Czichos H., Saito T., Smith L.E. (Eds.) Handbook of Metrology and Testing
Подождите немного. Документ загружается.


668 Part D Materials Performance Testing
12.7 Hydrogen-Induced Stress Corrosion
Cracking .............................................. 714
12.7.1 Electrochemical Processes ............ 715
12.7.2 Theories of H-Induced Stress
Corrosion Cracking....................... 716
12.7.3 Environment
and Material Parameters.............. 717
12.7.4 Fractographic and Mechanical
Effects of HISCC............................ 717
12.7.5 Test Methods .............................. 718
12.8 High-Temperature Corrosion ................. 718
12.8.1 Main Parameters
in High-Temperature Corrosion..... 718
12.8.2 Test Standards or Guidelines......... 719
12.8.3 Mass Change Measurements ......... 721
12.8.4 Special High-Temperature
Corrosion Tests............................ 729
12.8.5 Post-Test Evaluation of Test Pieces 731
12.8.6 Concluding Remarks .................... 732
12.9 Inhibitor Testing and Monitoring
of Efficiency ......................................... 732
12.9.1 Investigation and Testing
of Inhibitors ............................... 733
12.9.2 Monitoring of Inhibitor Efficiency.. 734
12.9.3 Monitoring Inhibition
from Corrosion Rates ................... 736
References .................................................. 738
12.1 Background
According to ISO 8044 [12.2] corrosion is defined as an
interaction between a metal and its environment that re-
sults in changes in the properties of the metal, and which
may lead to significant impairment of the function of
the metal, the environment, or the technical system,
of which these form a part. This definition has solved
a conflict because previously the term corrosion had
been used to mean the process, results of the process
and damage caused by the process. In most cases the
interaction between the metal and the environment is
an electrochemical reaction where thermodynamic and
kinetic considerations apply. From a thermodynamic
point of view the driving force as in any electrochem-
ical reaction is a potential difference between anodes
and cathodes in a short-circuited cell.
The result of corrosion is a corrosion effect which
is generally detrimental and may lead to loss of ma-
terial, contamination of the environment with corrosion
products or impairment of a technical system. It is im-
portant to notice that corrosion damage resulting from
an attack to the metal has to be distinguished from cor-
rosion failure which is characterized by the total loss of
function of the technical system. Corrosion protection
measures are available to influence corrosion process
with the objective of avoiding corrosion failures.
Corrosion and protective measures against corro-
sion result in costs. Many attempts have been made
to estimate the financial expenditure to the commu-
nity caused by corrosion. These include the costs which
can arise in the form of corrosion protection mea-
sures, through replacement of corrosion-damaged parts
or through different effects deriving from corrosion,
such as shut-down of production or accidents which
lead to injuries or damage to property. Several estima-
tions have arrived at the conclusion that the total annual
corrosion costs in the industrialized counties amount to
about 4% of the gross national product. Part of these
costs is unavoidable since it would not be economi-
cally viable to carry out the necessary precautions to
eliminate completely corrosion damage. It is, however,
certain that one can reduce losses considerably solely
by better exploiting the knowledge we have today and,
according to one estimate, about 15% of corrosion costs
are of this type [12.3].
Due to the technical, scientific and economic impor-
tance of the problem numerous textbooks [12.1, 4–18]
and publications in journals or proceedings from confer-
ences are available. As mentioned before the corrosion
reaction is in most cases of electrochemical nature.
Therefore many of the published information deal with
the electrochemical reactions and especially the kinet-
ics of the corrosion process. For the engineer durability
aspects in a technical plant or equipment or the safety
of a process play a more important role. However, both
approaches follow the same route in yielding informa-
tion about the corrosion rate of a material in a specific
environment [12.4].
The schematic diagram in Fig. 12.1 [12.4]showsthe
various reactions that can occur sequentially and simul-
taneously in the corrosion process. Material transport
and chemical reactions can supply or remove impor-
tant reaction components. In addition to adsorption or
desorption, a phase-boundary reaction occurs which
mostly involves electrochemical reactions and which
can be affected by external currents. An example is the
formation of hydride on lead where the partner to the
Part D 12.1
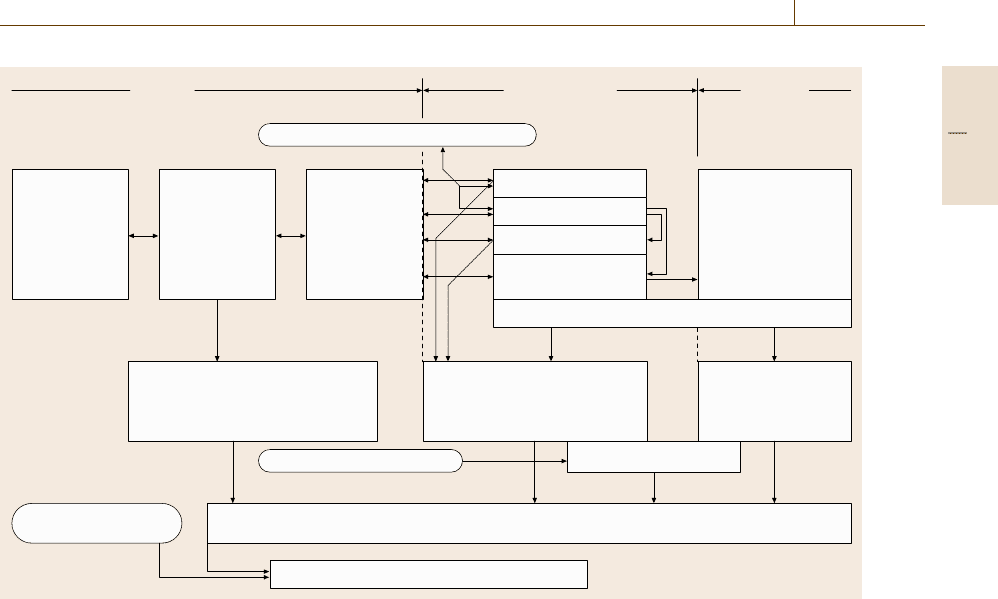
Corrosion 12.1 Background 669
Medium Phase boundary Material
Transport
(diffusion,
convection,
migration)
Pre- and
post-reactions
General degradation
(demaging resultant reactions,
contaminants, etc.)
Weight loss
uniform
localized
Microstructural
changes,
internal cracks
Adsorption
and
desorption
Anodic reaction
Cathodic reaction
Chemical reaction
Absorption
Liquid metal reactions
Metal-physical
and
chemical
processes
in the
material
External and galvanic currents
Mechanical stresses
Cracking
Corrosion effects
Corrosion damage
Requirements for
the system
Fig. 12.1 Flow diagram of corrosion processes and types of damage (after [12.4])
hydrogen reaction can arise from the cathodic process.
Hydrogen can also penetrate the metal microstructure,
where it can have a physical or chemical effect, causing
a degradation of the mechanical properties of the ma-
terial. In this type of corrosion cracks can form during
mechanical testing, leading to fracture without the loss
of any metal.
12.1.1 Classification of Corrosion
Numerous concepts have been applied to classify cor-
rosion into categories. Due to the complex interaction
between a material and its environment different clas-
sification schemes have developed to classify corrosion
by the type of attack, the rate of attack, the morphol-
ogy of the attack, or the properties of the environment,
which may change in the ongoing corrosion process.
Type of damage have been predominantly used.
This can be classified as uniform or localized metal re-
moval, corrosion cracking or detrimental effects to the
environment. Local attack can take the form of shallow
pits, pitting, selective dissolution of small microstruc-
ture regions or cracking. It is usual, where different
results of reactions lead to definite forms of corrosion
effects, to classify them as particular types of corrosion.
The most important are uniform corrosion, pitting cor-
rosion, crevice corrosion, intergranular corrosion, and
those with accompanying mechanical-stress corrosion
and corrosion fatigue. The two latter are most feared.
Most failures arise from pitting corrosion. Uniform cor-
rosion almost always occurs in practice but seldom
leads to failure.
From [12.19] it can be taken that there are generally
eight forms of corrosion.
1. General (uniform) corrosion;
2. Localized corrosion;
3. Galvanic corrosion;
4. Cracking phenomena;
5. Intergranular corrosion;
6. Dealloying;
7. High-temperature corrosion.
The eight forms of corrosion can be divided into three
categories.
Group I Those readily identified by visual examina-
tion (forms 1, 2 and 3);
Part D 12.1
670 Part D Materials Performance Testing
Group II Those which may require supplementary
means of examination (forms 5, 6 and 7);
Group III Those which usually should be verified by
microscopy, optical or scanning electron
microscope, although they are sometimes ap-
parent to the naked eye.
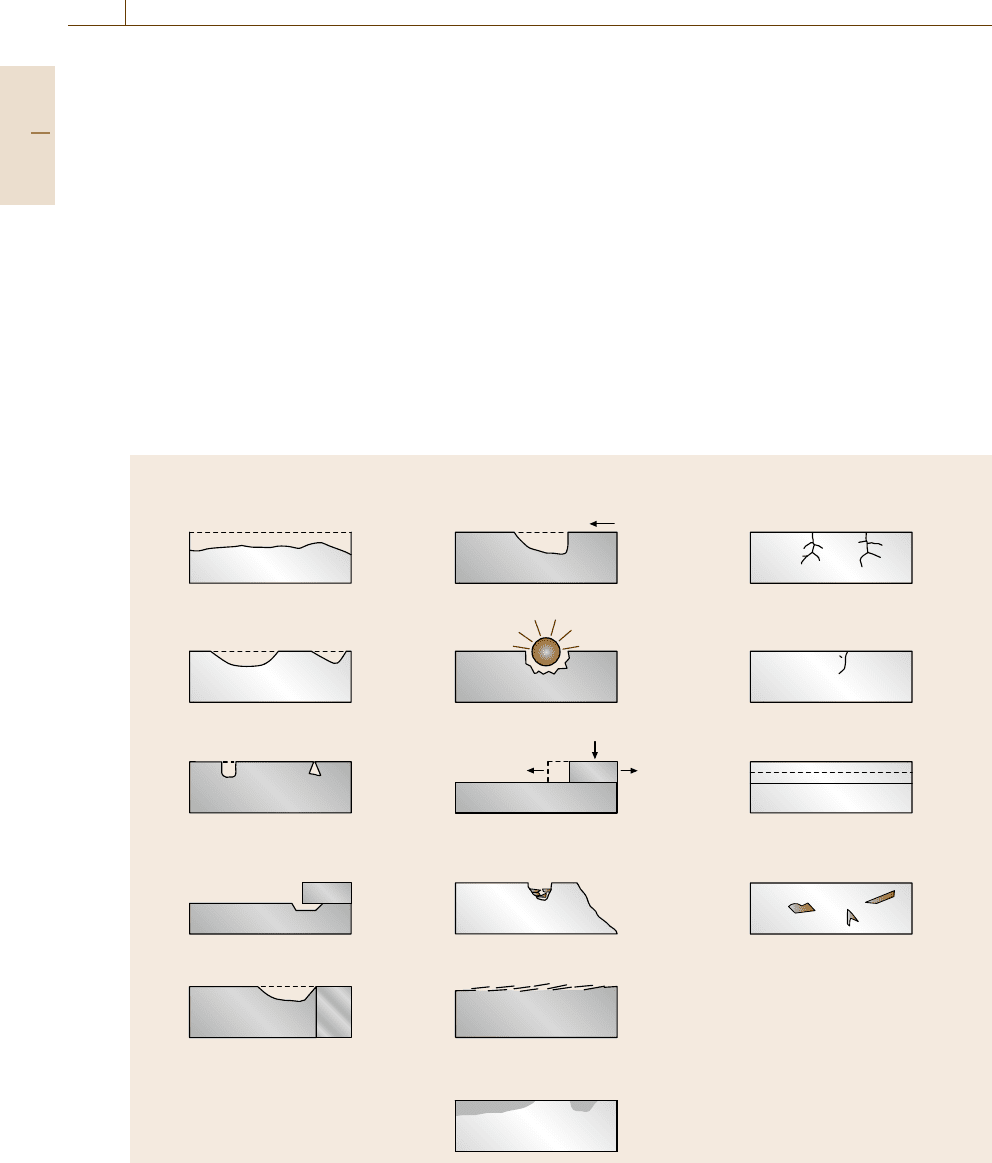
The individual phenomena are illustrated in Fig. 12.2.
12.1.2 Corrosion Testing
The safe use of materials in technical plants, structures
or equipment requires sufficient information for assess-
ing corrosion risks – especially if mechanical stresses
are superimposed on the electrolytic load and failure
of components due to stress corrosion cracking (SCC)
Group I
General attack
Original surface
Velocity phenomena
Intergranular attack
Erosion
Cracking phenomena
Static
stress
Dynamic
stress
Scale
Fissure
High-temperature attack
Stress corrosion cracking
Corrosion fatigue
Scaling
Internal attack
Dealloying attack
Layer Plug
Cavitation
Fretting
Load
Vibration
Weld
Weld decay
Exfoliation
HAZ
Bubbles
Flow
Localized attack
Galvanic attack
Base
metal
Noble
metal
Localized corrosion
Pitting
Crevice corrosion
Group II Group III
Fig. 12.2 Types of corrosion
cannot be excluded. If sufficient knowledge about the
corrosion behavior is not available then tests to deter-
mine this under practical conditions will be required.
In this context a distinction must be made between
the terms corrosion investigation and corrosion test.
According to the International Standards Organization
(ISO) standard ISO 8044 [12.2] a corrosion investiga-
tion includes corrosion tests and their evaluation and is
directed towards the following objectives.
•
The explanation of corrosion reactions,
•
Obtaining knowledge on corrosion behavior of ma-
terials under corrosion load,
•
Selecting measures for corrosion protection.
This enables statements about the properties of a corro-
sion system to be made. A corrosion test is a special
Part D 12.1
Corrosion 12.2 Conventional Electrochemical Test Methods 671
case of a corrosion investigation; the corrosion load
and the assessment of the results being prescribed by
certain regulations (standards, test sheet) and/or agree-
ments such as, for example, delivery conditions.
Corrosion tests mainly help in quality control dur-
ing production and further treatment of materials as well
as for the assessment of corrosion protection measures.
The significance of the corrosion tests selected for an
investigation differs. While corrosion tests under loads
that are characteristic of service conditions give the best
estimation of the real corrosion behavior, tests with in-
creased corrosion load or accelerated corrosion tests are
less suitable to give a reliable prognosis of behavior in
practice as the corrosion mechanism could differ from
that under practical conditions. Nevertheless, acceler-
ated corrosion tests are more suitable for determining
certain material properties (e.g. resistance to intergranu-
lar corrosion or stress corrosion cracking) although they
can be reliably used only if sufficient experience exists
for the transfer of the test results to practical situations.
12.2 Conventional Electrochemical Test Methods
Corrosion is the degradation of materials by gaseous
or liquid environments. Atmospheric corrosion involves
the influence of both media on the surface of a solid, of-
ten with alternating wet and dry periods. Any kind of
materials may be attacked, including metals, semicon-
ductors and insulators as well as organic polymers. This
chapter describes corrosion of metals in electrolytes and
therefore some fundamentals of electrochemistry and
related test methods are discussed.
Large losses are caused by corrosion in modern
industrial societies. One estimate is that 4.0% of the
gross national product is lost by corrosion, a third of
which may be avoided if all knowledge is taken into
account. Traditionally corrosion is subdivided into at-
tack by dry and hot gases and by electrolytes. In this
section corrosion in aqueous electrolytes only is de-
scribed, although solutions with organic solvents may
play an important role in praxis. Corrosion is a reaction
at the metal/electrolyte phase boundary. Although the
bulk properties of the solid and the solution may play an
important role for the degradation of the materials, the
properties of the metal surface and the reactions at the
solid/electrolyte interface are most important. Therefore
corrosion science and corrosion engineering apply the
concepts of electrochemistry and electrode kinetics. In
Sect. 12.2.1 a basic introduction to the equipment and
experimental procedures is given. Corrosion involves
electrode processes which are ruled by the electrode
potential and thermodynamic and kinetic factors. For
this reason the equilibria and the related thermody-
namic properties of the metal/electrolyte interface and
their electrode kinetics are discussed. In Sect. 12.2.3
a short introduction to electrochemical thermodynam-
ics and equilibrium is given followed by an introduction
to electrode kinetics in Sect. 12.2.5. Finally this chap-
ter is completed by a description of the application of
electrochemical transient measurements and some elec-
troanalytical methods.
12.2.1 Principles of Electrochemical
Measurements and Definitions
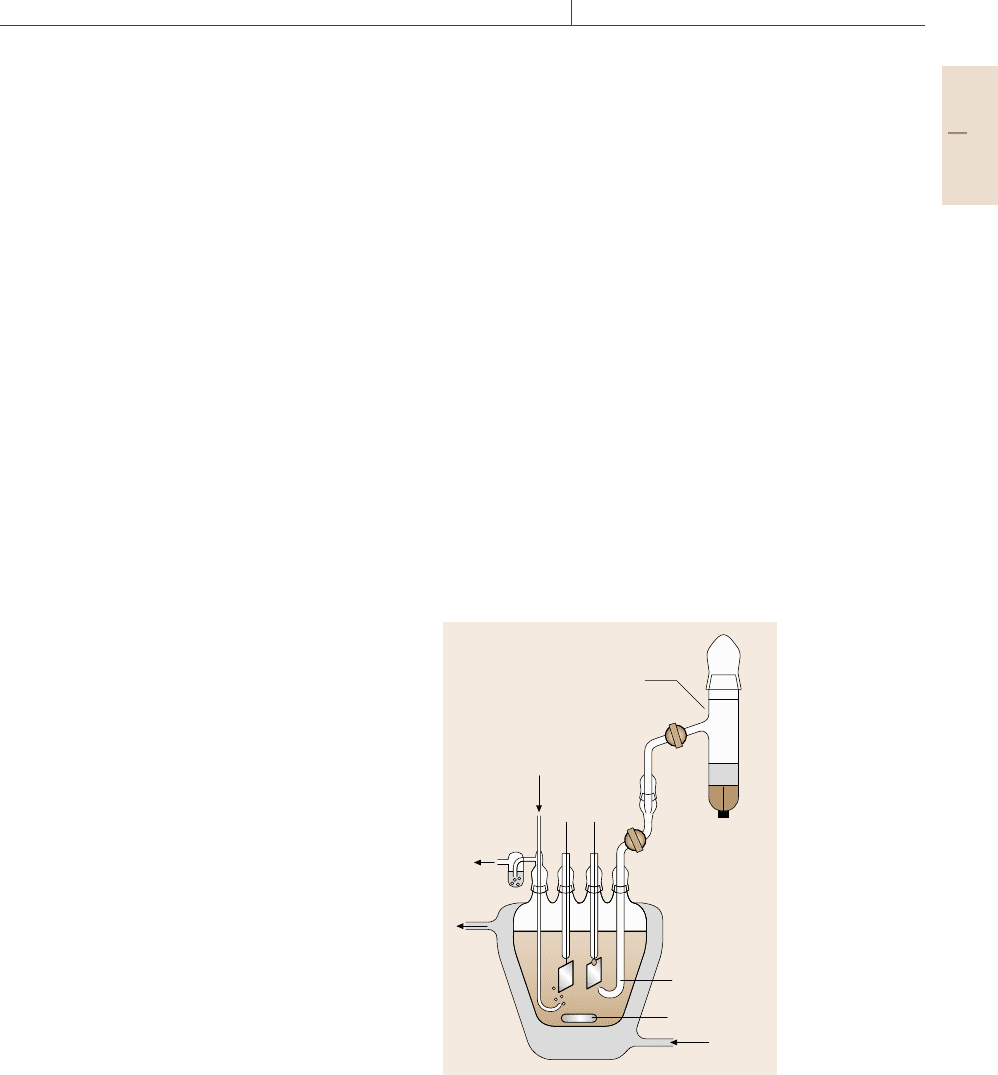
Electrochemical measurements are performed in an
electrochemical cell, which contains a three-electrode
arrangement in its most simple form Fig. 12.3. The
N
2
N
2
TW
out
TW
in
MS
HL
Hg
Salt
RE
CE WE
Fig. 12.3 Standard electrochemical cell with working elec-
trode (WE), counter-electrode (CE), reference electrode
(RE), Haber–Luggin capillary (HL), magnetic stirring
(MS), water jacket with thermostat water (TW) and nitro-
gen inlet
Part D 12.2
672 Part D Materials Performance Testing
Table 12.1 Some commonly used reference electrodes and their related standard potential E
0
from [12.20]
Electrode E
0
/V Nernst equation
Calomelelectrode:Hg/Hg
2
Cl
2
/Cl
−
0.268 E = E
0
– 0.059 log [Cl
−
]
Hg
2
SO
4
electrode: Hg/Hg
2
SO
4
/SO
2−
4
0.615 E = E
0
– 0.059 log [SO
2−
4
]
HgO electrode: Hg/HgO/OH
−
0.926 E = E
0
– 0.059 log [OH
−
]
AgCl electrode: Ag/AgCl/Cl
−
0.222 E = E
0
– 0.059 log [Cl
−
]
PbSO
4
electrode: Pb/PbSO
4
/SO
2−
4
−0.276 E = E
0
– 0.029 log [SO
2−
4
]
working electrode (WE) of the material under study,
the counter electrode (CE) of a metal which is not at-
tacked by the electrolyte, in most cases a platinum or
gold electrode, and finally the reference electrode (RE)
with an electrolytic contact to the bulk electrolyte via
the Haber–Luggin (HL) capillary. Usually such an elec-
trochemical cell contains an inlet for protective gases
such as nitrogen or argon, a stirrer for the electrolyte
and a water jacket to keep the electrolyte at the de-
sired temperature using a thermostat. Numerous special
designs for electrochemical cells have been applied de-
pending on the special problem under study. In some
cases small electrolyte vessels are used as e.g. for mi-
croscopic in situ investigations or in situ measurements
with a scanning tunneling microscope (STM). Another
approach is cells that restrict the investigated surface
area to the size of droplets in the μm range. In all these
cases a three-electrode set up is used with the excep-
tion of in situ STM investigations. Here two working
electrodes are required, the material under study and the
STM tip. The potentials of both electrodes have to be set
independently and therefore a bipotentiostat is required.
A similar situation with two or even more working
electrodes exists for a rotating ring-disc electrode. This
analytical method will be described in Sect. 12.2.11.
The reference electrode is usually an electrode of the
second kind. Its potential is fixed by a weakly soluble
compound of the metal and an anion of the solution.
Table 12.1 gives some examples of mercury and silver
RE
RE
OA
CE
WE
V
ΔU
RI
C
R
Fig. 12.4 Block diagram of potentiostat with operational amplifier
(OA), potential setting ΔU, resistance R for current measurement
I
C
as RI
C
and connections to working electrode (WE), counter-
electrode (CE) and reference electrode (RE)
electrodes [12.20]. These reference electrodes are easy
to prepare and to handle and their electrode potential is
very stable.
For most experiments the electrode potential is
fixed by an electronic potentiostat and the current is
measured. Potentiodynamic polarization curves involve
a linear change of the electrode potential with time. Po-
tentiostatic measurements keep the potential constant.
Potentiostatic transients require its rapid change. With
a fast potentiostat and some precautions a potential
change can be achieved in a few microseconds so that
fast electrode processes can be followed with a high
time resolution starting in the μs range. In most cases
of corrosion studies a ms resolution of the current mea-
surement is sufficiently fast.
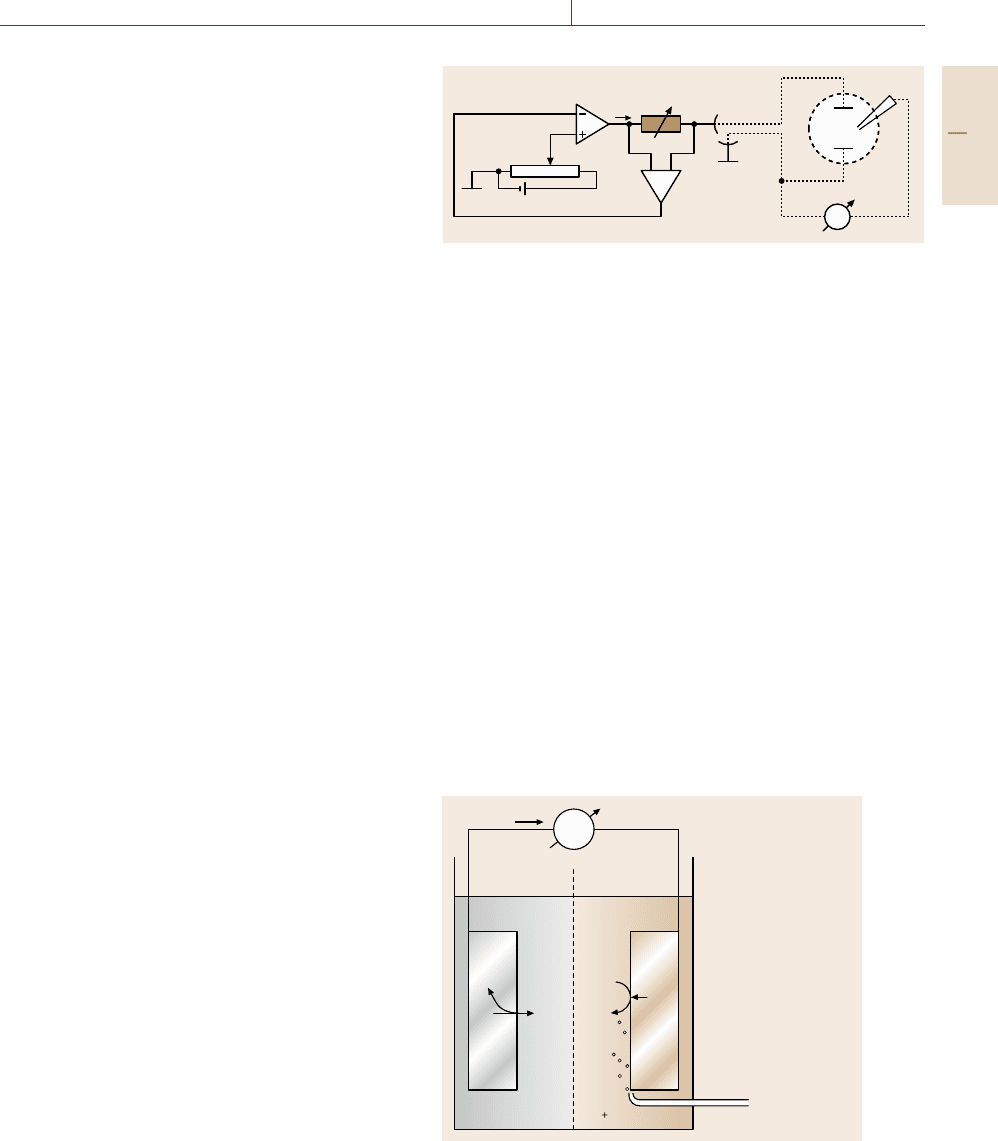
Figure 12.4 explains with a simple block diagram
the characteristics of a potentiostat built with an op-
erational amplifier (OA). The reference electrode, RE,
is given directly to the inverting input of OA and the
desired electrode potential is determined by the set-
ting of the voltage ΔU from a power source fed into
the noninverting input. The working electrode WE is
set to common. The high gain of the operational am-
plifier (factor 10
5
) ensures a negligibly small deviation
of both inputs. Thus RE has a potential difference ΔE
to WE equal to ΔU. The input resistance is very high
(10
7
Ω) so that almost no current flow occurs into the
OA. Its output resistance is very low so that it may sup-
ply a sufficiently high current I
C
to WE via CE and the
electrolyte which may be required for the rate of the
electrode processes related to the chosen potential. I
C
is
measured via its ohmic drop RI
C
at the resistor R. This
simple electronic circuit stabilizes the chosen electrode
potential automatically, independent of the required cur-
rent density I
C
. However, a good potentiostat contains
additional booster units to provide high currents and to
improve the setting of potential changes with a fast re-
sponse. Usually potentiostats contain additional circuits
built with operational amplifiers for necessary options
such as, e.g., an adder which permits the addition of var-
ious potentials forming a potential program with ramps
and pulses for potentiostatic transients. A special circuit
Part D 12.2
Corrosion 12.2 Conventional Electrochemical Test Methods 673
compensates for ohmic drops within the electrolyte be-
tween WE and HL which may occur in the case of high-
current densities and electrolytes with low conductivity.
Galvanostatic measurements require the setting of
a current I
C
while the change of the electrode potential
ΔE is followed with time. This is achieved by a gal-
vanostat (constant-current source) depicted as a simple
block diagram in Fig. 12.5. I
C
is set via a proportional
voltage ΔU from a built-in power source. ΔU is given
to the noninverting input of the operational amplifier
OA, which is compared to the ohmic drop RI
C
across
the resistor R at the output. This voltage drop passes
a differential amplifier (DA), which avoids the setting of
a second point of the circuit to common. As both inputs
of OA have to be at the same potential as a consequence
of its high magnification, I
C
is adjusted to the desired
value RI
C
= ΔU. Usually galvanostats are built in to
potentiostats. Appropriate voltages ΔU may be given
to the input with specific time characteristics to permit
also galvanodynamic measurements including galvano-
static transients controlled by appropriate voltage pulses
given to OA.
12.2.2 Some Definitions
An electrochemical cell usually consists of two elec-
trodes. Figure 12.6 givesasanexampleaniron
electrode in a Fe
2+
solution in contact with a hydro-
gen electrode via a diaphragm. The H
+
/H
2
electrode
consists of a platinum electrode in a solution of a given
pH with the introduction of pure hydrogen. If the solu-
tion is 1 M for the H
+
concentration, i. e. if pH is 0, and
the gas pressure of H
2
is 1 atm =1.013 bar, this is the
standard hydrogen electrode (SHE). Potential drops at
the electrode–electrolyte interface cannot be measured
separately in principle. The contact of a voltmeter to
the electrolyte introduces automatically a second inter-
face so that the measured voltage necessarily includes
the potential drop of two electrodes. The potentials of
any electrode is therefore given relative to the SHE.
The electrode potential of the standard hydrogen elec-
trode is thus set to 0 V by definition. Its value relative
to the zero point of the potential scale in physics, i. e.
the charge-free vacuum level, has been determined to
−4.6 V [12.21,22], so that both scales may be converted
to each other.
There are two main kinds of electrodes, metal/metal-
ion electrodes and redox electrodes. For the first case
the electrode reaction requires the transfer of metal ions
across the electrode–electrolyte interface. The Fe/Fe
2+
electrode is an example. Redox electrodes involve the
RE
OA
DA
V
CE
WE
ΔU
ΔU =RI
C
ΔE
I
C
Fig. 12.5 Block diagram for galvanostat with operational amplifier
(OA), differential amplifier (DA), voltage supply ΔU for current
setting I
C
and connections to working electrode (WE), counter-
electrode (CE) and reference electrode (RE)
transfer of electrons, which is the case for the hydro-
gen electrode. A Pt electrode within a solution of Fe
2+
and Fe
3+
ions or Fe(CN)
4−
6
and Fe(CN)
3−
6
are other
examples. If no reaction occurs, the electrode is in
electrochemical equilibrium, i. e. both reactions – Fe
2+
dissolution or Fe deposition – compensate each other.
The same is the case for the redox reaction.
One has to distinguish anodic and cathodic pro-
cesses. An anodic process involves the transfer of
positive charge from the electrode to the electrolyte,
such as Fe dissolution to Fe
2+
, or the transfer of
negative charge in the opposite direction, e.g. the ox-
idation of Fe
2+
to Fe
3+
. A cathodic process involves
the transfer of positive charge from the electrolyte to
the electrode, such as the deposition of Fe
2+
from the
solution as Fe metal or the transfer of negative charge
in the opposite direction, e.g. the reduction of Fe
3+
to
Fe
2+
. The currents of anodic processes get a positive
2e
–
2e
–
2e
–
Fe
2+
2H
+
H
2
p
H
2
= 1.013 bar
[H
+
]=1M
Fe
Fe Pt
V
Fig. 12.6 Electrochemical cell with an Fe/Fe
2+
and
H
2
/H
+
electrode
Part D 12.2
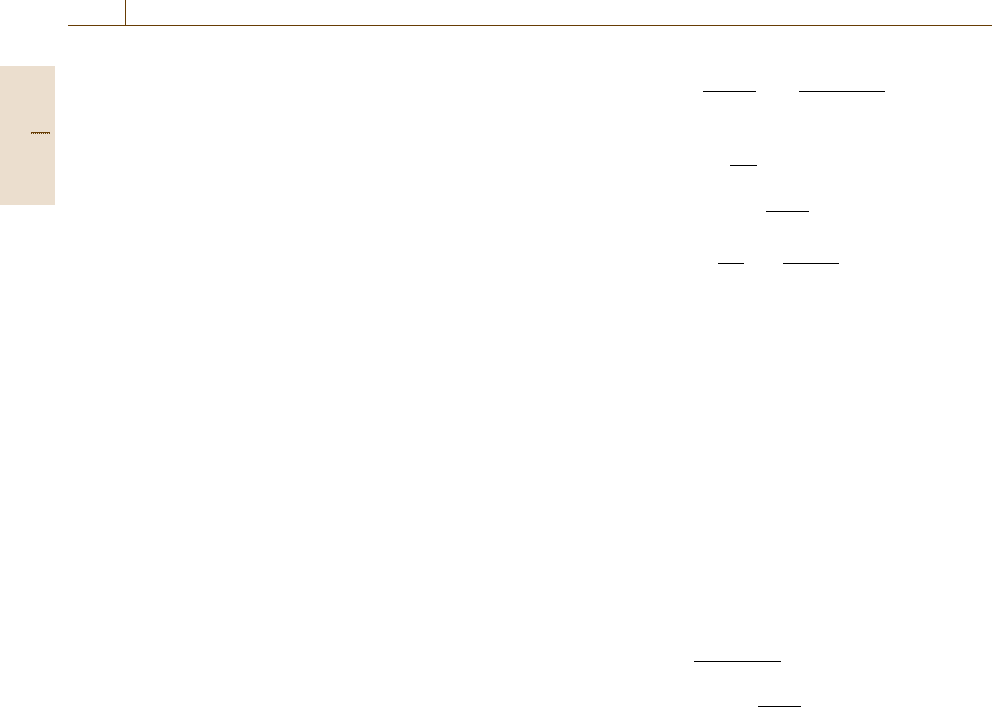
674 Part D Materials Performance Testing
sign, those for cathodic reactions a negative sign. In
conclusion, it is the kind of process which determines
whether an electrode acts as an anode or a cathode. De-
pending on the applied electrode potential and further
electrochemical conditions an electrode may be either
an anode or a cathode.
If anodic and cathodic currents of a redox or
a metal/metal-ion electrode compensate each other the
electrochemical equilibrium is established at the equi-
librium potential E
0
. A positive deviation η = E −E
0
leads to an anodic current. η is called the overvoltage.
A deviation in the negative direction with a negative
overvoltage η = E −E
0
< 0 causes a cathodic reaction.
If two different electrode processes compensate each
other, such as an anodic Fe dissolution and cathodic
hydrogen evolution with vanishing current in the ex-
ternal circuit, the electrode is at its rest potential E
R
.
A positive deviation π = E −E
R
> 0 is called a positive
polarization and π = E −E
R
< 0 a negative polariza-
tion.
12.2.3 Electrochemical Thermodynamics
The equilibrium potential E
0
is related to the change of
the Gibbs free energy for standard conditions ΔG
0
of
the related electrode process, i. e. for activities of so-
lutes of a = 1 M and gas pressures of p = 1.013 bar.
As all electrode potentials are referred to the stan-
dard hydrogen electrode its reaction has been chosen
as a compensating process for thermodynamic calcula-
tions. Equations (12.1, 12.2) describe the process of an
electrochemical cell with the Fe/Fe
2+
and the H
2
/H
+
electrode; (12.3) describes their combination. ΔG
0
for
the electrode process of interest has to be calculated for
the cathodic direction and the compensating process of
the reference electrode in the anodic direction to avoid
mistakes in the sign (12.4). The relation between ΔG
0
and the electrode potential is given by (12.5). The con-
centration dependence of the equilibrium potential E
0
is
given by the Nernst equation (12.6, 12.7) for both elec-
trodes. Equation (12.7) describes the pH dependence of
the hydrogen electrode
2e
−
+Fe
2+
→Fe , (12.1)
H
2
→2H
+
+2e
−
, (12.2)
Fe
2+
+2H
+
→Fe +2H
+
, (12.3)
ΔG
0
298
=
i
ν
i
G
0
i,i
=−G
0
Fe
2+
=78.9kJmol
−1
,
(12.4)
ΔG
0
298
=−nFE
0
, (12.5)
E
0
=−
ΔG
0
298
2F
=−
78.94 × 10
3
2F
=−0.409 V , (12.6)
E
0
= E
0
+
RT
nF
ln a(Fe
2+
)
=−0.409+
0.059
2
lg a(Fe
2+
)/V , (12.7)
E
0
=0+
RT
2F
ln
a(H
+
)
2
p(H
2
)
=−0.059 pH/V , (12.8)
with pH =−lg
a(H
+
)
. (12.9)
Electrode reactions that involve solid compounds are
treated in a similar way. As an example the equilibrium
of Fe with Fe
3
O
4
isgivenin(12.10–12.13). This equi-
librium describes the electrochemical formation of an
oxide layer at the metal surface. These equilibriums are
important for the calculation of potential–pH diagrams,
so called Pourbaix diagrams, which will be discussed in
the next section.
Fe
3
O
4
+8H
+
+8e
−
→3Fe+4H
2
O (12.10)
ΔG
0
298
=1013.23−4(237.13) =64.71 kJ/mol
(12.11)
E
0
=−
64.71 × 10
3
8F
=−0.084 V (12.12)
E
0
=−0.084+
0.059
8
lg
a(H
+
)
8
=−0.084−0.059 pH/V (12.13)
Thermodynamic data and especially standard Gibbs
free energies ΔG
0
f
for the formation of compounds
from the elements are listed in special publications for
T = 298 K, but also for other temperatures [12.23, 24].
They permit the calculate of data for electrochemical
equilibriums as shown for (12.1–12.13). For most equi-
libriums the standard electrode potentials E
0
are listed.
Their dependence on the concentrations of the reacting
species, i. e. their Nernst equations, are used to calculate
potential–pH diagrams [12.25]. Figure 12.7 shows the
diagram for iron, which is a very important example in
corrosion. The horizontal line (1) at E =−0.589 V de-
scribes the pH-independent Fe/Fe
2+
equilibrium with a
10
−6
M, i. e. a negligibly small concentration of Fe
2+
.
At E = 0.771 V the Fe
2+
/Fe
3+
equilibrium is given
as a further horizontal line (2). Line (3) depicts the
pH-dependent formation of Fe
3
O
4
according to (12.10–
12.13). Line (4) corresponds to the Fe
3
O
4
/Fe
2
O
3
equilibrium. Lines (5) and (6) refer to the oxidation
Part D 12.2
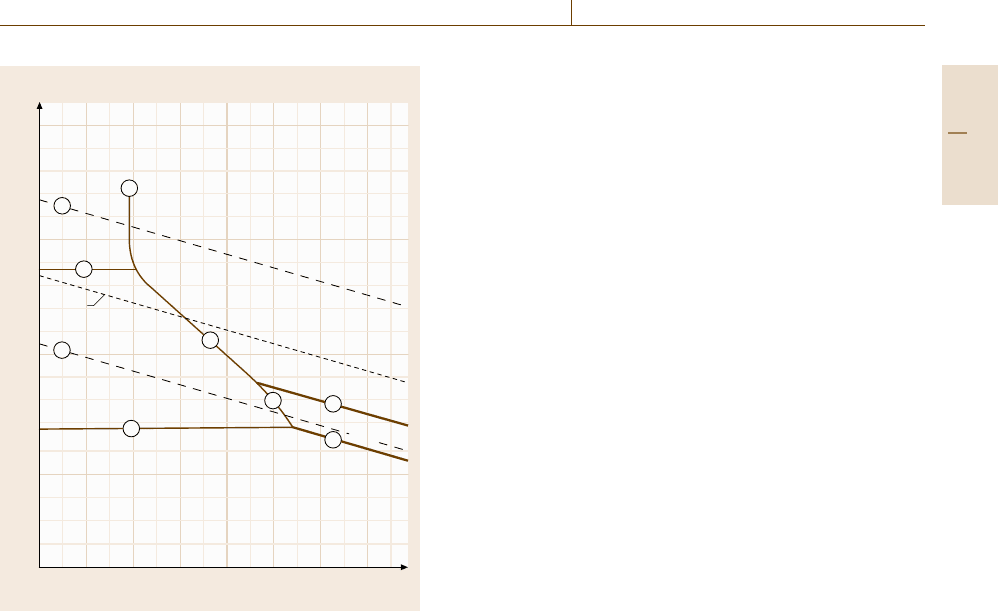
Corrosion 12.2 Conventional Electrochemical Test Methods 675
E (V)(SHE)
Fe
3+
Fe
2+
Fe
Immunity
Passivation
Flade
potential
Corrosion
Fe
2
O
3
Fe
3
O
4
2
1.8
1.6
1.4
1.2
1
0.8
0.6
0.4
0.2
0
–0.2
–0.4
–0.6
–0.8
–1
–1 1211109876543210–2 13
pH
–1.2
–1.4
–1.6
2.2
–1.8
b
a
1
6
5
4
3
7
2
Fig. 12.7 Potential–pH diagram of Fe with equilibria (1–
7) and (a) and (b) and domains of corrosion, immunity
and passivation, as described in the text, Flade potential
describing passivation in acidic electrolytes
of soluble Fe
2+
to Fe
3
O
4
and Fe
2
O
3
respectively. The
vertical line (7) describes the potential-independent
dissolution equilibrium of Fe
2
O
3
/Fe
3+
that is related
to a specific pH. According to this diagram one has
to distinguish three main fields. Corrosion, i. e. metal
dissolution, should occur where soluble products are
stable, i. e. at the upper left end of the diagram Fig. 12.7.
At the lower end of the diagram one has the field of
immunity where Fe is the stable species. In the upper
right part the surface should be protected by anodic ox-
ide layers. Here thin anodic films should form at the
metal surface, which block further oxidation and lead to
passivity. The lines (a) and (b) describe the equilibrium
potential of the hydrogen and oxygen electrode, which
are important redox systems for aqueous corrosion.
These diagrams have been calculated at 298 K for all
elements, metals, semiconductors and elements with in-
sulating properties [12.26,27]. More recently they have
also been calculated for elevated temperatures. They
provide an excellent possibility to get a first idea of the
corrosion properties of a metal. However, one should
always keep in mind that they are calculated on the ba-
sis of thermodynamic data only. Any kinetic properties
are not included. This means that conclusions on the
basis of these diagrams may be misleading. Pourbaix
diagrams tell us whether a reaction may occur or not.
Whether the predictions really happen or not depends
on the kinetic parameters of the related reactions. A typ-
ical exception is the passive behavior of iron in strongly
acidic electrolytes such as 0.5MH
2
SO
4
or 1 M HClO
4
.
Iron should dissolve at potentials above E =−0.409 V.
However, passivation occurs when the potential gets
above E = 0.58–0.059 pH[V]. This condition is de-
picted as a dashed line in Fig. 12.7. Surface analytical
investigations have shown that Fe forms a poreless few-
nm-thick layer of Fe
2
O
3
a spinell-type oxide which is
very slowly dissolving in these acidic electrolytes. De-
tailed electrochemical studies come to the conclusion
that the transfer of Fe
3+
cations from the oxide surface
to the electrolyte is an extremely slow process, although
the oxide layer is far from its dissolution equilibrium.
As a consequence this surface film passivates the metal
underneath. It has been shown that the passivation po-
tential may be explained by the thermodynamic data for
the anodic oxidation of Fe
3
O
4
to Fe
2
O
3
[12.28]. Appar-
ently the structure and the electrochemical properties
of Fe
2
O
3
provide sufficient stability for dissolution,
i. e. the activation energy to transfer Fe
3+
ions from
the potential well of the O
2−
matrix of an oxide is
very high. Similar arguments hold for passive layers
onCr.HereaCr
2
O
3
film of only 1–2 nm thickness
dissolves in acidic solutions so extremely slowly that
Cr is protected very effectively against corrosion in
strongly acidic electrolytes [12.29–31]. This is also the
case for Cr-containing alloys, such as Ni/Cr or Fe/Cr.
The strong bonds between Cr
3+
ions and its ligands
in its complexes are well known from inorganic chem-
istry. The exchange of ligands is so slow that many of
these Cr
3+
compounds are almost insoluble, although
this description is misleading; they dissolve extremely
slowly, which has the same effect. As an example the
extremely slow dissolution of CrCl
3
should be men-
tioned, which leads to its description as an insoluble
compound. However, this behavior is only caused by an
extremely slow dissolution rate [12.31]. This becomes
faster at elevated temperatures when the related high
activation energy for the exchange of ligands has been
overcome.
In conclusion the Pourbaix diagrams provide ex-
cellent initial information on the corrosion behavior of
metals on the basis of thermodynamic data. However,
a reliable discussion has to include kinetic parameters
Part D 12.2
676 Part D Materials Performance Testing
Au
–1.0 0 1.0 2.0
Au
Au/Au(CN)
–
2
Au/Au
+
E
0
E
R
E
0
E
0
E
R
E (V)
I (A/cm
2
)
O
2
/H
2
O
Cu/Cu
2+
Fe/Fe
2+
Fe Cu
Fig. 12.8 Schematic diagram of E
0
, E
R
, and anodic dissolution of
Fe, Cu and Au and the shift of E
0
of Au by complexation with
cyanide with oxygen reduction as counter-reaction
Preceding reaction
Adsorption
Charge transfer reaction
Desorption
Following reaction
Diffusion
Diffusion
Reaction overvoltage η
r
Charge transfer overvoltage η
d
Reaction overvoltage η
r
Diffusion overvoltage η
D
Diffusion overvoltage η
D
η =η
D
+ η
r
+ η
d
Fig. 12.9 Elementary reaction steps of an electrode process with
related overvoltages
in order to avoid misleading conclusions which might
contradict experimental results.
12.2.4 Complex Formation
The formation of strong complexes of dissolved cations
with anions has an important influence on the dissolu-
tion of metals; hey shift the concentration of free cations
to very small values and thereby shift the equilibrium
potential of the related metal/metal-ion electrode to
very negative values [12.20, 32, 33](Fig.12.8). A well-
known effect is the strong complexation of Au
3+
by
Cl
−
or of Au
+
by CN
−
as depicted in (12.14, 12.15).
The standard potential of Au dissolution is shifted by
Cl
−
from 1.42V to 0.994 V or by CN
−
from 1.68 V
to −0.60 V, which is a consequence of the small value
of the dissociation constant K
D
= 2.2×10
−22
of the
AuCl
−
4
and K
D
= 2.3×10
−39
for the Au(CN)
−
2
com-
plex. Therefore Au dissolution occurs in a 1 :3 mixture
of HCl and HNO
3
(aqua regia) whereas pure HNO
3
does not attack. Similarly Au may be dissolved in CN
−
-
containing solution by air oxidation (Fig. 12.8). The
latter example is shown by (12.16, 12.17) in detail, and
has been applied for extraction of Au from minerals.
AuCl
3−
4
→Au
3+
+4Cl
−
, K
D
=2.2×10
−22
(12.14)
Au(CN)
−
2
→Au
+
+2CN
−
, K
D
=2.3×10
−39
(12.15)
a(Au
+
) = K
D
a(AuCN)
−
2
a
2
(CN
−
)
(12.16)
E = E
0
Au
+
RT
F
ln a(Au
+
)
=1.68+0.059 log K
D
+ 0.059 log
a[Au(CN)
−
2
]
a
2
(CN
−
)
(12.17)
E
0
AuCN
=1.68−0.059 log 2.3×10
−39
=1.68−2.28 =−0.60 V (12.17a)
Complexation of cations also plays a role in the en-
hanced dissolution of passivating layers. The formation
of complexes of cations with halides has been proposed
as a mechanism for the breakdown of passivity and lo-
calized corrosion [12.29–31].
12.2.5 Electrochemical Kinetics
The kinetics of electrode processes influence decisively
corrosion phenomena. As usual in kinetics the rate of
a chemical reaction at the electrode surface is ruled by
the activation energy of the slowest of a sequence of
elementary reaction steps. This could be the charge-
transfer process or any preceding or following reaction
step. Any electrode process requires the transport of
the reacting species to the electrode surface, a possi-
ble chemical reaction within the solution in front of the
electrode, the charge-transfer process, a possible fol-
lowing chemical reaction and finally the transport of
the product from the electrode to the bulk electrolyte.
In addition adsorption and desorption processes may
be involved. Any of those steps may be the slowest
and thus may be rate-determining for the overall reac-
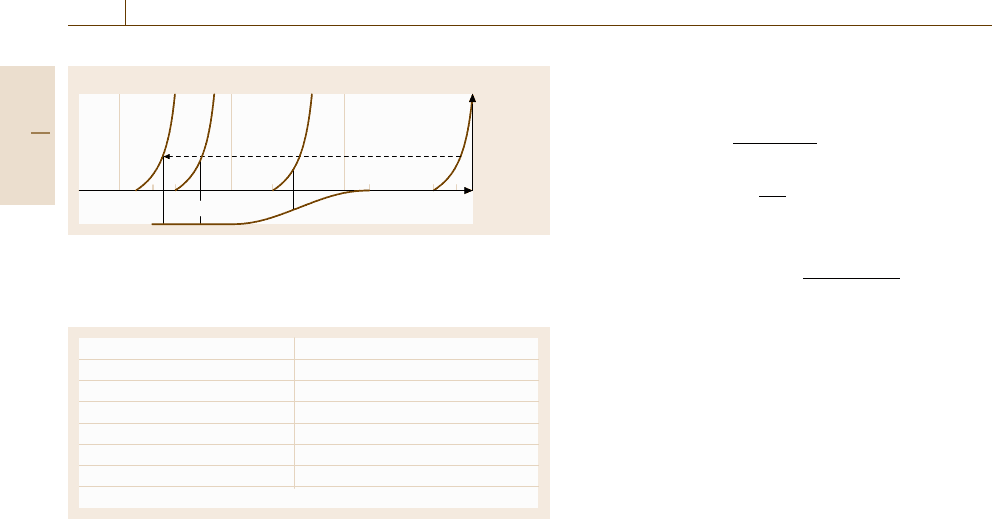
tion. Figure 12.9 describes the sequence of elementary
reaction steps, including the charge-transfer reaction,
that together form the overall reaction. Each of these
steps may contribute with its special overvoltage η
i
to
the total overvoltage η =
η
i
of the whole reaction
(Fig. 12.9). Usually one reaction step is the slowest and
thus rate-determining for the overall reaction. Its over-
voltage η
i
dominates η.
12.2.6 The Charge-Transfer Overvoltage
If the charge transfer is rate-determining the related
current density follows an exponential relationship, the
Butler–Volmer equation. As mentioned earlier an ion or
Part D 12.2
Corrosion 12.2 Conventional Electrochemical Test Methods 677
an electron may be the transferred species. This process
occurs within the Helmholtz layer, i. e. within a dis-
tance of the radius of solvated ions of typically less than
1 nm. The charge transfer of (12.18) occurs via a tunnel-
ing process that is seriously influenced by the potential
drop Δφ = φ
M
−φ
S
within this layer. Combining the
usual rate equation with Faraday’s law one obtains a re-
dox process (12.19) which includes the two opposite
parts due to the anodic and the cathodic reactions. At
the equilibrium potential these are equal and compen-
sate each other. F is the Faraday constant and n is the
number of charges transferred for this reaction, which
is usually 1 and may be larger if several one-electron
processes occur in a sequence, k
+
and k
−
are the rate
constants for the anodic and cathodic reaction steps and
c(Red) and c(Ox) are the concentrations of the oxidized
and reduced species of the redox system involved. For
metal dissolution equivalent (12.20, 12.21) hold where
Θ
Me
is the surface coverage for metal atoms which is
proportional to their surface concentration, and c(Me
z+
)
is the concentration of the cations within the electrolyte.
One has to distinguish in general between the charges
n which are involved in the total electrode reaction of
(12.18, 12.20), and the charge z of the species which
is transferred across the electrode–electrolyte interface
during the charge-transfer process. In the case of a sim-
ple metal dissolution, according to (12.20) n equals z.If
the dissolving cation forms a complex with anions from
the solution in a reaction before the charge-transfer step
these number may be quite different. For a redox reac-
tion z equals 1. If however, more than one electron is
transferred in a fast sequence of several steps, i. e. in the
case of a charge-transfer reaction with more electrons n
will be larger
Red →Ox+ne
−
, (12.18)
i = nF
−dc(Red)
dt
−
−dc(Ox)
dt
=nF
k
+
c(Red)−k
−
c(Ox)
, (12.19)
Me →Me
z+
+ne
−
, (12.20)
i = nFk
+
Θ
Me
−nFk
−
c(Me
z+
) . (12.21)
The rate equations of both types of charge-transfer
processes may contain the concentration of additional
species that are involved in their mechanisms. An ex-
ample is the catalysis of active Fe dissolution by OH
−
ions. The exponent of the concentrations of OH
−
,i.e.
its electrochemical reaction order, is 1.5 [12.34]. Re-
action orders may be any number for a given reaction
depending on the details of its mechanism.
Energy
Helmholtz layer
Distance
0
ΔE
0,A–
ΔE
0,A+
ΔE
A+
ΔE
A–
FΔφ
FΔφ
αFΔφ
H
(1 – α)FΔφ
H
αFΔφ
H
Distance
Metal Electrolyte
Fig. 12.10 Activation energy ΔE
0,A
and its change to ΔE
A
for the anodic (+) and cathodic reaction (−) due to the po-
tential drop Δφ across the electrode–electrolyte interface
As usual, both rate constants k
+
and k
−
depend
exponentially on their activation energy ΔE
0,A+
and
ΔE
0,A−
(12.22, 12.23). According to Fig. 12.10 the ac-
tivation energies ΔE
A,+
and ΔE
A,−
for the transfer of
charged species across the electrode–electrolyte inter-
face are influenced by the potential drop Δφ.However,
only a fraction of Δφ will modify ΔE
0,A+
. The part
α takes into account the fraction of Δφ which is kineti-
cally effective, i. e. which part will act on the species up
to the maximum of the energy–distance curve; α usu-
ally has a value of 0.2–0.8. If α refers to the anodic
process 1 −α will hold for the cathodic reaction. Thus
the activation energy ΔE
A,+
will change by −αzFΔφ
for the anodic and ΔE
A,−
by +(1 −α)zFΔφ for the
cathodic process, respectively; z is the charge of the
species which is transferred across the double layer,
where z = 1 for electrons, i. e. for charge-transfer pro-
cesses, and z may be larger for metal ions, e.g. z = 2
for Fe
2+
. Equations (12.22, 12.23) describe the influ-
ence of Δφ on the rate constants of both processes
and (12.24) gives the full kinetic equation for a re-
dox reaction. An equivalent equation is obtained for
a metal/metal-ion electrode. The unknown potential
drop Δφ within the double layer is replaced by the elec-
trode potential E and the drop Δφ
H
within the double
layeroftheSHE (Δφ = E −Δφ
H
). As Δφ
H
is con-
stant, any changes of Δφ will equal that of the electrode
Part D 12.2
