Czichos H., Saito T., Smith L.E. (Eds.) Handbook of Metrology and Testing
Подождите немного. Документ загружается.

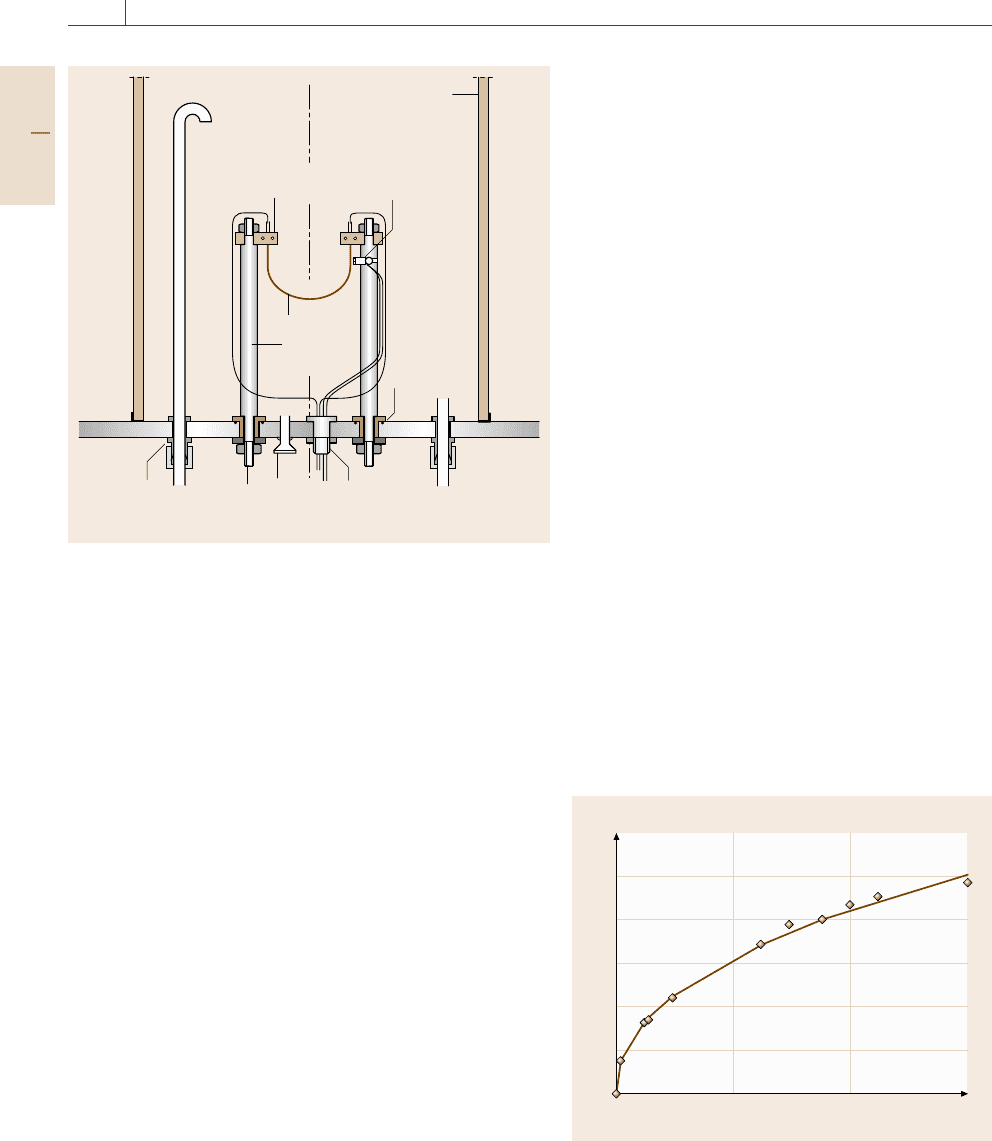
728 Part D Materials Performance Testing
Gas inlet
Bell jar
Semiconductor
pyrometer
Insulated
feedthrough
Base plate
Swagelock
10 mm bulkhead
Power
terminal
Vacuum
fitting
Four way
compensating
feedthrough
Testpiece
clamp
Testpiece
Testpiece
support
Fig. 12.55 Schematic of a test rig for thermal cycling oxidation
testing using ultrashort temperature cycles
recommended test cycle for ultrashort-dwell-time test-
ing consists of 5 min hot dwell and 2 min cold dwell.
For this specific type of test a special test rig is needed,
as described in detail in [12.112]. A schematic of this
rig is shown in Fig. 12.55.
Generally the test duration in thermal cycling test-
ing should be at least 300 h of accumulated hot dwell
time to allow significant oxidation or corrosion of the
test pieces. For more reliable results it is however rec-
ommended to extend the accumulated hot dwell time to
at least 1000 h.
Evaluation of the Mass Change
After the tests, or during the tests at intervals when
the specimens are cold, the mass has to be determined
to quantify the corrosion kinetics. For this weigh-
ing procedure the specimens have to be taken out of
the equipment but they should never be touched with
hands, to eliminate any contamination (grease, salts).
Generally the use of tweezers is recommended. Af-
ter removing from the furnace the test-piece supporters
containing the test pieces should be settled in the weigh-
ing room for 15 min to allow them to acclimatize.
The test pieces should not be descaled unless speci-
fied. For each mass-change determination the sum m
ST
of the mass of the test-piece support (m
S
) contain-
ing one test piece (m
T
) and the spalled scales (m
sp
),
i. e. m
ST
=m
S
+m
T
+m
sp
,themassm
s
+m
sp
of the
test-piece support including the spalled scales, and the
mass m
T
of the test piece including the adherent scales
should be measured. The following values have to be
determined.
1. Gross mass change Δm
gross
, i. e. mass change of test
piece after cooling including collected spall
Δm
gross
=m
ST
−[m
S
(t
0
) +m
T
(t
0
)]; (12.91)
2. Mass of spalled oxide Δm
sp
, i. e. scale flaked from
the test piece
Δm
sp
=m
S
−m
S
(t
0
) ; (12.92)
3. Net mass change Δm
net
, i. e. mass change of test
piece after cooling without spall
Δm
net
=m
T
−m
T
(t
0
) . (12.93)
The net mass-change results of the test pieces from
these measurements are plotted against time, as shown
in Fig. 12.56. To determine the quantitative key pa-
rameters characterizing the corrosion behavior of the
material a double logarithmic plot (log net mass change
versus log time) as shown in Fig. 12.57 is used. From
the y-axis intercept the oxidation rate constant k(T) can
be calculated as
a = log k(T)
1/n
→a =1/n log k(T) →k(T)
=10
an
. (12.94)
Δ m/A (mg/cm
2
)
0.3
0.25
0.2
0.15
0.1
0.05
0
0 15010050
Time t (h)
Fig. 12.56 Example of a plot of net mass change versus
time as a direct plot
Part D 12.8
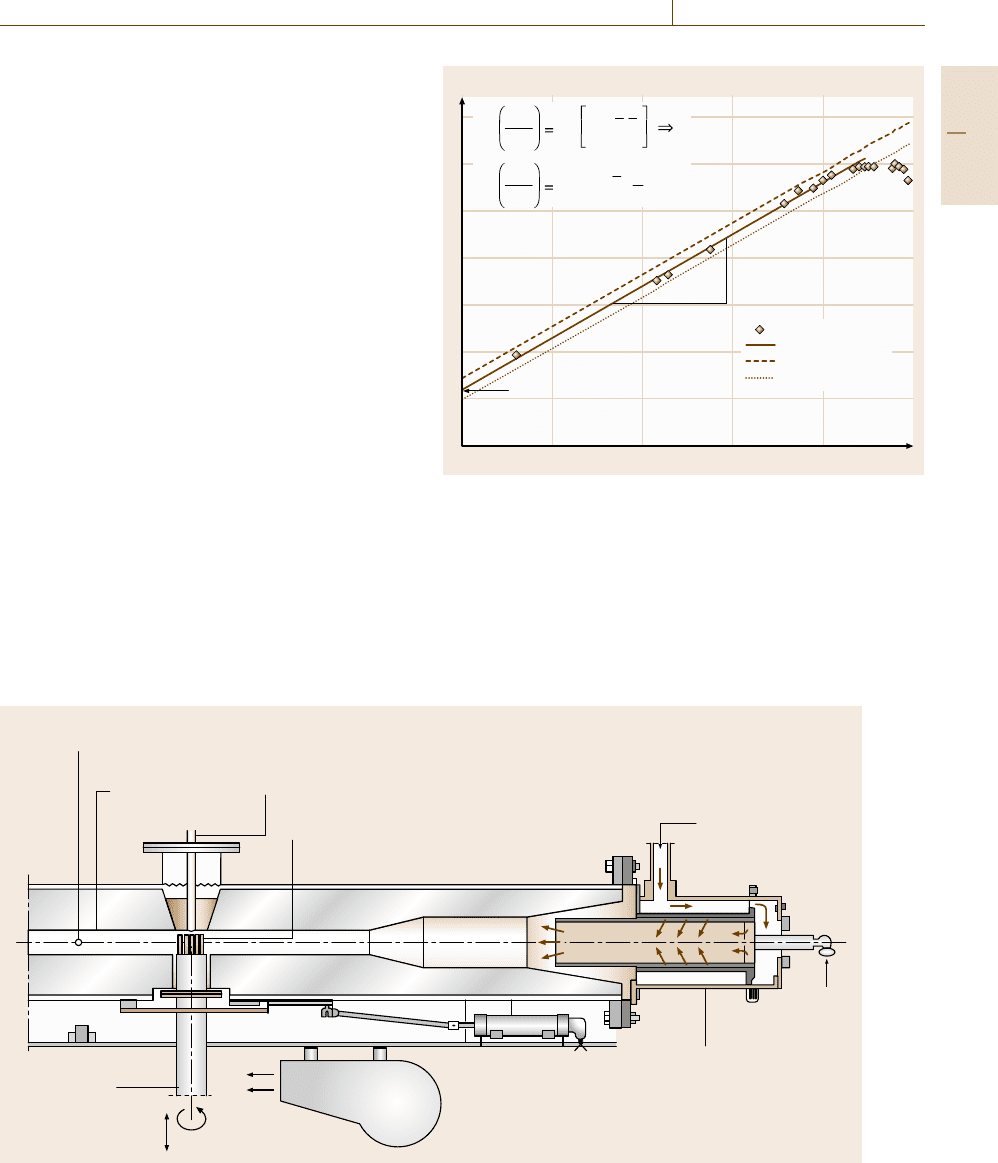
Corrosion 12.8 High-Temperature Corrosion 729
If spalling or breakaway oxidation occur, the time at
the beginning of these mechanisms can also be of sig-
nificant interest. In [12.109] a detailed procedure is
described that allows the quantitative determination of
these parameters.
12.8.4 Special High-Temperature Corrosion
Tests
Burner Rig Tests
In addition to the standard tests described so far sev-
eral special tests have also been developed that try
to take the specific loading situation under practical
conditions into account. Especially in the gas-turbine
manufacturing industries the so-called burner rig test
has become of some importance. In this test the test en-
vironment is produced by combustion of fuel with air
(Figs. 12.58 and 12.59). In Fig. 12.58 a schematic of
the so-called high-velocity burner rig is shown, where
the gas stream on the specimen surface can reach veloc-
ities of up to mach 0.3. In the case of a low-velocity
burner rig, combustion in a zone in the vicinity of
the specimen supporter produces the test gas, which
flows at a relatively low rate through a large tube to the
specimens (Fig. 12.59). Especially in the case of a low-
velocity burner rig, impurities are often added to the
combustion environment, first of all in the form of salt
water [12.87].
Thermocouple for
steady-state control
Thermocouple for recording
specimen temperature history
Compressed
inlet air 425 °C (800°F)
Fuel
Combustor
Blower
(thermal shock)
Rotating shaft
Thermal cycle
Specimen temperature measured
by pyrometer
50 mm
2
insulated
flame tunnel
Fig. 12.58 Schematic of a high-velocity burner rig (after [12.87])
log (Δ m/A)
log log
k(T)
n
t
n
log t
Δm
A
log log log t+
k(T)
n
a = log k
(1/n)
b = 1/n
Δm
A
1
1
1
1
n
Experimental data
Fit of the exp. data
0.97 limit
1.03 limit
Fig. 12.57 Double logarithmic plot of the data of Fig. 12.56 for the
evaluation of the parameters k(T)andn
Typical test specimens have the shape of a rod with
a rotating specimen carousel in the test rig carrying
a larger number of these rods. Using this carousel,
corrosion of rotating parts in engines is simulated. Fur-
thermore all specimens are subjected to the same test
conditions. In most cases with the burner rig test a ther-
Part D 12.8
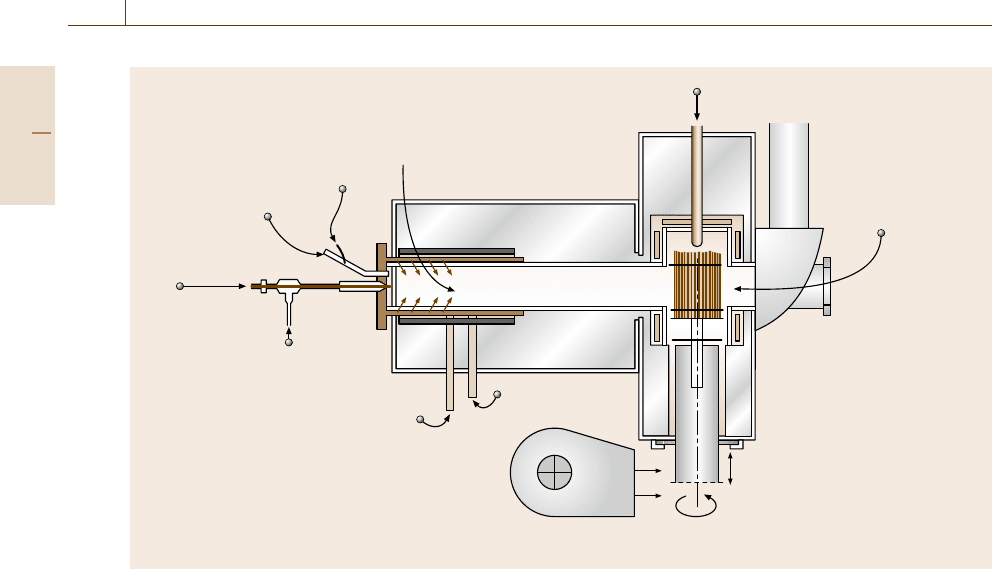
730 Part D Materials Performance Testing
Atomizing air
Salt water
Atomizing air
Fuel
Secondary air
Thermocouple
Controlling
thermocouple
Specimen
temperature
by pyrometer
Thermal shock cycle
Rotating
shaft
Blower
(thermal shock)
Combustion zone
Fig. 12.59 Schematic of a low-velocity burner rig (after [12.87])
mal cycling test is performed, where the carousel with
the specimens is automatically moved out of the hot
zone once every 30 or 60 min and then cooled before
reinsertion.
Corrosion with Mechanical Loading
A parameter which is neglected in standard high-tempe-
rature corrosion tests is the role of mechanical stresses,
which are omnipresent in practical operation. Their role
has been investigated extensively over the past two
decades and it turned out that significant effects on the
life time of high-temperature components can be possi-
ble [12.109,113–115]. For this reason different types of
tests have also been developed that combine mechanical
stresses with attack by high-temperature corrosion. In
most cases the equipment for such kinds of tests is basi-
cally the same as for the determination of the mechanical
properties of materials at high temperatures. In other
words, standard creep test [12.116] or constant-strain-
rate test [12.117] as well as low cycle fatigue (LCF)
and high cycle fatigue test (HCF) [12.118]rigshave
been used. In some cases even creep fracture mechanics
approaches have been combined with high-temperature
corrosion investigations [12.119, 120]. For these me-
chanical high-temperature corrosion tests the standard
mechanical test rigs were equipped with an environmen-
tal chamber that can hold different types of test gases.
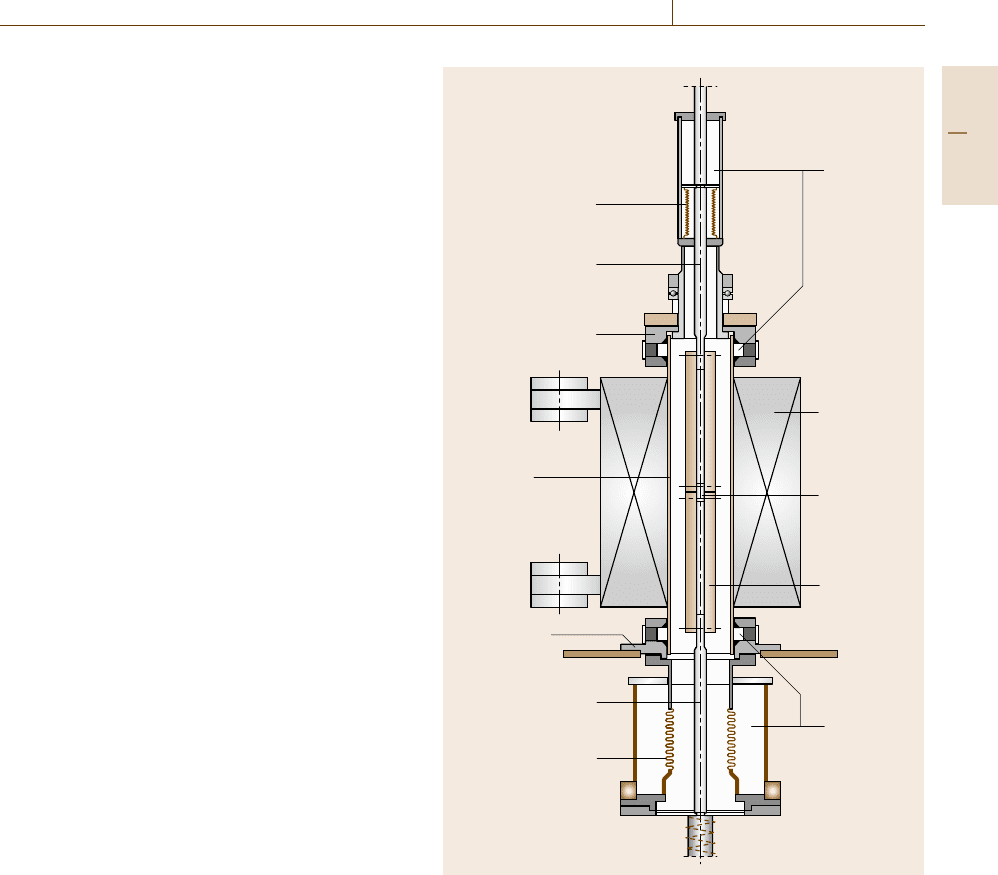
Examples of the design of such environmental chambers
are shown in Figs. 12.60 and 12.61, where Fig. 12.61
shows the environmental equipment to be inserted into
a constant-load creep testing machine with two separate
specimens, where the strain can be monitored by ei-
ther mechanical or electronic strain gages. Figure 12.60
shows the schematic of a creep fracture mechanics envi-
ronmental equipment for CT specimens where ceramic
pull rods had been used. The information received from
these experiments is the mechanical properties under
the influence of corrosive attack, i. e. strain versus time,
strain rate versus time, or stress versus strain curves. In
the LCF and HCF test rigs it is usually the number of
cycles to failure and the cyclic deformation curve that
are measured. There are also examples in the literature
where crack growth rates at high temperatures have been
measured under corrosive conditions [12.119–121].
An important aspect of these tests is post-test eval-
uation by metallographic sectioning and microanalysis
of the corrosion products. The results from such in-
vestigations can reveal whether internal formation of
corrosion products at grain boundaries or at crack tips
can significantly reduce the life time of components
Part D 12.8
Corrosion 12.8 High-Temperature Corrosion 731
by corrosion-assisted crack initiation and crack growth.
A number of examples have been studied in the litera-
ture [12.113].
12.8.5 Post-Test Evaluation of Test Pieces
Macroscopic Evaluation
The macroscopic appearance of the surface of the
test piece should be photographed if necessary using
a low magnification. Color photographs are preferred
over black and white photographs since coloring may
already give some information about the type of oxida-
tion/corrosion of the surface.
Metallographic Cross Section
For scale thickness measurements by metallographic
cross sections it is recommended that the recommen-
dations in [12.88] and the new draft ISO document
ISO/CD 26146 (Table 12.4) be followed. Care has to be
taken in mounting the specimen orthogonally to the pri-
mary axis of the test piece. The cross section of the test
pieces is analyzed using conventional light microscopy.
The measurements consist of
•
deposit thickness,
•
scale thickness,
•
depth of internal penetration inside the grains and at
grain boundaries,
•
depth of any depleted zone,
•
remaining cross section of unaffected material.
A minimum of eight measurements per test piece should
be taken. In addition the position of maximum attack is
measured.
Reporting
The report of each test should contain the following
information.
•
Test material: manufacturer, name of the mater-
ial (manufacturer designation, ASTM designation,
Deutsches Institut für Normung (DIN) designation,
etc.), grade or symbol, heat number/batch number,
chemical composition, processing conditions, heat-
treatment conditions.
•
Test piece: designation of test piece, dimensions and
surface area of test piece, surface finish condition of
test piece, degreasing method of test piece, method
of test-piece support, initial mass.
•
Temperature and testing environments: test temper-
ature (and maximum and minimum temperatures
Axial expansion joint
Water cooling
Water cooling
Furnace
CT specimen
Ceramic
pull rod
Metal pull rod
Axial expansion joint
Metal pull rod
Flange system
Quartz tube
Flange system
Fig. 12.60 Schematic of a closed system for crack growth measure-
ments in high-temperature corrosion environments (after [12.119])
during the test), test duration, hot dwell time, cold
dwell time, heating time, cooling time, dew point
temperature of humidified test gas or humidity of
laboratory air, flow rate of the test gas, type of sys-
tem (open or closed).
•
Test results: plot of net mass change per area
Δm
net
/A in mg/cm
2
versus time t, results of any
metallographic investigations, amount of spalled
scale Δm
sp
in mg, photograph of appearance af-
ter testing, photograph of the metallographic section
Part D 12.8
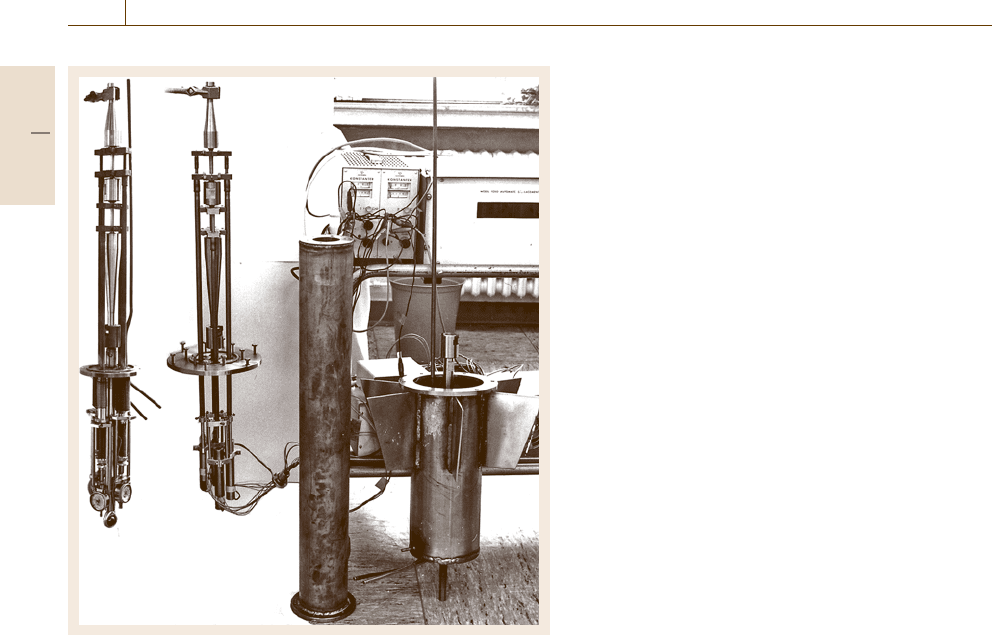
732 Part D Materials Performance Testing
of the test piece including the surface layer af-
ter testing, oxidation rate constant k(T ), exponent
of growth law n, the number of cycles N
B
and
Fig. 12.61 Two examples of closed systems for constant
load creep testing in high-temperature corrosion environ-
ments (after [12.122])
the accumulated hot dwell time t
B
that correspond
to the onset of spallation or breakaway oxidation.
If existing the report should also contain analyti-
cal results from scanning electron microscopy/EDS
(Energy Dispersive Spectrometer), electron probe
microanalysis/WDS (Wavelength Dispersive Spec-
trometrer) or x-ray diffraction.
12.8.6 Concluding Remarks
The aim of this section has been to provide a concise
and informative overview of the measurement meth-
ods used today in high-temperature corrosion testing.
Two of the more commonly used test methods were
described in some detail while the others were only dis-
cussed very briefly. For more detailed information it
is recommended to read the literature given in the list
of references. It should, however, be pointed out that
there is still significant development work going on in
these test methods. A certain settlement of this situ-
ation will be reached after finalization of the present
work items of work group 13 in ISO technical commit-
tee 156. In the meantime it is recommended to follow
the work of this work group closely when planning
the set up of new high-temperature corrosion testing
facilities.
12.9 Inhibitor Testing and Monitoring of Efficiency
The addition of small amounts of chemicals capable of
reducing corrosion of the exposed metal is a preventa-
tive measure known as corrosion inhibition. According
to the ISO 8044 definition corrosion inhibitors are
chemical substances that decrease the corrosion rate
when present in the corrosion system in sufficient
concentration, without significantly changing the con-
centration of any corrosive agent.
Inhibitors may act on both cathodic and anodic par-
tial reactions and are termed accordingly either cathodic
or anodic inhibitors. A cathodic inhibitor shifts the cor-
rosion potential in a negative direction, while the anodic
inhibitor causes a positive change; in both cases the
corrosion rate decreases.
Investigating corrosion inhibitor efficiency aims to
gather information on the profile of the functional ad-
ditive, including its inhibition mechanism. Corrosion
experiments as well as electrochemical and surface
analytical methods are the major tools in such inves-
tigations. In this context it is of utmost importance
to note that electrochemical measurements are not
corrosion experiments and cannot replace real cor-
rosion experiments. On the other hand, testing tries
to find out if and how well a functional substance
performs under specific experimental conditions. Per-
formance in a test may not correlate with performance
under service conditions. There are numerous exam-
ples where functional compounds have been developed
and designed to pass specific tests but have failed
in service application. Monitoring in this context in-
tends to create online information on the time-related
status of the corrosion intensity of an inhibited cor-
rosion system. Appropriate correlation between mon-
itoring data and the real corrosion status of a system
Part D 12.9
Corrosion 12.9 Inhibitor Testing and Monitoring of Efficiency 733
needs a detailed knowledge of the corrosion system
itself.
12.9.1 Investigation and Testing
of Inhibitors
As corrosion inhibitors are active at the electrified
interface their performance can be investigated by elec-
trochemical means.
Various electrochemical measuring methods have
been developed and applied for investigations of inhib-
ited systems. The questions arise: which method gives
which information and what are the application limits of
the methods? Before applying any of the methods one
has to consider the actual task of the investigation. Is
it aimed mainly at scientific research or is information
needed on the performance of inhibitors under service
conditions.
In scientific research it is important, which of the
two partial reactions (Fig. 12.62), the anodic metal dis-
solution,
Me →Me
n+
+n e
−
, (12.95)
or the cathodic reduction of an oxidizing agent,
Ox+n e
−
→Red , (12.96)
is influenced by the inhibitor. Is it an anodic, cathodic
or anodic–cathodic inhibitor (Fig. 12.63a–c)? Is the cor-
ne
–
Me
n+
Ox Ox
Inhibitor
Inhibitor
a) b) c)
Inhibitor
Inhibitor
Me
ne
–
Me
Ox
ne
–
Me
Red
Fig. 12.63a–c Schematic representa-
tion of inhibitor action on bare metal
surface: (a) anodic inhibition, (b) ca-
thodic inhibition, (c) anodic–cathodic
inhibition
ne
–
Ox
Inhibitor film Inhibitor film Scale
Scale-
pore
a) b) c)
( = Inhibitor)
Me
Me
n+
Red
ne
–
Ox
Me Me
n+
Red
ne
–
Ox
Me
Red
Fig. 12.64a–c Schematic representation of inhibitor action: (a) mass transport control at films (b) charge-transfer control
at films
(c) inhibitor action on scaled surfaces
ne
–
Me
n+
Ox (e.g. O
2
, H
+
)
(e.g. OH
–
, H)
(e.g. Fe
2+
, Al
3+
)
Me
Red
Fig. 12.62 Schematic representation of electrochemical
corrosion
rosion rate controlled by charge transfer or by mass
transport (Fig. 12.64a–c)? Is the inhibition effect due
to the formation of films, adsorption on metal surfaces
(e.g. in corrosion product pores) or incorporation in cor-
rosion product layers? (Fig. 12.64c)?
For practical applications information is needed on
the effect of inhibitors on, e.g.,
•
the reduction of metal loss in corrosive environ-
ments,
•
the influence of hydrogen uptake of the metal (in
systems with hydrogen formation in the cathodic
partial reaction),
•
the likelihood of localized corrosion.
Before starting any investigation the corrosion system
must be carefully studied in terms of the appearance of
Part D 12.9

734 Part D Materials Performance Testing
corrosion (uniform, local, ability to passivation, charac-
ter of layers developed by corrosion, scaling etc.) and the
rate-determining step of the corrosion process. Particu-
larly in exposure testing the analysis of the electrolyte
over the period of testing can play an important role.
The methods of investigation were generally out-
lined in Sects. 12.1 and 12.2. All conclusions regarding
the applicability and interpretation of such tests also ap-
ply for testing of inhibitors. Special care has to be taken if
results from laboratory testing are transferred into prac-
tice. In any case it is advisable to carry out performance
monitoring during service. The aim of this section pri-
marily focuses on performance of systems affected by
corrosion. Therefore monitoring of efficiency will be the
main focus of this publication.
12.9.2 Monitoring of Inhibitor Efficiency
General Remarks
It is important to monitor the effectiveness of
a corrosion-inhibitor treatment. It is even more im-
portant to ensure that the conditions under which the
monitoring is carried out are representative and relevant
to the operational conditions. Monitoring should ide-
ally be carried out under the worst conditions that are
likely to be encountered (e.g. stagnant areas or very high
flow rates etc.), otherwise failures may occur while the
monitoring process indicates that inhibition is effective.
Variables that need to be considered when monitor-
ing include
•
environment – both normal and extraordinary con-
ditions fluid flow conditions, including multiphase
flow patterns,
•
temperature,
•
fluid velocity chemical analysis – to cover seasonal
variations,
•
type of system – closed, open evaporative cooling,
once-through.
Application of Monitoring Data
The broad objective of carrying out monitoring is to
manage the corrosion problem, in this case to control
the corrosion-inhibitor treatment so that it works prop-
erly. The management process thus requires objectives
to be set, and measurements to be made that can be com-
pared with these objectives, so that adjustments to the
treatment can be made as necessary to achieve the re-
quired performance. The objectives may include one or
more of the following, depending upon the corrosion
management philosophy
•
to achieve a specified degree of corrosion protec-
tion (corrosion rates), to correlate with previous
experience or tests (corrosion rates, inhibitor con-
centrations),
•
to achieve a required inhibitor efficiency E
i
E
i
=100(w
o
−w
inh
/w
o
) ;
where w
o
= weight loss or corrosion rate without
inhibitor, w
inh
= weight loss or corrosion rate with
inhibitor.
It is important that the type of measurement made pro-
vides the data required. Thus, if the objective is to
maintain a specified inhibitor concentration, corrosion
rate measurements will be of little value.
Measurement of Inhibition Parameters
to be Measured
A number of parameters relevant to corrosion inhibition
may be measured.
•
Actual inhibitor concentration,
•
Inhibitor efficiency by corrosion monitoring,
•
Operational parameters in the plant that may affect
inhibitor performance,
•
Chemical composition of the fluid containing the
inhibitor.
Frequency of Measurement
The frequency of measurement may depend upon cor-
rosion monitoring practice. In general it is important
to monitor immediately after the first addition of the
inhibitor, and then at least daily until the level has stabi-
lized, since some of the inhibitor will be removed from
the fluid by adsorption on surfaces within the system.
Open systems are more likely to suffer unplanned
upsets than closed ones, and therefore should be mon-
itored more frequently. For critical systems online
measurements are useful. Online measurement systems
have other advantages.
•
They allow more rapid detection of changes or
trends in corrosion, and data can be continually an-
alyzed to reveal longer-term trends.
•
They eliminate variations associated with sampling
processes.
Chemical Analysis of Inhibitor Concentration
Sampling Techniques. It is essential to take as repre-
sentative a sample of the fluid as possible. The location
of sampling points is important.
•
Avoid dead legs,
•
If possible, use isokinetic sampling techniques,
Part D 12.9

Corrosion 12.9 Inhibitor Testing and Monitoring of Efficiency 735
•
Avoid long sampling lines with high surface :
volume ratios.
When taking a sample
•
always run a volume of the fluid to be sampled to
waste before taking a sample,
•
wash out the container with fluid before filling with
the sample,
•
observe sample preservation requirements (e.g. air
exclusion, cool in ice).
Measurement of Inhibitor Concentration
The efficiency of an inhibitor is usually closely de-
pendent upon its concentration in solution, since it is
assumed that there is an equilibrium of the form: dis-
solved inhibitor +surface → inhibited surface.
Measurement of the concentration of the inhibitor
does not give direct information about its efficiency.
A minimum concentration is usually specified for the
inhibitor to provide the required performance, and this
depends upon the conditions. Changes in operating con-
ditions may therefore require adjustments to be made
in inhibitor concentration to maintain the same perfor-
mance.
Allowing the concentration to fall below the speci-
fied level can be very dangerous with anodic inhibitors,
since partial failure of anodic inhibition can lead to
a high-cathode-area low-anode-area situation, and in-
duce rapid localized corrosion.
Characteristics of Commercial Inhibitors
Measuring the concentrations of commercial inhibitors
may not be straightforward as
•
commercial inhibitors are rarely pure analytical-
grade chemicals,
•
they may be formulated with other chemicals to im-
prove their performance (solvents, surfactants),
•
their specification may allow a range of composi-
tions.
The variability in concentration and composition may
make it desirable to analyze the product before use,
Table 12.6 Field methods of analysis
Species Semiquantitative method Quantitative
CrO
−
4
Diphenylcarbazone complex Photometry of diphenylcarbazone complex
NO
−
2
Magenta azo dye (a) Photometry of magenta azo dye; (b) Acidimetric titration with sulfamic acid
PO
3−
4
Phosphomolybdenum blue Photometry of molybdenum-vanadate – phosphoric acid
SiO
2
/silicate Silicomolybdenum blue Photometry of silicomolybdenum blue
Zn Zn-thiocyanate-brilliant green Atomic absorption spectroscopy
unless a quality-assurance scheme is in operation. The
supplier’s recommendations for the concentrations to be
used should be adhered to; this avoids complications if
problems or failure occurs.
Laboratory Methods of Analysis
These have a number of advantages.
•
They are very versatile.
•
They can be very sensitive.
•
High selectivity is possible.
A wide range of techniques is available. Separation and
detection techniques are combined to increase sensitiv-
ity. Particularly useful are
•
ICPS – inductively coupled plasma spectrometry;
•
MS – mass spectrometry;
•
HPLC – high-performance liquid chromatography;
•
IC – ion chromatography.
The disadvantages are
•
they require special, usually expensive, equipment,
•
good performance requires well-trained operators,
•
samples have to be returned to the laboratory, result-
ing in a time delay.
Field Methods of Analysis
These have a number of common features.
•
They give almost instant results.
•
They employ preprepared and prepacked reagents
(the sell-by date should not be exceeded).
•
They usually involve color formation reactions.
•
They compare color to intensity scale or use
a battery-operated photometer.
Some common methods are specified in Table 12.6.
Some disadvantages are
•
individual kits cover limited concentration ranges;
choose the one you need,
•
look out for possible interferences (other colored
species present),
Part D 12.9
736 Part D Materials Performance Testing
Table 12.7 Summary of techniques
Technique type Information Time for Response Corrosion Application
measurement to change type interpretation
Optical Distribution of attack Fast, if, accessible Slow Localized Simple
Analytical methods Corrosion state,
total
Fairly fast Fairly fast General Relatively easy, needs
knowledge of corrosion in
system, inhibitor present
Corrosion coupons Average rate of
corrosion and form
Long exposure Poor General or localized Easy, simple
Polarisation
resistance
Corrosion rate Immediate Fast General, localized Simple to complex
Electrical resistance Integrated corrosion Immediate Moderate General Relatively simple
Corrosion potential Corrosion state Immediate Fast General or localized Easy to apply, needs
knowledge and experience
to interpret
ZRA = zero-
resistance ammetry
Corrosion state Immediate Fast Bimetallic galvanic
corrosion
Fairly easy, needs
corrosion knowledge
Ultrasonics Thickness of metal
left cracks, pits
Fast Fairly poor General or localized Easy to apply, care
with sensitivity
Eddy currents Cracks, pits Immediate Fairly poor Localized Easy
Acoustic emission Propagation of
cracks, leak detection,
cavitation
Immediate Fast Cracking, leaks Expensive, needs
specialist
Hydrogen probe Total corrosion Fast Poor General Easy, only in acid
Sentinel holes Go/nogoforslow
remaining thickness
Poor General Easy, relatively simple
Thin-layer
activation (TLA)
Integrated material
removal
Slow Va ria bl e General, localized Simple, special equipment
•
color intensity may vary with time, may bleach in
sunlight,
•
the number of species that can be detected is limited
(pH, nitrite, chromate).
Interpretation: Inhibitor Loss Processes
Lower than expected concentrations of inhibitor may be
the result of a number of processes.
•
Precipitation, which may depend on temperature,
solution composition,
•
Chemical reaction with other species, e.g. oxygen,
•
Adsorption on surfaces (metals, solids in suspen-
sion, corrosion products, precipitates),
•
Degradation by microorganisms,
•
Thermal degradation.
To assist in finding the reason for the loss of inhibitor,
measurements of other fluid chemistry parameters may
be useful, as suggested below.
Additional Measurements
•
Water chemistry: including pH, dissolved species –
chloride, iron etc.,
•
Mass balance: gross check of concentration – chem-
icals used, make-up water volume,
•
System features
– concentration of atmospheric impurities in open
systems,
– process-side leaks,
– inefficient blow-down microbiological activity,
– fouling of surfaces, including heat transfer sur-
faces.
•
Operational parameters
– fluid temperature surface temperature, including
hot-spots,
– flow velocities – periods of shut-down.
12.9.3 Monitoring Inhibition
from Corrosion Rates
General
The main objective of using inhibitors is to control cor-
rosion, therefore a measurement of the corrosion rate
can be the most direct way of assessing the effectiveness
of a corrosion control programme. However, a number
of points need to be borne in mind.
Part D 12.9
Corrosion 12.9 Inhibitor Testing and Monitoring of Efficiency 737
•
The technique must replicate the type of corrosion
(general, localized etc.).
•
The results must be presented simply and be easy to
interpret.
•
Some techniques are real-time online measure-
ments, others are not.
•
Most rates are measured with samples in the form
of a probe or coupon; the results will depend on the
geometry (flush, cylindrical, crevice etc.).
Electrical Techniques
Polarization Resistance.
•
These are based on the rates of cathodic and anodic
electrochemical corrosion reactions.
•
They may be difficult to interpret if protective layers
are formed.
•
Other redox reactions may occur at the same time as
the corrosion reaction.
•
Bimetallic and selective corrosion processes cannot
be detected.
Electrical Resistance.
•
This depends upon the change in resistance of a wire
or thin plate due to corrosion and/or erosion,
•
It can be very sensitive: 1–1000 mpy,
•
The metallurgical state of probe may be very un-
representative of the real material; geometry may be
important (e.g., flush, intrusive).
FSM – Electrical Fingerprint.
•
This is similar to electrical resistance in principle.
•
It uses the actual vessel, pipe.
•
It provides nonintrusive, real-time, on-line wall
thickness data.
•
It allows multiple (96) measurements at different
locations e.g. in multiphase flow.
•
Changes in thickness are measured from voltage
changes when current passed.
•
Computer display is provided.
•
It is relatively expensive.
Corrosion Coupons. These are the most widely used
means of monitoring corrosion. Their advantages are
•
cheap to buy,
•
available with standard finish, weighed, degreased,
•
can give information about localized corrosion,
•
bimetallic and welded coupons can be prepared,
•
effects of stress can be reproduced in C-rings or
four-point load probes can be inserted and retrieved
during operation through access fittings.
The measurements that can be made with coupons in-
clude
•
weight determination after drying
(look out for spalling of deposits, corrosion prod-
uct),
•
weight loss after pickling,
•
percent area of localized corrosion,
•
pit depth by sectioning, grinding,
•
analysis of corrosion products,
•
optical, SEM metallography of corroded surface.
They have a number of limitations.
•
They must be electrically insulated from plant com-
ponents they only provide integrated measurements
of corrosion;
•
They must be of reasonable area (1500–2000 mm
2
)
to avoid cut-edge effects;
•
The extent of localized corrosion may depend on
area;
•
The surface finish and material should be the same
as for components to be tested;
•
There is no standard precorroded surface;
•
The location of the coupon is important (fluid flow,
temperature effects).
A special type of corrosion coupon is required
for measurements under heat-transfer conditions. Such
specimens usually have tubular geometries. A number
of features have to be considered in choosing and setting
up such specimens
•
choice of electrical or sensible fluid heating,
•
electrical control is most convenient, and easiest,
•
most industrial heat transfer involves fluids,
•
conditions at the surface depend on the heat-transfer
coefficient,
•
the heat-transfer coefficient depends on the fluid and
the flow conditions, surface temperature or heat flux
control.
Table 12.7 provides information on techniques used in
the field and their most important features.
Part D 12.9
