the Haber–Luggin capillary of a reference elec-
trode and the working electrode of a potentiostatic
circuit. It may be compensated automatically to about
90% by an electronic feedback loop, a unit built into
potentiostats. However, this may still be a problem
for electrochemical measurements at very high cur-
rent densities of i ≥10 A/cm
2
or electrolytes with low
conductivity. Large electrodes will also be submitted
to a nonhomogeneous potential distribution due to the
presence of large local differences of the ohmic drops.
In the case of a microelectrode with a radius of
a =10
−4
cm one obtains ΔU
Ω,max
=4.5×10
−3
mV for
conditions otherwise the same as given above (12.60).
Furthermore the potential will be uniform in front of
its surface even for high current densities. Similar con-
ditions will hold for concave hemispherical surfaces
such as small corrosion pits with μm dimensions. Here
again the potential drop will be three times larger, i. e.
13.5×10
−3
mV for a =1 μm, due to the concave geom-
etry
ΔU
Ω
=iR
Ω
with R
Ω
=
d
κ
, (12.59)
ΔU
Ω
=iR
Ω
, R
Ω
=
ra
κ(r +a)
;
R
Ω,max
=
a
κ
for r →∞. (12.60)
Microelectrodes are of increasing interest. Many pro-
cesses occur on a very small scale so that micro- and
nanoelectrochemistry are actively developing branches
in electrochemistry. These fields are also important for
corrosion studies. Many corrosion processes occur on
a small scale and still cause huge damage. Mecha-
nistic studies of localized corrosion of passive metal
electrodes may follow the formation and growth of cor-
rosion pits to micrometer and nanometer scales with
appropriate methods such as scanning force microscopy
(SFM) and scanning tunneling microscopy (STM).
These methods use very sharp tips that may also be
seen as ultramicroelectrodes. Another important exam-
ple is corrosion of integrated circuits, which is a serious
problem for their life time and reliability. There are al-
Part D 12.2
690 Part D Materials Performance Testing
ways electrolyte residues on metallic connections on
chips and the encapsulent gives imperfect protection
against the ingress of moisture. The voltage supply of
5 or 15 V is large for any electrochemical process and
thus dissolution of metal at positive potentials and its re-
deposition at negative potential causes disconnection of
vapor-deposited metal connections with μm dimensions
and short circuits by the deposition of dendrites. The in-
vestigation of biological processes and the development
of sensors also need electrochemistry on a micrometer
scale. Further miniaturization pushes electrochemistry
to a nanometer scale. Thus micro- and nanotechnol-
ogy require studies of electrochemical processes with
the help of micro- and ultramicroelectrodes. Corro-
sion research necessarily has to address these small
and extremely small scales. Modern electroanalytical
chemistry uses these micro- and ultramicroelectrodes
for scanning electrochemical microscopes (SECM). It
should also be mentioned that the tip of an STM or an
SFM may be used for highly localized electrochemical
studies and surface structuring in combination with the
imaging of the electrode surface with mesoscopic and
atomic resolution. One possible application of these ul-
tramicroelectrodes is the structuring of metal surfaces
by dissolution and deposition of metal on a submicrom-
eter and nanometer scale.
12.2.13 Measurement of Ohmic Drops and
Potential Profiles Within Electrolytes
The ohmic drop in front of an electrode may be used
as a measure of localized electrochemical processes, as
e.g. in the case of local elements and other localized
corrosion phenomena. For an electrolyte with good con-
ductivity the potential in front of an electrode does not
change if the current densities assume moderate values,
although the rate of electrode reactions change locally
with the surface sites A and B due to their physical or
chemical differences. This leads to local anodes A and
cathodes B as discussed in Sect. 12.2.9 with no potential
drop between them (Fig. 12.16). However for a low-
conductivity electrolyte large potential drops ΔU
Ω
may
occur, which shift the rest potentials of these sites to the
values E
RA
and E
RB
apart from each other (Fig. 12.24).
For a vanishingly small conductivity κ the potential
profile parallel to the electrode surface within the elec-
trolyte follows the shape of the related areas A and
B with steep changes at their contact (Fig. 12.25). For
0 <κ<∞ a smoothed profile is found which disap-
pears for κ →∞. Thus the potential in close vicinity to
the electrode surface will change within the electrolyte
Metal
V
Electrolyte
RE
II
RE
I
ΔU
Ω
Fig. 12.26 Measurement of the potential profile ΔU
Ω
within the electrolyte with two reference electrodes RE
I
and RE
II
with location. However, these differences will smear out
with increasing distance from the electrode due to the
spread of the current lines.
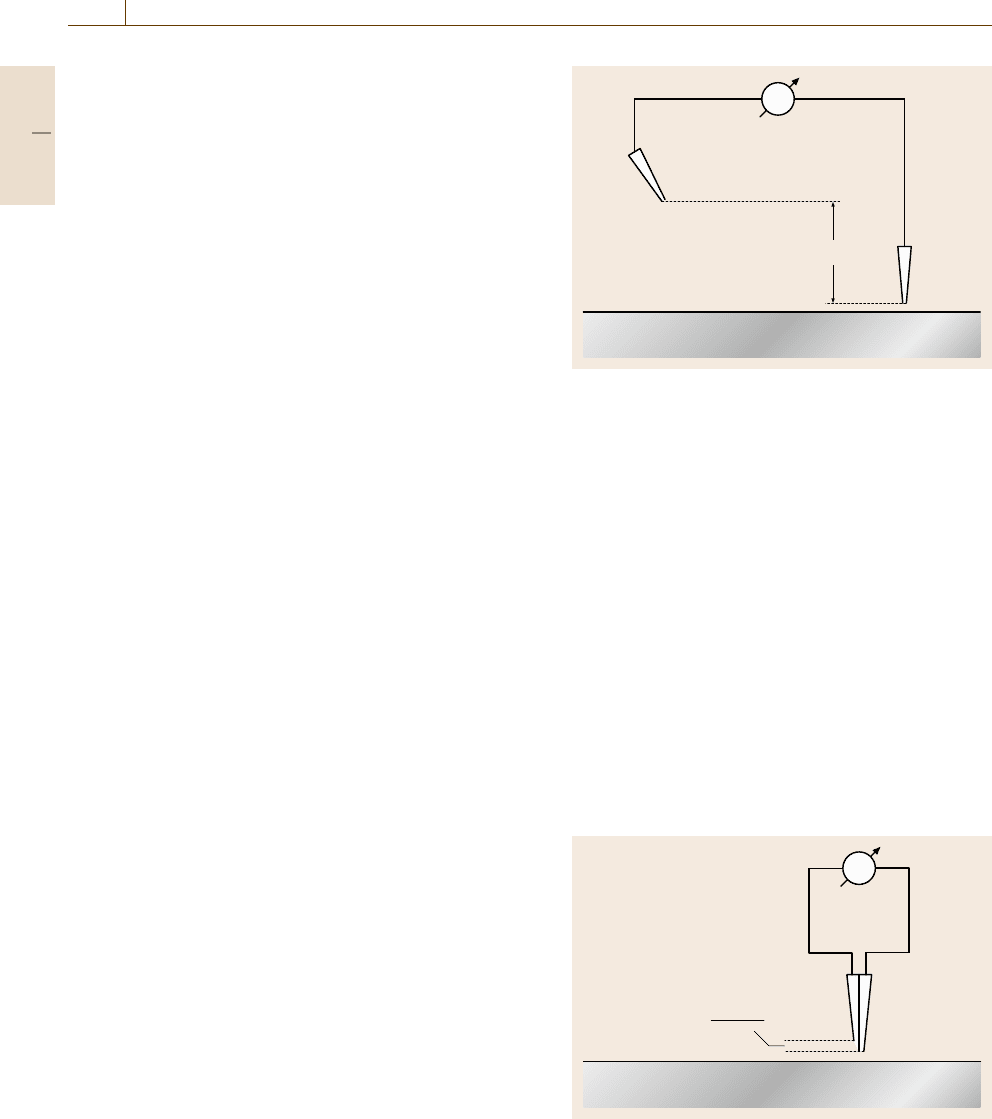
The related ohmic potential drop ΔU
Ω
may be
measured with two reference electrodes, e.g. two
calomel electrodes, one close to the surface moving to
the locations of interest (RE
I
) and the other at large dis-
tance within the bulk electrolyte (RE
II
). This arrange-
ment measures the total drop ΔU
Ω
with superimposed
changes when moving the sampling electrode parallel
to the surface (Fig. 12.26). More sensitive measure-
ments of small changes of ΔU
Ω
are performed when
two reference electrodes are fixed with their Haber–
Luggin capillaries close to each other (Fig. 12.27). If
they are moved together across the metal surface one
measures the potential difference referring to their close
Metal
V
Electrolyte
RE
II
RE
I
d(ΔU
Ω
)
dx
Fig. 12.27 Measurement of the profile of the potential gra-
dient d(ΔU
Ω
)/dx within the electrolyte with two close
reference electrodes RE
I
and RE
II
Part D 12.2
Corrosion 12.2 Conventional Electrochemical Test Methods 691
distance as a function of position. Their potential dif-
ference changes with their common position in front
of the electrode surface and thus samples local poten-
tial gradients without a large background voltage. In
conclusion, with the second electrode separation within
the bulk electrolyte one measures the integral poten-
tial drop ΔU
Ω
whereas with two closely interconnected
reference electrodes, i. e. with the so called scanning
reference electrode technique (SRET) [12.46–48], the
local potential gradient dΔU
Ω
/dx is obtained. One
may improve the spatial resolution with two small plat-
inum tips that are isolated at their sides and which may
be arranged closer to each other.
An even better arrangement works with a vibrating
reference electrode, i. e. the scanning vibrating elec-
trode technique (SVET) [12.46, 47]. Here one uses
a small vibrating metal tip and a second reference
electrode far away in the bulk electrolyte. The small
amplitude of vibration measures the related local poten-
tial gradient corresponding to the small local changes
during vibration. The measurement of the related small
potential changes may be improved by a lock-in tech-
nique that separates the signal from the background and
disturbing signals of different frequencies from other
sources. With an applied scan of the vibrating electrode
parallel to the electrode surface one samples the related
potential profile and thus images the sites of different
electrochemical activity. The potential change may also
be investigated perpendicular to the surface and thus
three-dimensional potential profiles may be obtained.
Useful SVET equipment is available commercially. One
may even calculate from these potential gradients the lo-
cal current densities when the size of the surface sites of
different activity and a possible change of the conduc-
tivity of the electrolyte in front are taken into account.
At present the vibrating electrode is used mainly to map
surface sites of different activity. Examples of frequent
interest are local elements on a corroding metal surface
with cathodic and anodic areas, inclusions at a metal
surface causing localized corrosion or corrosion pits
with high metal dissolution rates within a passivated
metal surface.
a)
WTW
E
FW
e(φ
W
– φ
T
)
eφ
W
eφ
T
eΔU
E
FW
E
FT
E
FT
T
b)
Fig. 12.30a,b Contact of specimen W
and tip T with work functions eΦ
W
and eΦ
T
and (a) the resulting contact
potential difference e (Φ
W
−Φ
T
),
(b) compensation of e (Φ
W
−Φ
T
)by
eΔU. E
FW
and E
FT
are the Fermi
levels of the specimen and the tip
V
RE
II
Ground
Pipeline
RE
I
Fig. 12.28 Detection of localized corrosion sites on under ground
pipelines by measurement of potential profiles with two reference
electrodes (RE)
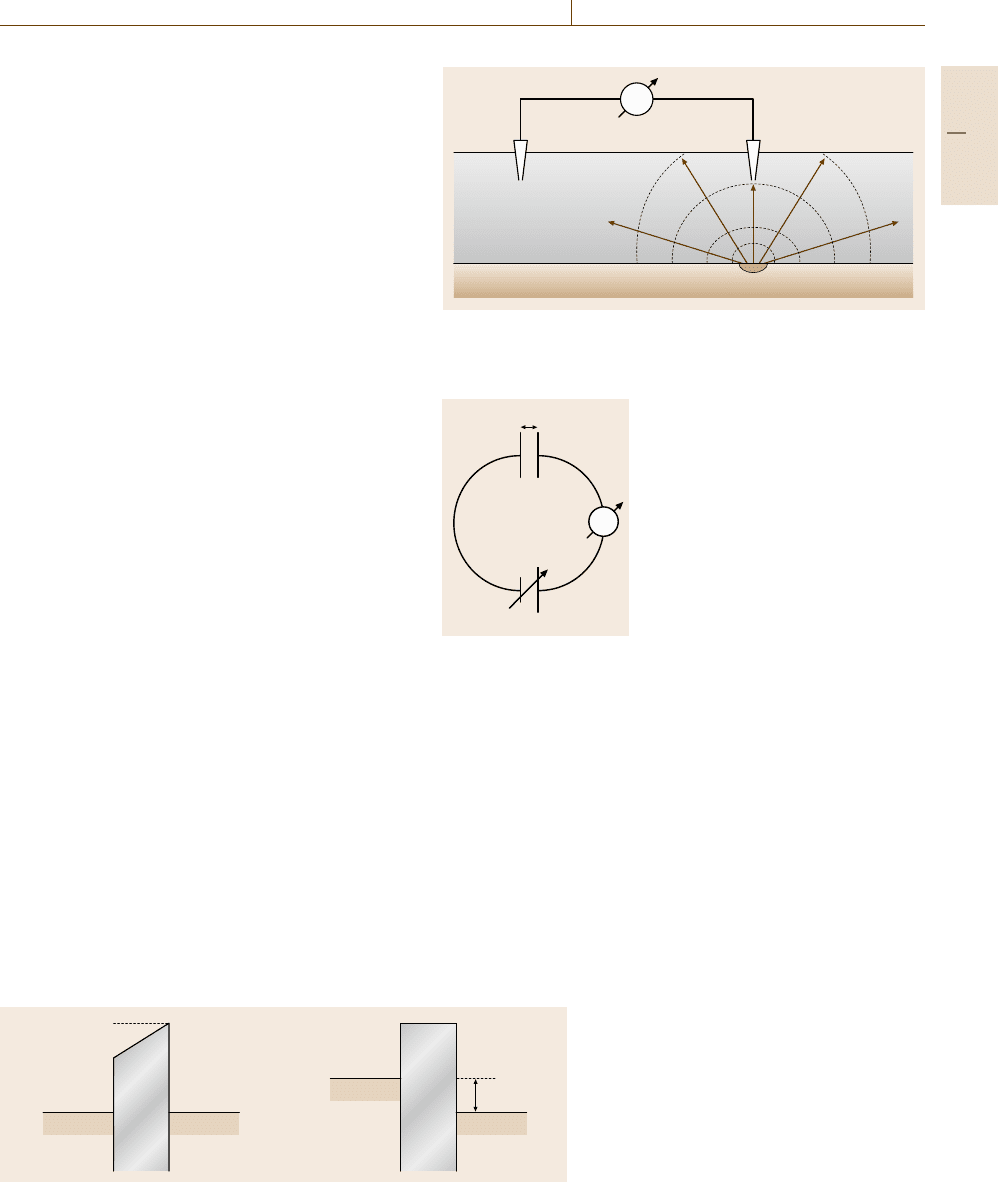
C
d(t)
ΔU
A
Fig. 12.29 Equivalent circuit of
a Kelvin probe with the capacity C
of the vibrating tip/specimen combi-
nation, the compensation voltage ΔU
and current measurement A
In industry the investigation of local corrosion dam-
age by potential measurements is a very important
method. Corrosion damage of metal constructions un-
der ground may be detected by screening with two
reference electrodes (Fig. 12.28). Similarly to the above
discussion, localized corrosion of a pipeline will cause
a spread of currents and related potential drops within
the ground. The center of the damage may be found by
two references which are in contact with the ground.
One of these will be placed at different sites, thus mea-
suring the potential difference as a function of location.
The position of large potential differences should be
close to the site of active corrosion damage.
The scanning Kelvin probe provides another pos-
sibility to investigate surface sites with varying elec-
Part D 12.2
692 Part D Materials Performance Testing
trochemical activity. Its application to surface studies
is complementary to the two aforementioned methods
for corrosion studies. Its advantage is that it enables
a potential measurement without direct contact with the
medium of interest. Two different metals, the work-
ing electrode W and a vibrating tip T in its vicinity,
form a condenser (Fig. 12.29). Their direct contact in
the outer circuit produces a contact potential differ-
ence which equals the difference of the work functions
e
0
(Φ
W
−Φ
T
) = e
0
ΔΦ of both metals (Fig. 12.30a).
This potential difference ΔΦ causes a small alternat-
ing current during the vibration of the tip to the surface
of the working electrode due to the change of the ca-
pacity C =
εε
0
A
d
with distance d according to (12.62).
This current disappears when an externally applied
voltage ΔU compensates ΔΦ = (Φ
W
−Φ
T
)((12.61),
Figs. 12.29, 12.30b). Thus the difference of the work
functions is measured by a disappearing current den-
sity i of a Kelvin probe. The work function is closely
related to the energy of the Fermi level of a metal, which
changes with the potential drop within the electrolyte in
front of an electrode, i. e. due to charging of its dou-
R
CT
log (
|
Z
|
/Ω)
3
2
1
4
0
321–1 4 5
log ( f/Hz)
0
60
40
20
0
80
C
R
Ω
ΔΦ(deg)
Φ phase
Z impedance
a)
b)
Fig. 12.31 (a) Equivalent circuit of an electrode surface with
a double-layer capacity C = 10 μF, a charge-transfer resistance
R
CT
=1kΩ and an ohmic resistance R
Ω
=10 Ω within the elec-
trolyte.
(b) Bode diagram of the impedance Z in dependence on the
frequency of the added alternating voltage (solid) and the related
phase shift ΔΦ (dashed)
ble layer. Any change of the potential drop across the
electrode–electrolyte interface affects the work function
and thus the compensating voltage of the Kelvin probe.
With a vibrating small metal electrode T one may scan
the electrode surface and thus measure the changes of
the difference of the work functions of W and T as
a function of its position and consequently the poten-
tial distribution in front of a corroding metal. The main
advantage is the measurement of potential differences
without making contact with the electrolyte in front of
the electrode. This may be very important when the
electrode–electrolyte interface is buried below a layer
of polymer. For these cases the Kelvin probe allows
one to follow electrochemical reactions and corrosion
processes, including the delamination of polymer lay-
ers on metals via potential measurements that otherwise
would not be accessible [12.49, 50]. Another example
is atmospheric corrosion when only a very thin film of
electrolyte covers the surface, to which one cannot eas-
ily make a conducting contact via the Haber–Luggin
capillary of a reference electrode.
ΔU
tot
=e
0
ΔΦ −ΔU =e
0
(Φ
W
−Φ
T
)−ΔU = 0 ,
(12.61)
dC
dt
=
εε
0
A
dd
=
dQ
dt
ΔU
tot
=
i
ΔU
tot
, (12.61a)
i =
dC
dt
ΔU
tot
=
εε
0
A
dd
ΔU
tot
=0 . (12.62)
12.2.14 Nonstationary Methods,
Pulse Measurements
The sequence of several elementary reaction steps and
their influence on the overall electrochemical corrosion
process requires their separation. This could be done by
electrochemical transient measurements. A very rapid
change of the electrode potential leads to a sequence of
reaction steps (Fig. 12.31) that may be separated in the
time domain. The ohmic drop within the electrolyte be-
tween the reference electrode and the working electrode
is established within less than a microsecond. This may
be measured with fast galvanostatic transients. For this
discussion a useful equivalent circuit for an electrode
within the electrolyte consists of a capacity C in paral-
lel to a charge-transfer resistance R
CT
. A resistance R
Ω
in series to this combination takes care of the electrolyte
resistance between the Haber–Luggin capillary and the
working electrode (Fig. 12.31). A galvanostatic pulse
with a rise time in the range of about 100 ns may easily
be applied between working and counter-electrode. As
Part D 12.2
Corrosion 12.2 Conventional Electrochemical Test Methods 693
a consequence the ohmic drop will be detected imme-
diately, within less than 1 μs. The subsequent charging
of the double layer, i. e. of the capacity C with time
causes an increase of the measured voltage across the
equivalent circuit. Extrapolation of this voltage increase
back to t = 0 yields at the intersection the ohmic drop
ΔU
Ω
, which should increase linearly with the galvano-
statically applied current density i. The thus measured
ohmic drop ΔU
Ω
= R
Ω
i may be used for an appropri-
ate setting of the resistance R
Ω
at the compensation
unit of a potentiostat. Thus one may compensate ΔU
Ω
automatically during potentiostatic transients, which in-
crease proportionally to the current density i.
The electronic properties of the electrode–electro-
lyte interface and its related equivalent circuit may
be determined by measurement of the impedance and
its dependence on frequency, which is presented in
Fig. 12.31 as a so-called Bode plot. For impedance mea-
surements the electrode potential is varied by a small
superimposed amplitude of a few mV in order to jus-
tify the approximation of the electrochemical system
by the proposed equivalent circuit. A linear i–E re-
lation in the sense of Ohm’s law is valid for small
potential changes only. In general the i–E relation
is exponential according to the Butler–Volmer equa-
tion of (12.26). Figure 12.31 also contains the phase
shift ΔΦ. For the presented example R
CT
= 1kΩ,
R
Ω
= 10 Ω and C = 10 μF are chosen. These values
are reasonable for the electrode–electrolyte interface.
At low frequency (ω = 2π f ), i. e. below 1 Hz, the
current passes the ohmic resistances leading to approx-
imately Z = R
CT
+R
Ω
because the parallel capacitive
resistance 1/ωC becomes very large. The phase shift
is vanishingly small. At very high frequencies of
f =100 kHz the capacitive resistance is very low with
a short circuit across C. In this case the impedance
equals R
Ω
with ΔΦ = 0. At intermediate frequencies
of f =100 Hz, Z =1/ωC will hold approximately with
a maximum of ΔΦ. In the example of Fig. 12.31a,b one
obtains 1/ωC = 100 Ω for f =158 Hz, which refers to
the maximum of the phase shift ΔΦ.
For potentiostatic transients one has to discuss
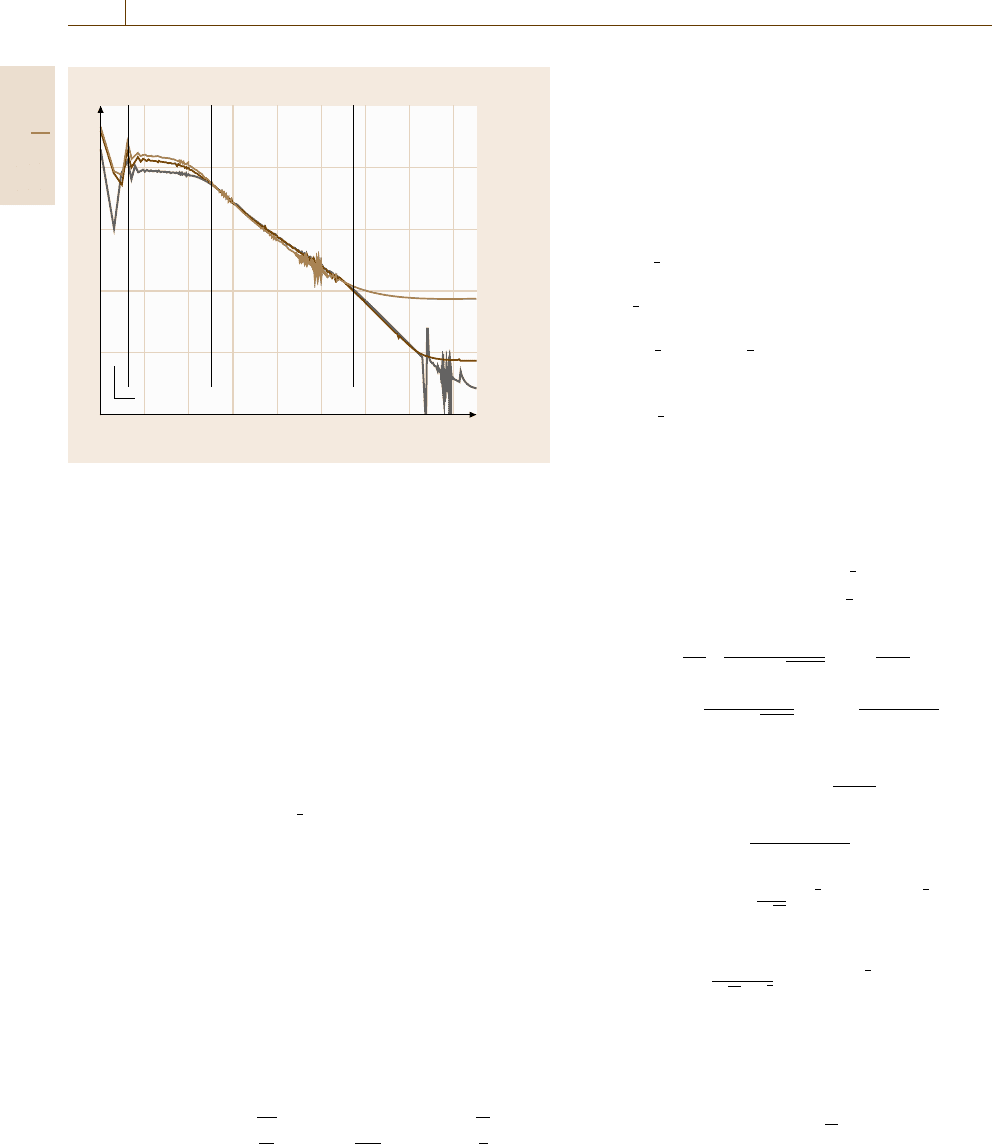
a sequence of time ranges. Figure 12.32 presents an
example for a rotating Au disc electrode in a solution
of 0.5×10
−3
M, K
3
[Fe(CN)
6
]and K
4
[Fe(CN)
6
],which
are dominated by the following processes: (1) charging
of the double layer, (2) charge-transfer control of the
electrode process (3) increasing diffusion control and
(4) full diffusion control. This charge-transfer process
of a redox system does not involve complicating chem-
ical reaction steps within the electrolyte and adsorption
and desorption at the electrode surface (Fig. 12.11).
Therefore it is still a relatively simple situation.
1. The charging of the double layer via the R
Ω
-C com-
bination in series occurs according to (12.63, 12.64).
The total applied voltage is the sum of the potential
drop at the ohmic resistance R
Ω
and the capacity C
of the double layer i. e. ΔU = ΔU
Ω
+ΔE. With
a typical capacity of 20 μF/cm
2
for a free metal
surface and a resistance of R
Ω
= 0.1 Ω cm
2
for
a well conducting electrolyte at the short distance
between the metal surface and the Haber–Luggin
capillary of the reference electrode the charging
of the double layer occurs with a time constant
of R
Ω
C =2×10
−6
s =2 μs. For potentiostatic tran-
sients the measured current density drops according
to (12.63) and the voltage across the double layer,
i. e. across the capacitance C, increases according to
(12.64). After about 8 μs the electrode potential ΔE
should be fully established. A larger R
Ω
value will
increase the time constant. This is the case for low-
conductivity electrolytes. However in most cases the
charging time for the double layer is short enough
to follow corrosion processes with a sufficient time
resolution
i =
ΔU
R
Ω
exp
−
t
R
Ω
C
, (12.63)
ΔE = ΔU
1 −exp
−
t
R
Ω
C
. (12.64)
2. After about 10 μs of double-layer charging one
may measure the electrode reaction under charge-
transfer control with the related charge-transfer
overvoltage η
d
. For these conditions the electrode
process is ruled by the Butler–Volmer equation
(12.26). If the current density is small enough
no further complications are involved. This sit-
uation refers to the plateau currents in time
range 2 of Fig. 12.32. The current density as-
sumes a constant value of 10
−2
A/cm
2
for about
0.3 ms, which corresponds to a charge of 3 μC/cm
2
and thus to 3 ×10
−11
mol/cm
2
of K
3
[Fe(CN)
6
].
For the 5 × 10
−4
M solution this amount of re-
acting ions is within a layer of 600 nm. This
distance is close to the mean diffusion path
¯
x =
√
2Dt =
√
2×5×10
−6
×3×10
−4
=
√
30 × 10
−10
=
5.5×10
−5
cm =550 nm of dissolved ions within the
electrolytes.
3. If diffusion becomes rate-determining for a suf-
ficiently large current density the change of the
electrolyte composition immediately at the elec-
Part D 12.2
694 Part D Materials Performance Testing
log i (A/cm
2
)
Charge
transfer
control
Rise time of potentiostatic circuit
Increasing
diffusion
control
Stationary
diffusion
control
–2
–3
–4
–5
–1
–6
–2–3–4–5 –1 102
12
i
Di
(η
d
)
i
Di
(η
D
)i
Di
(η
d
+ η
D
)
34
–6
log t (s)
1000 rpm
10 rpm
0rpm
ω =
Fig. 12.32 Potentiostatic transient for an Au RDE, with the dif-
ferent time domains, change of i with the frequency of rotation
ω =2π f/(min
−1
)
trode surface leads to a diffusion overvoltage η
D
.
Consequently the reaction rate becomes determined
by the diffusion overvoltage after some time when
the concentrations of the reacting species at the
electrode surface deviate from their bulk values. If
only the diffusion overvoltage is effective, Cottrell
derived the current density as a function of time,
as shown in (12.65) [12.51]. i depends exponen-
tially on the diffusion overvoltage η
d
and (12.65)
is similar to (12.53), which holds for stationary con-
ditions. For a potentiostatic transient i also depends
on time with 1/
√
t. The bulk concentration c
B
and
the diffusion constant D refer to a species that is
involved in the electrode process and which causes
diffusion overvoltage by its limited transport. It
could be a reduced or oxidized species and the cur-
rent density then refers to the anodic or cathodic
process, respectively. Equation (12.65) is not valid
for t = 0 because i goes to infinity for this condi-
tion. However, charge-transfer overvoltage cannot
be excluded, which limits the current density for
short times. This however, is not included in this
treatment.
i = nF
D
π
c
B
exp
nF
RT
η
D
−1
1
t
.
(12.65)
If both, charge transfer and diffusion are rate-de-
termining the total overvoltage equals η = η
d
+η
D
.
For these conditions the change of the current den-
sity for potentiostatic transients has been deduced
as (12.66) [12.52, 53]. i(0) is the current density for
t → 0 when the double-layer charging is finished
and the concentration of any reacting species is still
unchanged. This corresponds to (12.26)whendiffu-
sion control is still not effective. For short times, i.e.
for λ
√
t 1, the approximation of (12.67) has been
derived which may be used to determine i(0) from
i–
√
t plots of the current values of potentiostatic
transients. Thus i(0) is obtained by extrapolation
of i–
√
t plots to
√
t → 0 for each electrode poten-
tial. The i(0) values may then be compared to the
polarization curve for pure charge-transfer control.
For λ
√
t 1 the approximation of (12.68) has been
derived with a t
−0.5
dependence of i. This con-
dition corresponds to dominant diffusion control.
The double logarithmic plot of the current transient
of Fig. 12.32 shows a slope d log i/dlogt =−0.5.
This result is expected for the t
−0.5
dependence of
(12.65). A slope of −0.5 is also expected from the
approximation of (12.68)forλ
√
t 1
i =i(0) exp
λ
2
t
erfc
λ
√
t
, (12.66)
λ =
i
0
zF
1
c
B,Red
√
D
Red
exp
αzF
RT
η
+
1
c
B,Ox
√
D
Ox
exp
−
(
1 −α
)
zF
RT
η
,
(12.66a)
i(0) =i
+
+i
−
=i
0
exp
αzFη
RT
−exp
−(1 −α)zFη
RT
, (12.26)
i =i(0)
1 −
2
√
π
λ
√
t
for λ
√
t 1
,
(12.67)
i =i(0)
1
√
πλ
√
t
for λ
√
t 1
.
(12.68)
4. For times longer than 0.5 s the transients of
Fig. 12.32 are in the regime of total diffusion con-
trol with a current density which depends on the
rotation speed of the RDE. In this time range the
current densities follow the
√
ω dependence accord-
ing to the Levich equation (12.57) demonstrating the
full diffusion control of the process.
Part D 12.2

Corrosion 12.3 Novel Electrochemical Test Methods 695
12.2.15 Concluding Remarks
Corrosion within electrolytes or under the influence
of thin electrolyte films is an electrochemical process.
For an understanding of its leading mechanisms the
application of electrochemical methods is a necessary
requirement. They allow the simulation of corrosion
phenomena under well-controlled electrochemical con-
ditions with a systematic variation of the related
parameters. For a detailed understanding of the mecha-
nisms and the condition of the metal surfaces one needs
additional information, which is obtained through the
application of surface analytical methods. For a chem-
ical analysis of corroding surfaces and surface films
methods working in the ultrahigh vacuum such as x-ray
photoelectron spectroscopy (XPS) [12.54, 55] or Auger
electron spectroscopy (AES) [12.56] are valuable an-
alytical tools that provide qualitative and quantitative
information. The structure of surfaces and surface lay-
ers may be investigated with synchrotron methods such
as x-ray diffraction (XRD) and x-ray absorption spec-
troscopy (XAS), which yield the parameters of the long-
or short-range order of surfaces or surface films, respec-
tively [12.57]. A direct image of surface structures even
down to atomic resolution is obtained by the in situ ap-
plication of scanning methods such as scanning force
microscopy (SFM) and scanning tunneling microscopy
(STM). There are numerous other in situ methods such
as infrared (IR) or laser Raman spectroscopy, each of
them having its specific advantages and providing some
insight into a corroding system. All these methods to-
gether give information on the chemical composition,
structure and the properties of surfaces and surface
films. Electrochemistry remains incomplete and rather
arbitrary without the surface methods, whereas these
analytical tools without a detailed understanding of the
electrochemical reactions and a well-controlled electro-
chemical specimen preparation give no reliable results.
One therefore has to apply many of these methods in
combination in order to get reliable results without too
much speculation for the usually complicated systems
in corrosion, whether in theory or practice.
12.3 Novel Electrochemical Test Methods
During the last 30 years new electrochemical techniques
have arisen which give more and faster information
about corrosion reactions and also provide data on
corrosion in geometric orders at the nanoscale. An-
alyzing the dynamic behavior of a corrosion system
requires special techniques, which are essentially dif-
ferent from conventional direct-current (DC) techniques
(Sect. 12.1), such as measurement of the open-circuit
potential, polarization curves, weight loss, or other
physicochemical parameters. Based on linear system
theory (LST), electrochemical impedance spectroscopy
(EIS) is one of the most powerful techniques.
Electrochemical noise analysis (ENA) is a relatively
new method. Stochastic fluctuations of the electrode
potential or the cell current are often referred to as
electrochemical noise, analogous to the word noise,in-
dicating random fluctuations of incoherent acoustic or
electrical signals. Noise analysis is a well-developed
technique in many fields, and it is being applied in-
creasingly to electrochemical systems, in particular in
corrosion science and engineering.
Another important and widely used tool for corro-
sion investigations is the scanning electrode technique.
The inherent advantage of scanning reference electrodes
is the possibility to measure the initiation, distribution,
and rate of local corrosion processes in situ with spatial
resolutiondownto20μm.
12.3.1 Electrochemical Noise Analysis
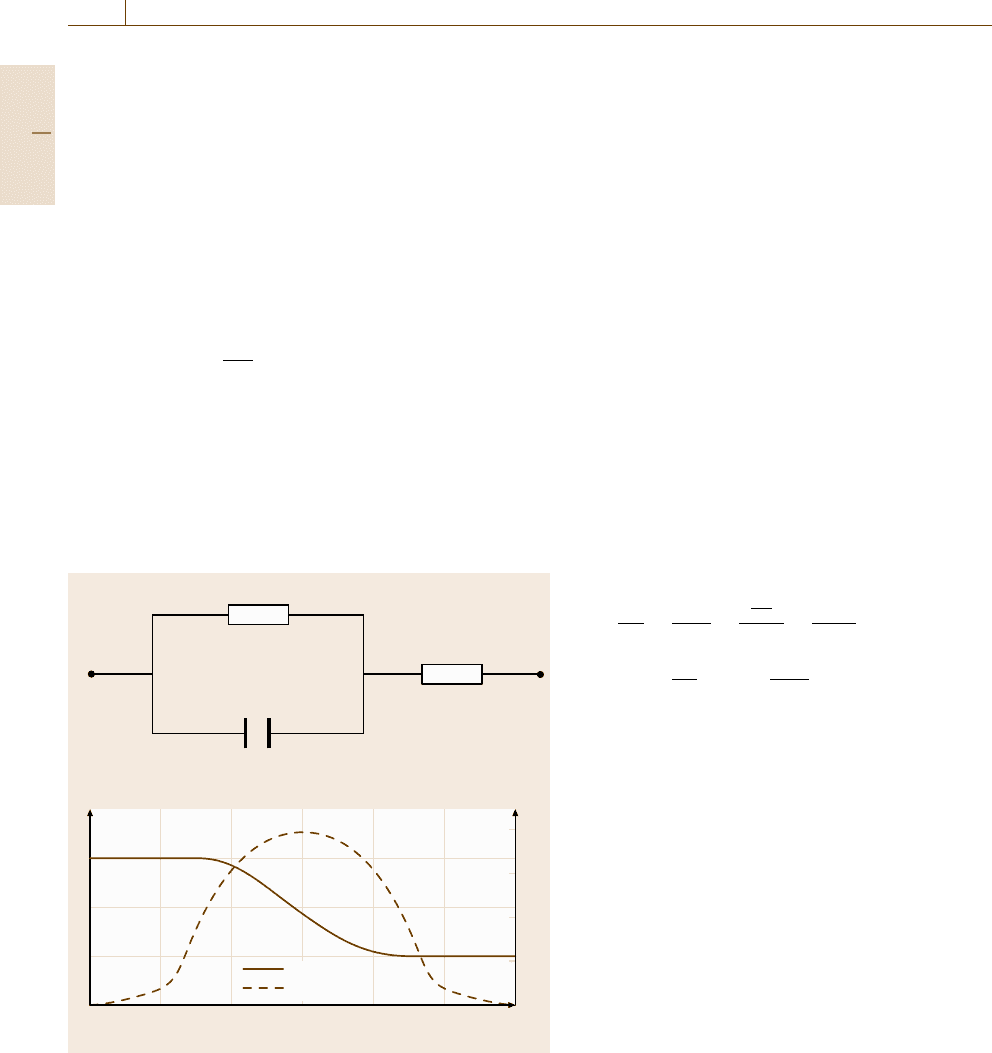
Within corrosion research, the analysis of electro-
chemical noise offers a simple, sensitive and virtually
nondestructive measuring technique for assessment of
the corrosion susceptibility of metallic materials and
for the investigation of corrosion processes. The present
status of knowledge concerning noise diagnostics in
corrosion processes permits the application of this
method not only to experimental tasks in the laboratory,
but also to special problems in the context of practical
corrosion monitoring. Furthermore, specific advantages
of the technique enable its use to an increasing extent
in supporting or improving conventional corrosion test-
ing. The advantages here include obtaining additional
information and shortening testing times, thus resulting
in state-of-the art corrosion testing.
ENA is an electrochemical method that offers great
potential for measuring and monitoring localized corro-
sion. This technique needs no external signal to obtain
corrosion data. The principle is based on the fact that
two identical electrodes exposed to the same medium
Part D 12.3
696 Part D Materials Performance Testing
(Current noise)
Current
output
(Potential
noise)
Zero resistance
ammeter (ZRA)
Reference
electrode
Identical
electrodes
Multi-
meter
E1
RE
RR
E
100 kΩ 100 kΩ
E2
E1 E2
Fig. 12.33 Experimental set up for potential and current
noise measurements
(environment) under free corrosion conditions show
stochastic fluctuations of the open-circuit potential in
the μV range and of the galvanic coupling current in the
nA range, generated by corrosion reactions on the elec-
trode surfaces [12.58–61]. These potential and current
fluctuations are known as electrochemical noise.
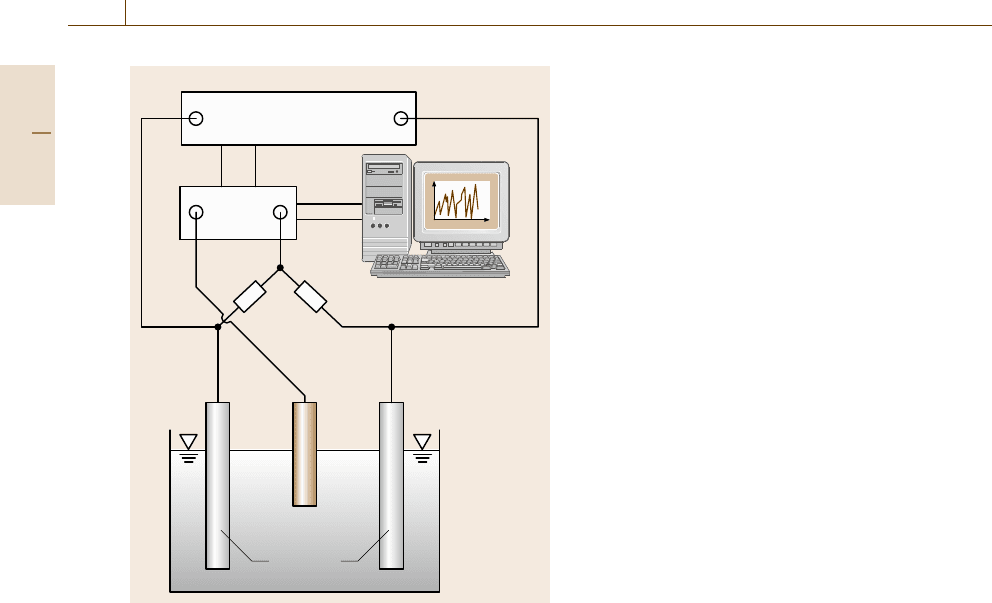
The experimental set up for measuring electro-
chemical noise is relatively simple (Fig. 12.33). The
two identical electrodes (made, e.g., from mild steel)
are connected over a zero-resistance ammeter (ZRA),
which feeds the current output (current noise) via a mul-
timeter into a computer. The two electrodes can be con-
nected over two ultraaccurate metal resistors (100 kΩ
each) in series to provide an average potential point:
since the resistance of the two resistors is much larger
than the solution resistance (generally not more than
100 Ω) no measurable disturbance is introduced into the
system by the potential and current measurements. The
potential between this average point and the reference
electrode (e.g. Saturated Calomel Electrode (SCE))
is sampled. For potential and current measurements
a 2 points/s sampling rate for 500 s (8.3min)ofmea-
suring time is sufficient. For corrosion monitoring this
procedure will be repeated in appropriate time intervals.
Figure 12.34 shows potential and current noise ver-
sus time plots of mild steel in two cooling waters with
different salt concentration [12.61]. Cooling water 2
contained higher chloride and sulfate levels, which ob-
viously resulted in a significantly different fluctuation
pattern of the electrochemical noise at mild steel in
these two waters. The electrochemical noise signals can
be processed in a digital or analogue manner. The digital
signal-processing technique involves the acquisition of
raw data and their subsequent numerical analysis, while
the analog processing method produces an output sig-
nal proportional to the root mean square (RMS)ofthe
noise using electronic filters and amplifiers. From these
data the noise resistance R, can be defined as
R
n
=δV/δI , (12.69)
where δV is the RMS of the corrosion potential fluctua-
tion and δI is the RMS of the current fluctuation [12.62,
63].
Data processing can also include transformation in
the frequency-domain (spectral density) curves using
the maximum-entropy method (MEM) or fast Fourier
transformation (FFT). The MEM inherently produces
smoother spectra without apparent loss of informa-
tion [12.64] and is simpler to use when the number
of data points is not a power of two. Analysis in the
frequency domain needs simultaneous collection of po-
tential and current noise data.
With FFT the spectral noise response R
S
n( f ) can be
calculated at each frequency f by
R( f ) = V ( f )/l( f )and (12.70)
R
sn
=
|
R( f )
|
=
R( f )
2
re
+R( f )
im
1/2
, (12.71)
where V( f )andI( f ) are complex numbers obtained
from the FFT. The spectral noise resistance R
sn
is de-
fined as the value of
R
sn
( f )at f =0 , (12.72)
R
0
sn
=lim f →0
R
sn
( f )
. (12.73)
An analysis in the frequency domain can yield informa-
tion on the type of corrosion at the electrodes.
The electrochemical current-noise power-spectral
density [PSD(I)] at high frequencies (100–1000 MHz)
can be indicative for general corrosion while the shape
and the repetition rate of transients of the electrochem-
ical potential and current determine the type of cor-
rosion [12.61]. The slope of the power-spectrum den-
Part D 12.3
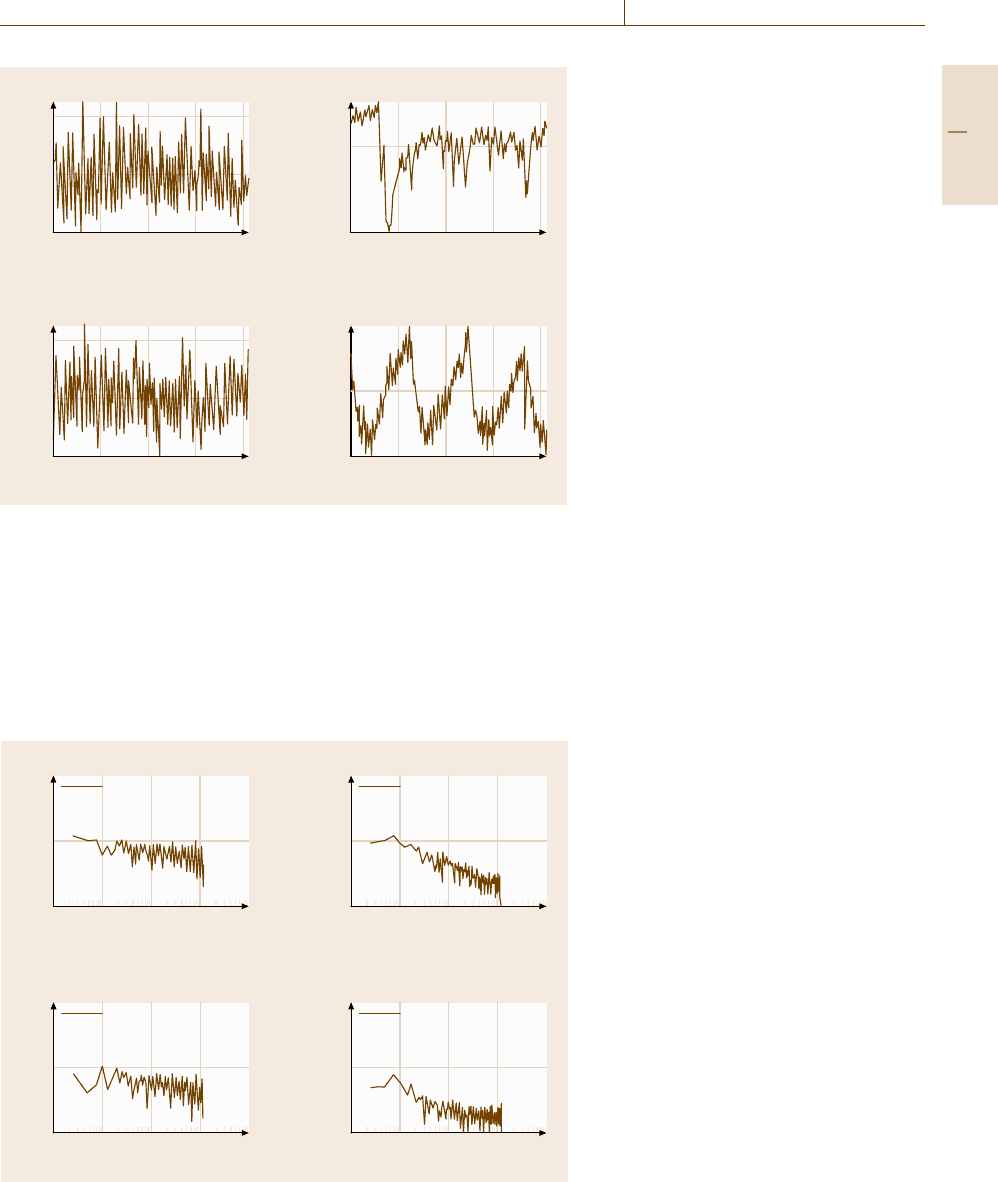
Corrosion 12.3 Novel Electrochemical Test Methods 697
Voltage (mV)
1.22
–0.96
1.14
Time (h)
0 0.57
Current (nA)
660
–476
1.14
Time (h)
0 0.57
Cooling water 1
Voltage (mV)
0.236
–0.447
1.14
Time (h)
0.570
Current (nA)
60
–43.5
1.14
Time (h)
0 0.57
Cooling water 2
Fig. 12.34 Potential and current noise
at mild steel in different cooling
waters (after [12.61])
sity of the electrochemical voltage-noise relationship
[PSD(U)] after the removal of the linear trend was rec-
ognized as a significant parameter for distinguishing be-
tween uniform and local corrosion. The electrochemical
noise generated by uniform corrosion is, white noise,
and therefore the slope is close to zero. Electrochem-
ical noise generated by localized corrosion consists of
PSD(U) [dB(V
2
/Hz)]
0
–120
–60
1000
Frequency (mHz)
0.1
PSD(U) [dB(V
2
/Hz)]
0
–120
–60
1000
Frequency (mHz)
0.1
PSD(I) [dB(A
2
/Hz)]
–60
–240
–120
1000
Frequency (mHz)
0.1
PSD(I) [dB(A
2
/Hz)]
–60
–240
–120
1000
Frequency (mHz)
0.1
Cooling water 1 Cooling water 2
PSD(U) = –73.8dB(V
2
/Hz)
Slope = 6.3dB(V
2
/Hz) decade
–1
PSD(I) = –140.1dB(A
2
/Hz)
Slope = 8.2dB(A
2
/Hz) decade
–1
PSD(U) = –94.8 dB(V
2
/Hz)
Slope = 15.3dB(V
2
/Hz) decade
–1
PSD(I) = –164.9dB(A
2
/Hz)
Slope = 3.1dB(A
2
/Hz) decade
–1
Fig. 12.35 Power-spectral density
(PSD) for potential and current noise
at mild steel in different cooling
waters (after [12.61])
exponentially decreasing transients and the slope of the
PSD(U) curve is higher (> 12 dB (V
2
Hz
−1
)) decade
−1
.
Figure 12.35 shows PSD(U) graphs that, for the
uniformly corroding steel in cooling water 1, the
slope is 6.3dB(V
2
Hz
−1
) decade
−1
, while the slope is
15.3dB(V
2
Hz
−1
) decade at the locally corroding steel
in cooling water 2.
Part D 12.3
