Canale L.C.F., Mesquita R.A., Totten G.E. Failure Analysis of Heat Treated Steel Components
Подождите немного. Документ загружается.

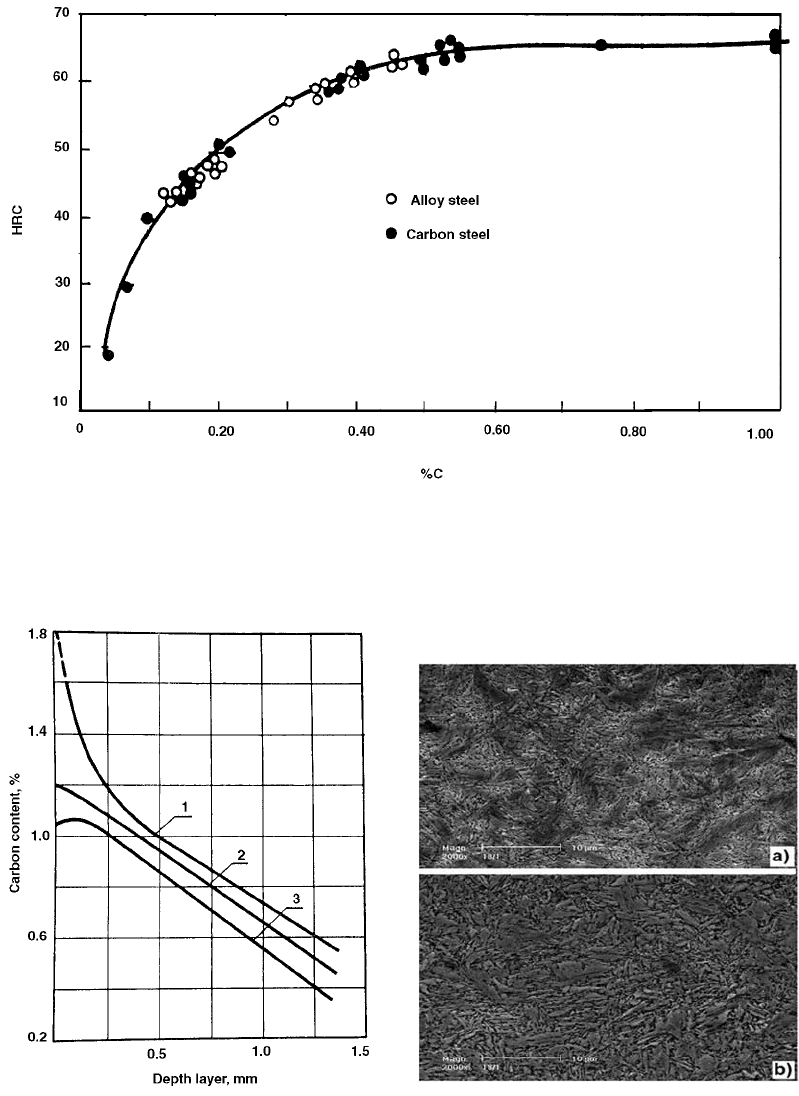
Fig. 43 Martensitic hardness as a function of carbon content in carbon and alloy steel. Source: Ref 80
Fig. 44
Dependence of the carbon gradient as a function
of case depth for three carburized steels that were
carburized under the same conditions: 925
C and 10 h. 1,
chromium-molybdenum steel (0.56% Cr, 0.16% Mo); 2, carbon
steel; 3, nickel steel (3.5% Ni)
Fig. 45
Microstructures of the carburized case structure
of two different samples of 20H steel that were
carburized in the same load. Carburizing temperature:
930
C for 7 h; hardening temperature: 860
C for 0.5 h. (a)
Surface. (b) Core. Etchant: 3% HNO
3
. Original magnification:
500·
214 / Failure Analysis of Heat Treated Steel Components
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_177-240.pdf/Chap_06/ 18/8/2008 3:20PM Plate # 0 pg 214
established between the gaseous atmosphere and
the steel surface:
C
Fe
2CO
C
Fe
+H
2
O CO+H
2
C
Fe
+2H
2
CH
4
When the reactions proceed from left to right,
decarburization will occur. These are the reverse
of the carburization process.
Some of the more commonly reported causes
of decarburization include a malfunctioning
endogas generator, such as soot accumulation
hindering catalyst activity; excess moisture in
Fig. 47
Illustration of lath martensite in the core of carbur-
ized SAE 1524 steel; water quenched from 925
C
(1700
F). Etchant: nital. Scale = 10 mm. Courtesy of G. Vander
Voort, Buehler Ltd., Lake Bluff, IL
Fig. 48
Illustration of lath martensite in the core of carbur-
ized SAE 8115 steel; water quenched from 925
C
(1700
F). Etchant: nital. Scale = 10 mm. Courtesy of G. Vander
Voort, Buehler Ltd., Lake Bluff, IL
Fig. 46
Microstructure of SAE 8620 case of a mold taken just
below the surface. Etchant: alkaline sodium picrate
boiling (60 s), area just below the surface. Original magnifica-
tion: 500·. Courtesy of G. Vander Voort, Buehler Ltd., Lake
Bluff, IL
Fig. 49
Micrograph of poorly carburized SAE 8620 mold
showing decarburization at the surface (note patches
of ferrite and pearlite). Below this zone is where the grain-
boundary carbides are seen. Original magnification: 500· .
Courtesy of G. Vander Voort, Buehler Ltd., Lake Bluff, IL
Fig. 50
This is a higher magnification of the decarburized
microstructure shown in Fig. 49 of the surface of a
poorly carburized SAE 8620 mold (note patches of ferrite and
pearlite). Original magnification: 1000·. Courtesy of G. Vander
Voort, Buehler Ltd., Lake Bluff, IL
Sources of Failures in Carburized and Carbonitrided Components / 215
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_177-240.pdf/Chap_06/ 18/8/2008 3:21PM Plate # 0 pg 215
the furnace atmosphere; air contamination and
leakage (Fig. 51); heating in aged (deoxidized)
salt baths; and improper carbon potential selec-
tion (Ref 94).
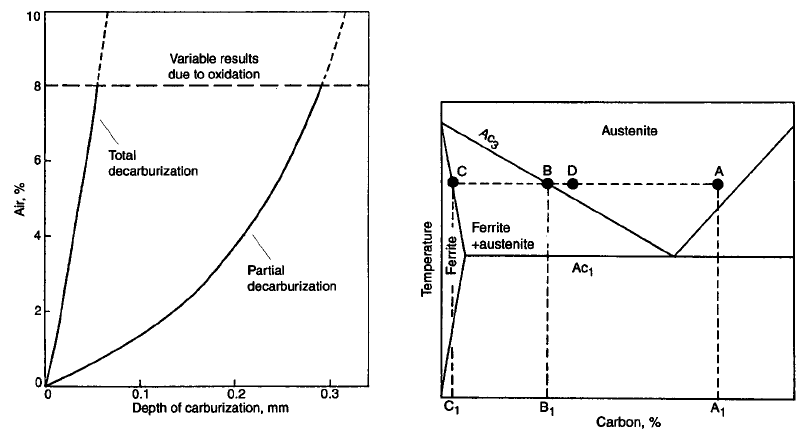
If a carburized steel is at a temperature above
the Ac
3
(approximately 900
C) in a decarbur-
izing atmosphere, the carbon potential will be
low and the surface carbon content will also be
low, since the carbon in the steel surface and the
related gaseous reactions shown previously will
be driven to this equilibrium condition. This will
result in a decarburized layer being produced,
and the depth of the decarburized layer will
depend on residence time in the furnace under
these conditions.
If the temperature of the steel is below Ac
3
and above Ac
1
(800 to 840
C), there is a dif-
ferent decarburization condition. In this case,
the carbon content rapidly decreases from “A”
to “B,” as shown in Fig. 52 (Ref 94). Further
decreases in carbon content will result in a
material of carbon content “C” in equilibrium
with material of carbon content “B”. Therefore,
further loss of carbon by decarburization must
result in the formation of ferrite containing
carbon content “C”.
If the atmosphere carbon content is controlled
to carbon potential “D”, then ferrite cannot form.
Instead, a gradient is formed between carbon
contents “A” and “D”.
Decarburization is typically classified as total
or partial. Figure 53(a) illustrates a case of total
decarburization of 1018 steel and is charac-
terized by a ferritic surface layer (Ref 94).
Usually, there is a gradient from total to partial
decarburization with increasing depth from the
surface. Partial decarburization is illustrated in
Fig. 53(b) and is often characterized by grain-
boundary ferrite at the surface (Ref 94). In this
case, nital etching will reveal a structure more
gray in color than would be achieved with a
higher-carbon martensite. The quenched surface
of partially decarburized steel is typically bai-
nitic or martensitic. The final microstructure
produced by a specific level of decarburization is
dependent on the steel alloy and cooling rate.
Decarburization is accompanied by surface
hardness reduction. However, partial or shallow
decarburization may not necessarily be detected
by macrohardness determination. If decarbur-
ization is suspected, microhardness determina-
tions, in view of their sensitivity to the presence
of such microstructures, should be used.
Decarburization can exhibit dramatic effects
on the residual stress of a component, as illu-
strated by Fig. 54 (Ref 94). In this example,
the surface carbon content of a carburized
3.5Ni-1.5Cr steel would be approximately 1%,
and the surface residual stresses would be
compressive at 4 392 MPa. After decarburiza-
tion reduces the carbon content to 0.64%,
the surface residual stresses were found to be
nearly 0. Finally, when decarburization re-
duced the surface carbon content to 0.35%,
Fig. 52
Iron-carbon equilibrium diagram to explain dec-
arburization
Fig. 51
Effect of air ingression into the carburization atmo-
sphere (N
2
/4% natural gas) on the decarburization of
SAE 8620 after 2 h at 850
C. Source: Ref 94
216 / Failure Analysis of Heat Treated Steel Components
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_177-240.pdf/Chap_06/ 18/8/2008 3:21PM Plate # 0 pg 216
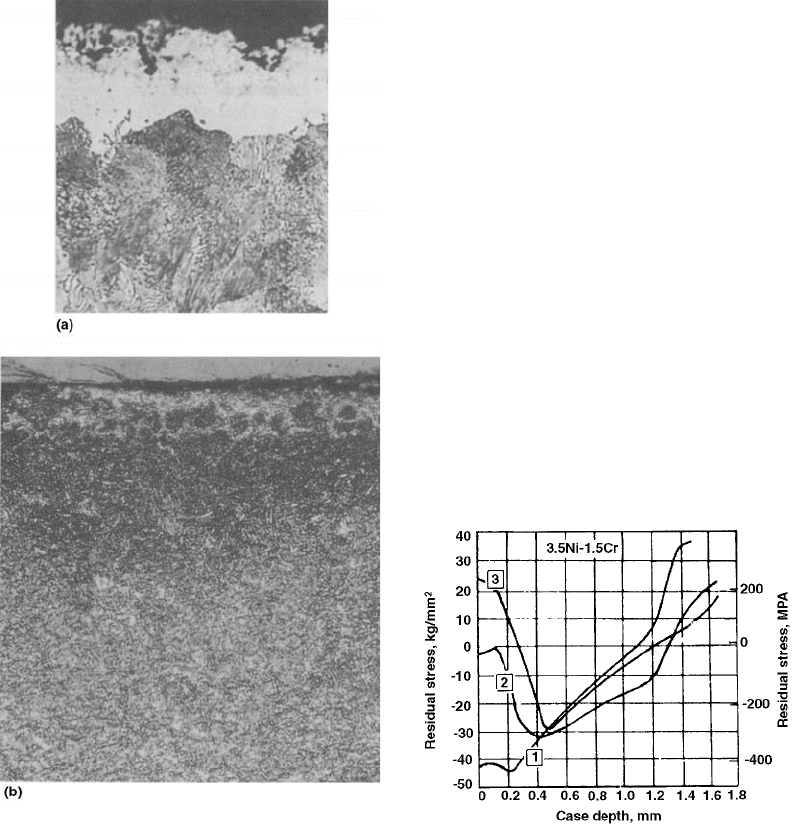
the surface residual stresses were tensile at
226 MPa.
Various reports have shown that decarbur-
ization can result in large decreases in bending
and contact fatigue strength (Ref 94). Since
wear-resistance properties are typically depen-
dent on achieving optimal hardness, reduction of
carbon content and thus surface hardness will
have a correspondingly adverse effect.
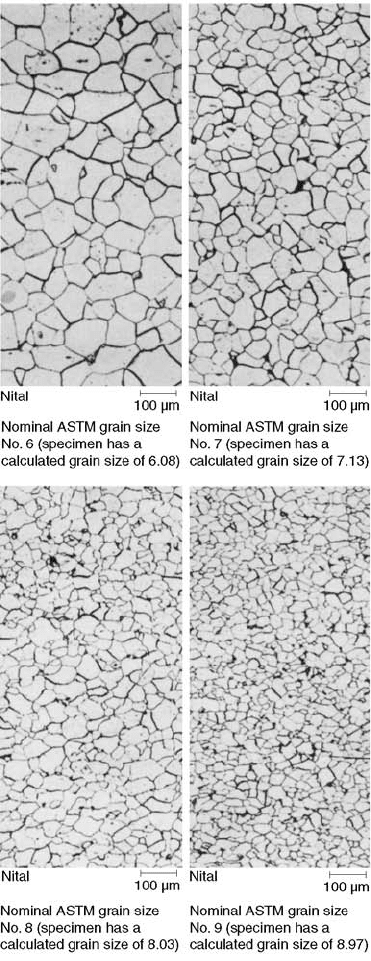
Influence of Grain Size
Grain size is one of the most important and
characteristic features of steel. Grain size in-
fluences mechanical and plastic properties,
especially impact resistance and also steel har-
denability. Grain size is characterized by the size
of the austenite grain, and it is dependent on
various factors, such as degree of cooling and the
deoxidation process during steelmaking (Ref
95). Generally, an ASTM grain size of 6 to 8 is
specified. Figure 55 illustrates grain sizes of
ASTM No. 6 to 9.
Grain growth is affected by temperature.
Typically, grain growth increases with tem-
perature and time at temperature. Aluminum
may be added to steel to provide resistance to
grain growth (grain refined). Alloying elements
such as nickel and molybdenum also provide
greater resistance to grain coarsening at typical
carburizing temperatures than plain carbon
steels. Although grain coarsening is usually not a
problem for carburizing temperatures up to
925
C, carburizing at temperatures greater than
1000
C is typically accompanied by some grain
coarsening, yielding a mixed-grain structure.
Reheating at 820 to 860
C can be performed to
refine the mixed-grain structure.
Fine-grained steels are less hardenable than
coarse-grained steels with the same composi-
tion, and this generalization is true for case
structure also. The slower the cooling of the steel
during steelmaking, the larger the grain, since
there are fewer nucleation sites formed. Steels
Fig. 53
Micrographs illustrating total and partial decarbur-
ization. (a) Total decarburization of 1018 steel
caused by a furnace air leak. Etchant: 1% nital. Original magni-
fication: 500·. (b) Illustration of partial decarburization. Original
magnification: 190·
Fig. 54
Effect of decarburization on the residual stresses of
carburized and hardened 3.5Ni-1.5Cr steel. The
carbon content at 0.002 mm was approximately 1% for curve 1,
0.64% for curve 2, and 0.35% for curve 3.
Sources of Failures in Carburized and Carbonitrided Components / 217
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_177-240.pdf/Chap_06/ 18/8/2008 3:21PM Plate # 0 pg 217
are typically deoxidized by silicon and manga-
nese prior to aluminum addition. This will
inhibit the undesirable formation of AlN or
Al
2
O
3
particles, which provide nucleation sites
for coarse-grained carbides that lead to the
formation of coarse ferrite/carbide (pearlitic)
grain structures on cooling.
Grain size is possible by control of the steel
composition during the steelmaking process.
Subsequent to this, control is by proper heat
treatment. Heating the steel to the upper critical
temperature, Ac
1
, will typically produce an
average minimum grain size. Heating to higher
temperatures will increase the grain size. Also,
quenching from the Ac
1
temperature will pro-
duce fine grain size, and quenching from a
higher temperature would yield a coarser grain
size.
The austenite grain size at the onset of trans-
formation during the quenching process will
influence the martensite platelet size and thus
will affect microcracking potential, the amount
of retained austenite formed, and the frequency
and depth of internal oxidation (Ref 95).
Coarse grain structure is observed on etched
microsections, most often in the form of coarse
grain structure of martensite relative to finer
retained austenite structure. Of the various fac-
tors affecting grain size, the primary factor is
furnace treatment. Although coarse-grained
steels exhibit better machinability, they gen-
erally possess lower toughness and ductility and
exhibit a greater tendency for distortion and
cracking than fine-grained steels. Coarse-
grained steel also exhibits a more limited range
of thermal treatment temperatures, and they
possess better hardenability with higher as-
quenched hardness. Additionally, coarse-
grained steels typically possess lower impact
resistance and a lower yield point.
Carburized steel case structures with a
coarse-grained structure, along with significant
amounts of overcarburizing, are characterized
by decreased mechanical properties. In this case,
the grain-boundary structure contains a con-
tinuous network carbide structure that is difficult
to remove, and within the grain structure there
are typically acicular carbides. Such micro-
structures are fatigue sensitive, with cracking
throughout the brittle network carbide structure.
The presence of these defective microstructures
can be prevented by controlling the carburizing
temperature and carbon potential or by using a
steel with characteristically fine-grained struc-
ture.
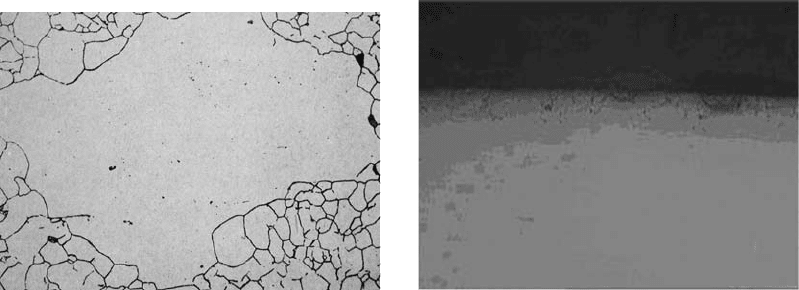
An example of grain size formation during
austenitization of steel 40 is shown in Fig. 56.
The irregular austenitic grain boundaries result
from the short heating time and also are related
to a prior normalizing and annealing process.
Fig. 55
Comparison of nominal ASTM No. 6 to 9 grain
sizes. Etchant: nital. Original magnification: 100· .
Source: Ref 96
218 / Failure Analysis of Heat Treated Steel Components
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_177-240.pdf/Chap_06/ 18/8/2008 3:21PM Plate # 0 pg 218
This thermal history produces the variable grain
size shown.
Isogai et al. reported that grain sizes of
approximately 8 were required for carburized
transmission gear steel to achieve the required
high fatigue strength, pitting strength, and
impact strength in severe-use environments (Ref
97). To assure a fine grain size, after the
carburizing process, the chromium-containing
steel, such as SCr420, was reheated to 820 to
870
C for 20 to 60 min in an atmosphere con-
taining the carburizing gas (carbon potential of
0.75%), quenched in oil until the steel was
120
C, and then tempered at 120 to 200
C.
Treatment of the carburized steel in this way
permits substantial reductions in grain size and
corresponding improvements in fatigue strength
and impact strength. Similar improvements were
achievable with carbonitrided steel (Ref 97).
Internal Oxidation (Ref 77)
The depth of internal oxidation that originates
the carburizing atmosphere may vary from 1 to
30 mm (Ref 93, 98, 99). Internal oxidation con-
sists of a continuous oxide layer on the surface,
on the order of 0.01 mm, due to oxygen reaction
with the carburized steel surface. In this region,
the oxygen content can be 10 to 20 times that of
the core. During the gas carburizing process,
oxides will not only form on the surface but also
penetrate into the steel surface. Since internal
oxide formation is a diffusion process, the depth
and extent of oxide penetration is dependent on
the square root of the total carburizing time and
temperature, as well as the steel alloy chemistry.
The total depth of internal oxidation can be
calculated from (Ref 100):
X
2
i
=½(2D
0
C
0
)=(n C
M
)t=k
p
t
X
i
=½k
p
t
0:5
where X
i
is the depth of oxygen penetration, D
0
is the diffusion coefficient of oxygen in the alloy,
C
0
is the oxygen concentration at the steel alloy
surface, C
M
is the concentration of the base
metal in the alloy (e.g., silicon, chromium,
manganese, titanium, vanadium), and n is the
stoichiometric factor.
Internal oxidation appears on the polished
metallographic specimen in the form of very
small inclusions concentrated in an austenite
grain or within the grain boundary. The prob-
ability of oxidation within the grain increases as
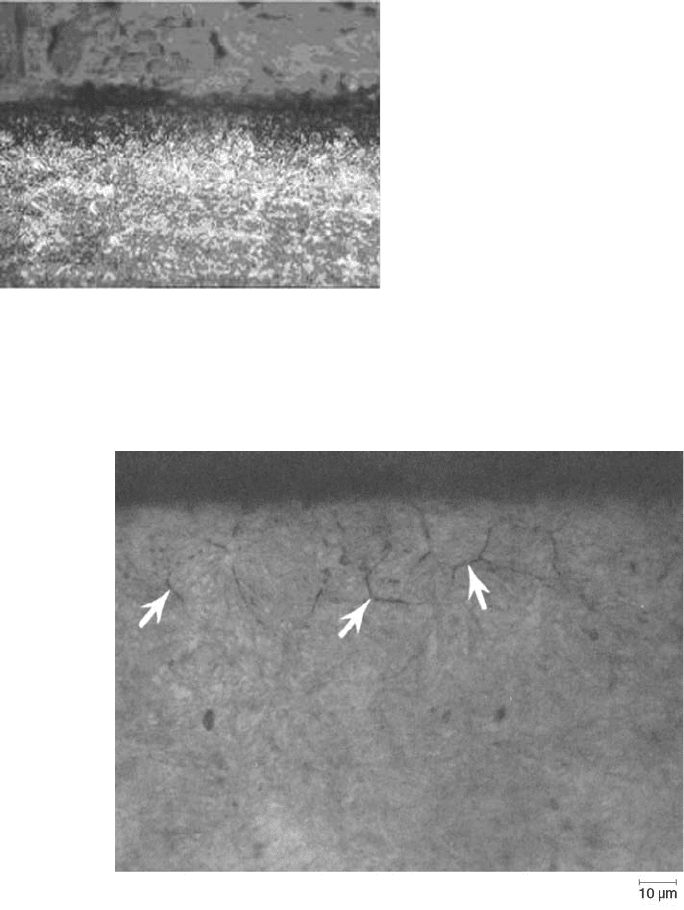
the grain size decreases (Ref 101). Figure 57
shows grain-boundary oxidation of carburized
20MnCr5 steel. Figure 58 also shows grain-
boundary oxidation, but it is accompanied by
nonmartensitic transformation products. Inter-
nal oxidation occurs in two zones: an inner zone
and an outer zone. Oxides of chromium-man-
ganese are typically formed in the outer zone,
both within the grain and in the grain boundaries.
In the inner zone, silicon-rich oxides are typi-
cally formed exclusively within the grain
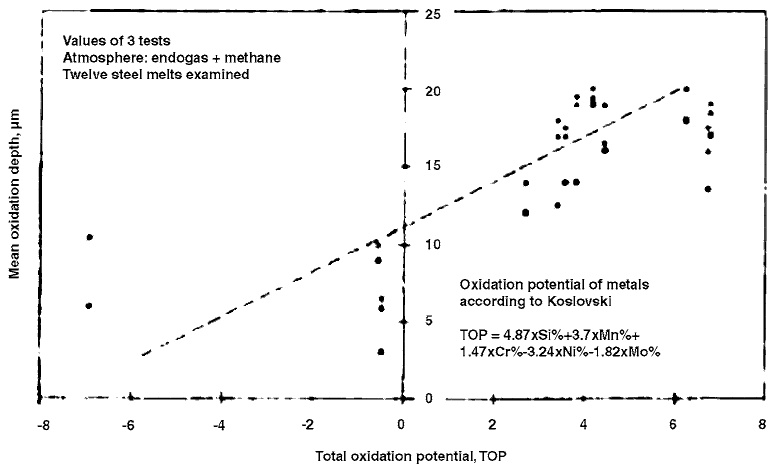
boundaries (Ref 102). Figure 59 also illustrates
intergranular oxidation of a gas-carburized steel
(Ref 79).
Typically, the greater the case depth, the
greater the degree of oxide formation at the
Fig. 56
Prior-austenite grains formed in hardened steel 40,
which were due to abnormal growth during the
austenitizing process, Etched: S. Bechet and L. Beaujurda. Ori-
ginal magnification: 500·
Fig. 57
Illustration of grain-boundary oxidation of carbur-
ized 20MnCr5 to a depth of 30 mm. Unetched. Ori-
ginal magnification: 200 · . Courtesy of Fluidtherm Technology P.
Ltd., Ambattur, India
Sources of Failures in Carburized and Carbonitrided Components / 219
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_177-240.pdf/Chap_06/ 18/8/2008 3:21PM Plate # 0 pg 219
surface and the greater the depth of internal
oxidation. The thickness of the internal oxida-
tion zone typically is approximately 5% of the
carburized layer thickness and, on occasion,
may be as high as 10%. The critical cooling rate
is greater in the internal oxidation zone. As a
result, an otherwise normal hardening produces
a greater amount of bainite, which will lead to
lower surface hardness and poorer abrasive wear
resistance.
Typically, the oxides in the outer surface
region are globular in form, while intergranular
oxides were formed further from the surface. In
one analyis of a carburized chromium-manga-
nese steel, larger globular oxides were formed in
the region closer to the surface (1.9 mm depth)
and intergranular oxides in the region farther
from the surface (2.49 mm) when the steel was
heated for 16.6 h (diffusion at 2 h at 800
C,
followed by 3 h at 930
C and a boost cycle at
930
C for additional heating times, in this case
11.6 h) (Ref 103). For this work, glow discharge
optical emission spectroscopy, in which a sput-
ter erosion process using ionized argon gas with
a voltage of 600 V and 25 mA was used to
quantify the degree of oxidation and elemental
distribution, was performed using energy-dis-
persive x-ray analysis. When the carburized
samples were subjected to shorter heating times,
intergranular oxides formed relatively farther
from the surface.
Transmission electron microscopy was used
by An et al. (Ref 103) to identify oxide type
and morphology. Chromium and manganese
globular oxides formed nearer the surface after a
total heat carburizing cycle of 5.8 h (5+0.8 h).
An agglomerated internal oxide of chromium
Fig. 58
Illustration of grain-boundary oxidation with non-
martensitic transformation products to a depth of
approximately 30 mm. Etchant: nital. Original magnification:
200·. Courtesy of Fluidtherm Technology P. Ltd., Ambattur,
India
Fig. 59
Intergranular oxidation of the surface along prior grain boundaries in a carburized steel. Original magnification: 1000· .
Source: Ref 78
220 / Failure Analysis of Heat Treated Steel Components
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_177-240.pdf/Chap_06/ 18/8/2008 3:21PM Plate # 0 pg 220
and manganese oxides in the core surrounded by
silicon oxides formed after heating for 16.6 h
(5+11.6 h) (Ref 103).
Internal oxide formation within grain bound-
aries provides sites for crack initiation (Ref 10).
This was shown in a study by Laue et al.
(Ref 104), who evaluated the fatigue behavior of
case-hardened SAE 5115 steel with internal
oxidation of the case structure. Fatigue studies
were conducted on this steel, and it was shown
that fatigue crack initiation occurred along the
oxidized grain boundaries of the steel (Ref 104).
Internal oxides form as a result of oxygen
diffusion into the surface, with subsequent for-
mation of metal oxides at carburizing tempera-
tures. The formation of these oxides is enhanced
by the presence of metals, chromium and man-
ganese and Cr-Mn-Ti, which possess a greater
affinity for oxygen than iron. The susceptibility
for internal oxidation increases with increasing
concentration of these oxide-forming elements.
Lohrmann et al., referring to earlier work by
Kozlovskii and co-workers, reported that the
depth of internal oxidation was dependent on the
total oxidation potential (TOP) of the steel alloy
(Ref 105, 106):
TOP=4:87 Si+3:7Mn+1:47 Cr 3:24 Ni
1:82 Mo
where the elemental compositions are given in
weight percent. Figure 60 provides a correlation
of the TOP and depth of internal oxidation (Ref
106).
In addition, Kozlovskii et al. reported that
most steels, when subjected to the gas carbur-
izing process, will undergo internal oxidation
with a corresponding surface formation of
troostite to a depth of 0.01 to 0.03 mm. If
troostite is formed at a depth greater than
0.014 mm, there is a substantial decrease in
fatigue strength. However, the potential for
internal oxide formation can be reduced by the
addition of 5 to 10% of ammonia to the furnace
for 10 min before the carburizing process is
completed or by using steels containing 0.5%
Mo and not more than 0.5% Cr (Ref 106).
Lohrmann showed that the form and type of
internal oxide obtained was dependent on the
alloy composition of the steel. For example,
depending on the steel alloy, spot-, liner-, or
lattice-type internal oxides could be formed
(Ref 105).
Kehr and Seese examined the effect of internal
oxidation during carburizing of investment-cast
ingot-iron test specimen steels containing var-
ious amounts of chromium (0.20, 0.45, 0.91,
1.85, and 4.74% Cr) (Ref 107). It was shown that
steels containing approximately 0.50% Cr, for
Fig. 60 Correlation of calculated total oxidation potential (TOP) and average depth of internal oxidation
Sources of Failures in Carburized and Carbonitrided Components / 221
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_177-240.pdf/Chap_06/ 18/8/2008 3:21PM Plate # 0 pg 221
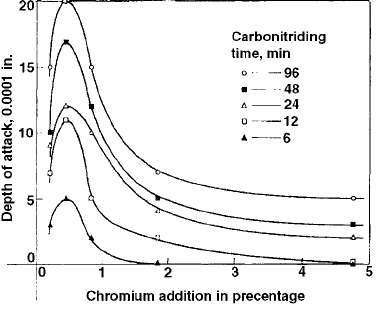
example, SAE 8620, were more susceptible to
internal oxidation than either plain carbon steel
or steels with greater amounts of chromium, such
as AISI 5120 (0.70 to 0.90% Cr). Figure 61
shows that the depth of oxide formation increases
as the chromium content increases up to 0.45%
(Ref 107).
The presence of internal oxidation on car-
burized steels greatly reduces bending and
contact fatigue strength and wear resistance
(Ref 93). However, surface oxides that are
formed may be removed by grinding or shot
peening. The potential for internal oxidation can
be reduced by heating steel to the carburizing
temperature under a nitrogen/hydrogen gas
mixture. During carburizing, these gases are
replaced by a carburizing atmosphere whose
oxygen activity is less than that required for the
formation of manganese II oxide or chromium
III oxide, and, in some cases, in the presence of
ammonia (Ref 100).
Finally, internal oxidation (and decarburiza-
tion) is known to lead to variations in the surface
compressive stresses in a carburized component
(Ref 108). There is one report of the presence of
internal oxidation leading to undesirable surface
tensile stresses that then led to subsequent
cracking of a carburized idler gear when used in
a diesel engine gearbox.
Carbides and Carbide Structure
Carbides formed during carburization are
treated as undesirable products to be avoided.
There are three types of carbides to be discussed
here: globular (or massive) carbides, network
carbides, and surface-film or flake carbides.
Carbides in steel are hard and brittle ceramic-
like interstitials with a high compressive
strength but low tensile strength (approximately
35 MPa, or 5000 psi).
Carbides in steel basically form when carbon
levels exceed the solubility limits of carbon in
the iron crystal structure. The allotropic nature
of iron also has different phase structures (i.e.,
crystal) with different solubility limits for car-
bon. For example, the maximum solubility of
carbon in the body-centered cubic (bcc) struc-
ture of ferrite is approximately 0.025 wt% at
723
C on the iron-carbon phase diagram (see
Appendix 9). For the face-centered cubic phase
of austenite (c), the maximum solubility limit of
carbon in c is approximately 2.06 wt% C at
1147
C. At still higher temperatures, another
type of bcc solid phase is d-ferrite. The max-
imum solubility of carbon in d-ferrite is
0.09 wt% C at 1493
C. A peritectic also occurs
at 0.16% C at 1493
C.
The iron-carbon system has eutectic trans-
formation at 1147
C during soldification, with
steel carbon levels of 2.06 to 6.67 wt% C. The
eutectic carbon concentration is 4.3%. Solid-
state transformations in steel include the well-
known eutectoid transformation at 733
C, with
a carbon concentration of 0.83%. At 733
C,
austenite transforms to pearlite. Pearlite is a
eutectoid mixture containing 0.83% C and is
characterized by a fine ferrite-cementite struc-
ture that forms upon austenite decomposition
during slow cooling.
The upper critical temperature (A
3
) is the
temperature below which ferrite starts to form
due to ejection from austenite in the hypoeu-
tectoid alloys. The lower critical temperature
(A
1
) is the temperature where the austenite-to-
pearlite eutectoid transformation occurs. Aus-
tenite does not exist below this temperature. A
2
is the temperature below which ferrite is ferro-
magnetic.
Cementite (Fe
3
C) is the principal carbide of
iron and carbon, with an orthrhombic crystal
structure. Cementite is harder and more brittle
than ferrite. Steel hardness increases with in-
creasing cementite content. Increasing carbon
content increases the amount of cementite but
does not affect the amount of ferrite present,
since ferrite is saturated with 0.22% C. If there is
less than 0.83% C, iron and carbon will combine
to form Fe
3
C until no carbon remains. The
Fig. 61
Effect of chromium content of steel on the depth of
oxidation
222 / Failure Analysis of Heat Treated Steel Components
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_177-240.pdf/Chap_06/ 18/8/2008 3:21PM Plate # 0 pg 222
cementite formed in this manner will combine
with the required amount of ferrite to form
pearlite, and any remaining ferrite will be in the
structure as free ferrite (proeutectoid ferrite).
Pearlite will form if the carbon content in the
austenite is 40.83%, and excess carbon will
form cementite. Excess cementite (proeutectoid
cementite) will deposit in the grain boundaries.
Because cementite (Fe
3
C) contains a specific
amount of carbon and iron, pearlite also contains
a specific amount of cementite and ferrite.
As a phase in steel, the chemical composition
of cementite will contain carbides of other car-
bide-forming elements, such as chromium and
manganese Alloying elements of chromium,
manganese, nickel, and other elements are, of
course, commonly used in alloy steels for
property improvement. They also impact the
properties of ferrite and cementite, because they
partition differently in the phases. For example,
chromium and manganese partition in cementite
instead of ferrite. However, nickel and silicon
tend to favor partitioning in ferrite. Chromium,
manganese, molybdenum, and titanium are thus
cementite stabilizers in steels, while nickel and
silicon are ferrite stabilizers. Interestingly, while
chromium, manganese, molybdenum, and van-
adium show no negative effect on cementite
formation, titanium, nickel, and silicon exhibit a
negative effect on cementite formation (Ref
109).
Cementite develops different morphologies
and distributions depending on the process of
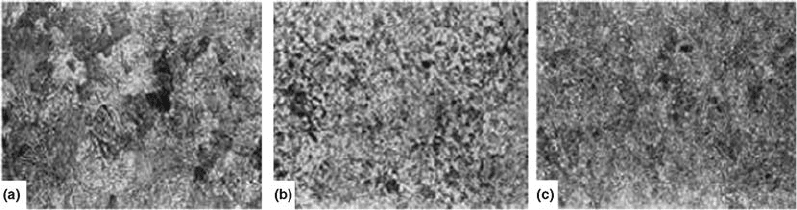
cementite formation. Figure 62 illustrates three
microstructural forms of cementite: lamellar,
mixed, and granular (Ref 110). Cementite may
also be classified as reticular, acicular, or gran-
ular (Ref 110). Reticular cementite, also known
as shell-type cementite, possesses a crack-
sensitive network or platelet structure. Acicular
cementite, or needlelike structure, refers to a
lamellar structure of cementite in ferrite, shown
in Fig. 62(b). Finally, cementite exhibits a
granular or grainy appearance, as shown in
Fig. 62(c).
Cementite forms during soldification from a
liquid or during solid-state transformations.
When cementite originates by crystallization
from a liquid melt, it is referred to as pri-
mary cementite (Fe
3
CI). Secondary cementite
(Fe
3
CII) is formed from austenite by hyper-
eutectoid alloys (carbon40.8%). Tertiary cem-
entite (Fe
3
CIII) is formed at temperatures below
723
C by precipitation in the grain boundaries
(which become richer in carbon with the
decreasing carbon content in a-iron).
Globular Carbides. Slowly heating a steel
to the carburizing temperature in the presence of
a carburizing atmosphere through the ferrite-to-
austenite temperature transformation region will
lead to unconnected globular carbide formation
either within the ferrite grains or at the former
ferrite grain boundaries, as shown in Fig. 63.
This process is favored by high carbon potentials
and also by reduction of normal heating rates
typically involved during carburizing, by
excessive furnace loading, or by a furnace mal-
function. Globular carbide formation may also
be enhanced by austenitic nuclei or by localized
concentrations of carbide-forming elements.
The problem of globular carbides may also
coexist with other problems, such as retaining
austenite or quench cracking.
When carburizing steels (0.15 to 0.25% C),
which are commonly ferritic with localized
areas of spheroidal carbides due to prior nor-
malizing and subcritical annealing, are heated
through the Ac
1
temperature, the high-carbon
regions begin to transform to austenite, resulting
in the formation of localized regions of carbon
and carbide-forming elements in addition to
undissolved carbides in the presence of the
Fig. 62 Cementite structures of CT60 steel with (a) lamellar, (b) mixed, and (c) granular cementite. Original magnification: 500·
Sources of Failures in Carburized and Carbonitrided Components / 223
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_177-240.pdf/Chap_06/ 18/8/2008 3:21PM Plate # 0 pg 223
