Askeland D.R., Fulay P.P. Essentials of Materials Science & Engineering
Подождите немного. Документ загружается.


backbone of carbon atoms; two hydrogen atoms are bonded to each carbon atom in the
chain. The chain twists and turns throughout space. In Figure 16-2, polyethylene has no
branches and hence is a linear thermoplastic. The simple two-dimensional model in
Figure 16-2(c) includes the essential elements of the polymer structure and will be used
to describe the various polymers. The single lines (aa) between the carbon atoms and
between the carbon and hydrogen atoms represent a single covalent bond. Two parallel
lines (bb) represent a double covalent bond between atoms. A number of polymers in-
clude ring structures, such as the benzene ring found in polystyrene and other polymers
(Figure 16-3).
In the structure shown in Figure 16-2(c), if we replace one of the hydrogen atoms in
CH
2
with a methyl group (CH
3
), a benzene ring, or chlorine, we get the structures of
polypropylene, polystyrene, and polyvinyl chloride (PVC), respectively. If we replaced
all H in CH
2
groups with fluorine (F), we get the structure of polytetrafluoroethylene or
Teflon
TM
. Like many other discoveries Teflon
TM
was also discovered by accident.
Many polymer structures can thus be derived from the structure of polyethylene. The
following example shows how di¤erent types of polymers are used.
Figure 16-3
Two ways to represent the benzene ring. In this
case, the benzene ring is shown attached to a pair
of carbon atoms, producing styrene.
EXAMPLE 16-1 Design/Materials Selection for Polymer Components
Design the type of polymer material you might select for the following appli-
cations: a surgeon’s glove, a beverage container and a pulley.
SOLUTION
The glove must be capable of stretching a great deal in order to slip onto the
surgeon’s hand, yet it must conform tightly to the hand to permit the max-
imum sensation of touch during surgery. A material that undergoes a large
amount of elastic strain—particularly with relatively little applied stress—
might be appropriate; this requirement describes an elastomer.
The beverage container should be easily and economically produced. It
should have some ductility and toughness so that it does not accidentally shat-
ter and leak the contents. If the beverage is carbonated, di¤usion of CO
2
is a
major concern (Chapter 5). A thermoplastic such as polyethylene terephthalate
(PET) will have the necessary formability and ductility needed for this appli-
cation.
The pulley will be subjected to some stress and wear as a belt passes over
it. A relatively strong, rigid, hard material is required to prevent wear, so a
thermosetting polymer might be most appropriate. It may also be possible to
use a material like nylon.
CHAPTER 16 Polymers500
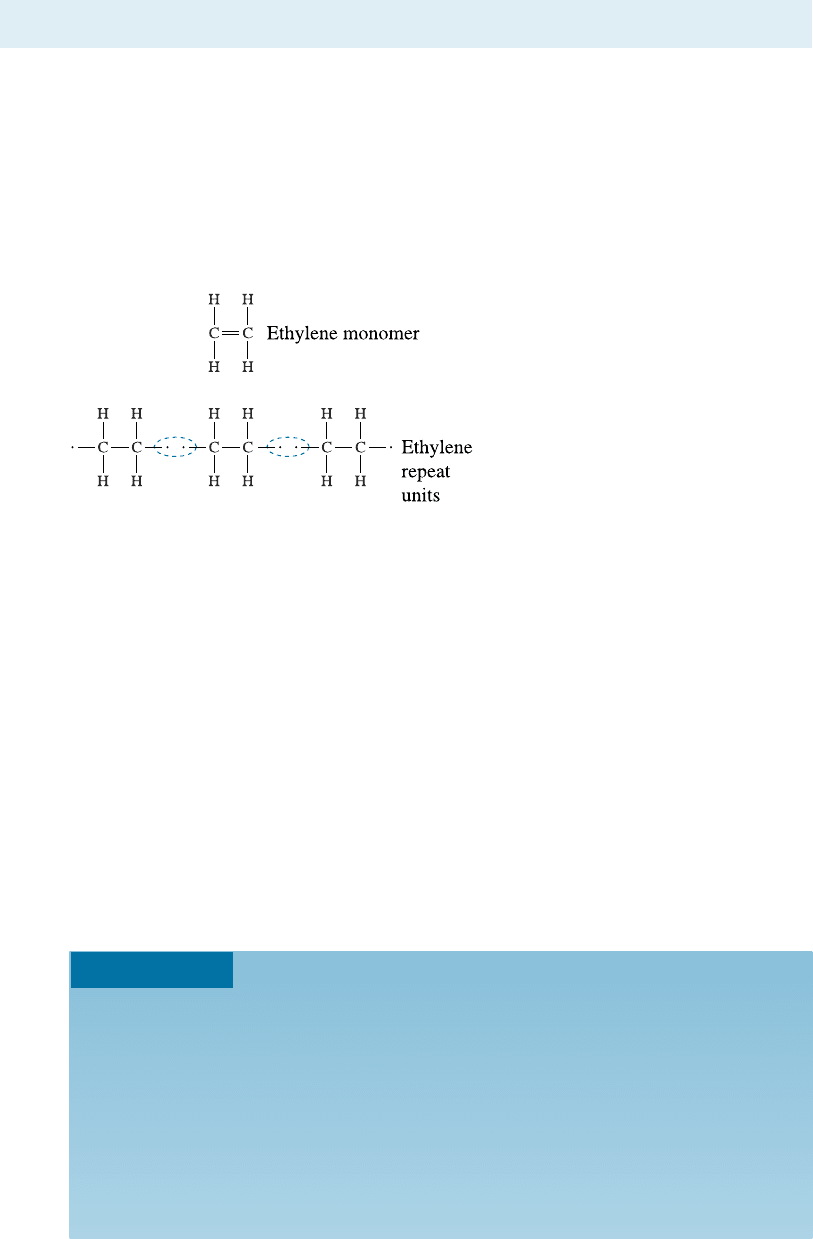
16-2 Addition and Condensation Polymerization
Addition polymerization and condensation polymerization are the two main ways to
conduct ‘‘polymerization’’ (creating a polymer). The polymers derived from these
processes are known as addition and condensation polymers, respectively. The for-
mation of the most common polymer, polyethylene (PE), from ethylene molecules is an
example of addition or chain-growth polymerization. Ethylene, a gas, is the monomer
(single unit) and has the formula C
2
H
4
. The two carbon atoms are joined by a double
covalent bond. Each carbon atom shares two of its electrons with the second carbon
atom, and two hydrogen atoms are bonded to each of the carbon atoms (Figure 16-4).
In presence of an appropriate combination of heat, pressure, and catalysts, the
double bond between the carbon atoms is broken and replaced with a single covalent
bond. The double bond is an unsaturated bond. After changing to a single bond, the
carbon atoms are still joined, but they become active; other repeat units or mers can be
added to produce the polymer chain.
We need polymers that have a designated average molecular weight and a molec-
ular weight distribution. Thus, the polymerization reactions must have an ‘‘o¤ ’’ switch
as well! The polymerization of chains may be terminated by two mechanisms. First, the
ends of two growing chains may be joined. This process, called combination, creates a
single large chain from two smaller chains. Second, the active end of one chain may
remove a hydrogen atom from a second chain by a process known as dis-
proportionation. This reaction terminates two chains, rather than combining two chains
into one larger chain. Sometimes, compounds known as terminators are added to end
the polymerization reactions. In general, for thermoplastics, the higher the average
molecular weight the higher will be the melting temperature and the higher will be the
Young’s modulus of the polymer (Section 16-3). The following example illustrates an
addition polymerization reaction.
EXAMPLE 16-2 Calculation of Initiator Required
Calculate the amount of benzoyl peroxide (BPO) initiator required to produce
1 kg of polyethylene with an average molecular weight of 200,000 g/mol. What
is the degree of polymerization? Assume that 20% of the initiator is actually
e¤ective and that all termination occurs by the combination mechanism.
SOLUTION
For 100% e‰ciency, we need one molecule of benzoyl peroxide for each poly-
ethylene chain. One of the free radicals would initiate one chain, the other free
radical a second chain; then, the two chains combine into one larger one. The
Figure 16-4
The addition reaction for producing
polyethylene from ethylene molecules.
The unsaturated double bond in the
monomer is broken to produce active
sites, which then attract additional
repeat units to either end to produce
a chain.
16-2 Addition and Condensation Polymerization 501
molecular weight of ethylene is ¼ (2 C)(12) þ (4 H)(1) ¼ 28 g/mol. Therefore,
the degree of polymerization is:
200;000 g=mol
28 g=mol
¼ 7143 ethylene molecules per average chain
ð1000 g polyethyleneÞð6:02 10
23
monomers/molÞ
28 g=mol
¼ 215 10
23
monomers
The combination mechanism requires the number of benzoyl peroxide mole-
cules to be:
215 10
23
ethylene molecules
7143 ethylenes=chain
¼ 0:03 10
23
chains
The molecular weight of benzoyl peroxide is (14 C)(12) þ (10 H)(1) þ
(4 O)(16) ¼ 242 g/mol. Therefore, the amount of initiator needed to form the
ends of the chains is:
ð0:03 10
23
Þð242 g=molÞ
6:02 10
23
¼ 1:206 g
However, only 20% of the initiator actually is e¤ective; the rest recombines or
combines with other molecules and does not cause initiation of a chain. Thus,
we need five times this amount, or 6.03 g of benzoyl peroxide per kilogram of
polyethylene.
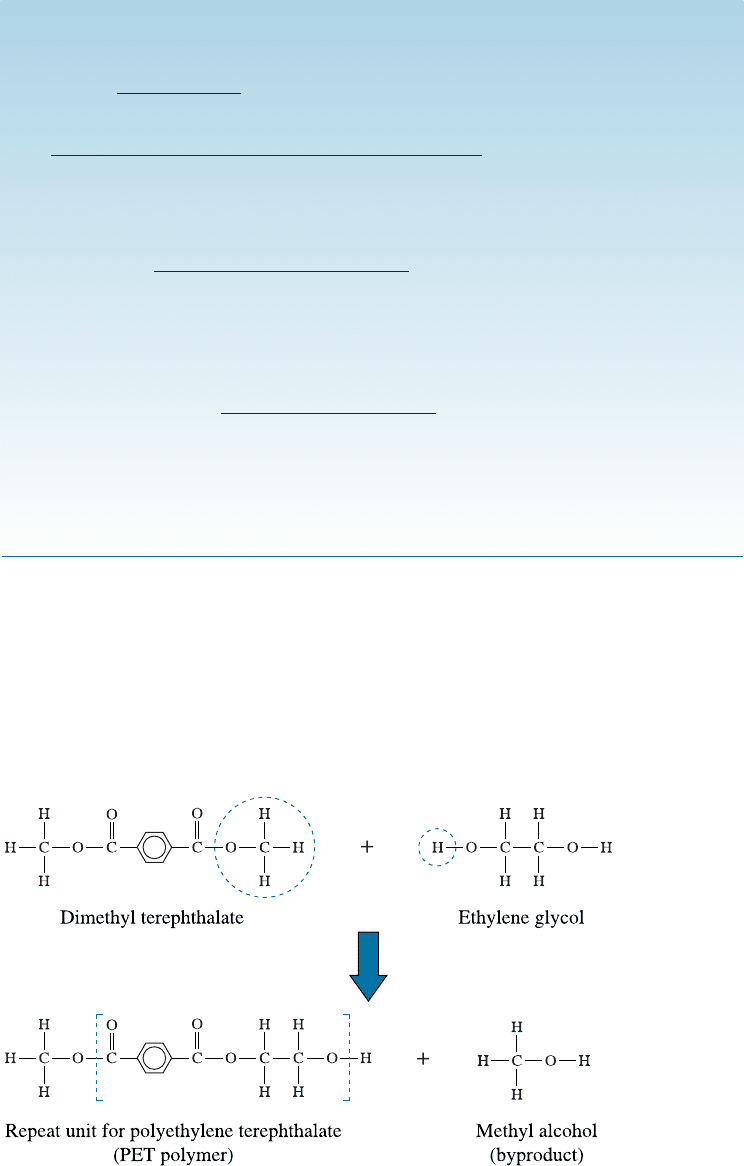
Condensation Polym erization Polymer chains can also form by condensation re-
actions, or step-growth polymerization, producing structures and properties that re-
semble those of addition polymers. In condensation polymerization, a relatively small
molecule (such as water, ethanol, methanol etc.) is formed as a result of the polymer-
ization reaction. This mechanism may often involve di¤erent monomers as starting or
precursor molecules. The polymerization of dimethyl terephthalate and ethylene glycol
(also used as radiator coolant) to produce polyester is an important example (Figure 16-5).
Figure 16-5 The condensation reaction for polyethylene terephthalate (PET), a common
polyester. The OCH
3
group and a hydrogen atom are removed from the monomers,
permitting the two monomers to join and producing methyl alcohol as a byproduct.
CHAPTER 16 Polymers502
During polymerization, a hydrogen atom on the end of the ethylene glycol monomer
combines with an OCH
3
(methoxy) group from the dimethyl terephthalate. A by-
product, methyl alcohol (CH
3
OH), is ‘‘condensed’’ o¤ and the two monomers combine
to produce a larger molecule. Each of the monomers in this example are bifunctional,
and the condensation polymerization can continue by the same reaction. Eventually, a
long polymer chain—a polyester—is produced. The repeat unit for this polyester con-
sists of two original monomers: ethylene glycol and dimethyl terephthalate. The length
of the polymer chain depends on the ease with which the monomers can di¤use to the
ends and undergo the condensation reaction. Chain growth ceases when no more mon-
omers reach the end of the chain to continue the reaction. Condensation polymerization
reactions also occur in sol-gel processing of ceramic materials.
The following example describes the discovery of nylon and calculations related to
condensation polymerization.
EXAMPLE 16-3
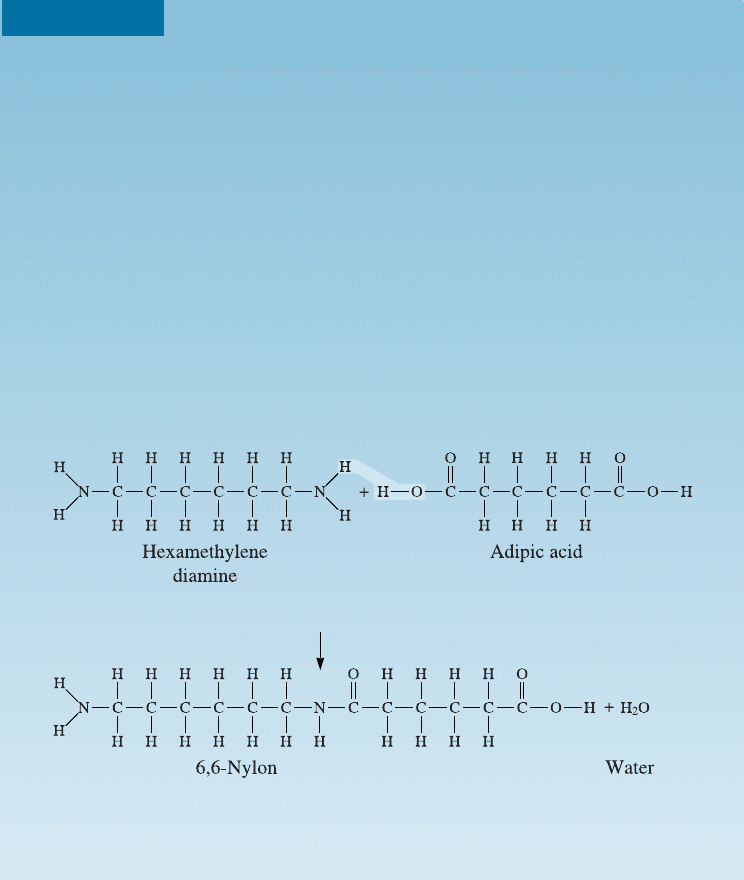
Condensation Polymerization of 6,6-Nylon
Nylon was first reported by Wallace Hume Carothers, of du Pont in about
1934. In 1939, du Pont’s Charles Stine reported the discovery of this first syn-
thetic fiber to a group of 3000 women gathered for the New York World’s
Fair. The first application was nylon stockings that were strong. Today nylon
is used in hundreds of applications. Prior to nylon, Carothers had discovered
neoprene (an elastomer).
The linear polymer 6,6-nylon is to be produced by combining 1000 g of
hexamethylene diamine (HMDA) with adipic acid. A condensation reaction
then produces the polymer. Show how this reaction occurs and determine the
byproduct that forms. How many grams of adipic acid are needed, and how
much 6,6-nylon is produced, assuming 100% e‰ciency?
SOLUTION
The molecular structures of the monomers are shown below. The linear nylon
chain is produced when a hydrogen atom from the hexamethylene diamine
combines with an OH group from adipic acid to form a water molecule.
Note that the reaction can continue at both ends of the new molecule;
consequently, long chains may form. This polymer is called 6,6-nylon because
both monomers contain six carbon atoms.
16-2 Addition and Condensation Polymerization 503

We can determine that the molecular weight of hexamethylene diamine
(HMDA) is 116 g/mol , of adipic acid is 146 g/mol, and of water is 18 g/mol.
The number of moles of hexamethylene diamine added (calculated below) is
equal to the number of moles of adipic acid:
1000 g
116 g=mol
¼ 8:621 moles ¼
x g
146 g=mol
x ¼ 1259 g of adipic acid required
The number of moles of water lost is also 8.621:
y ¼ð8:621 molesÞð18 g=molÞ¼155:2gH
2
O
But each time one more monomer is attached, another H
2
O is released.
Therefore, the total amount of nylon produced is
1000 g þ 1259 g 2ð155:2gÞ¼1948:6g:
16-3 Degree of Polymerization
Polymers, unlike organic or inorganic compounds, do not have a fixed molecular
weight. For example, polyethylene may have a molecular weight that ranges from
@25,000 to 6 million! The average length of a linear polymer is represented by
the degree of polymerization, or the number of repeat units in the chain. The degree of
polymerization can also be defined as:
Degree of polymerization ¼
average molecular weight of polymer
molecular weight of repeat unit
ð16-1Þ
If the polymer contains only one type of monomer, the molecular weight of the repeat
unit is that of the monomer. If the polymer contains more than one type of monomer,
the molecular weight of the repeat unit is the sum of the molecular weights of the
monomers, less the molecular weight of the byproduct.
The length of the chains in a linear polymer varies considerably. Some may be
quite short due to early termination; other may be exceptionally long. We can define an
average molecular weight in two ways.
The weight average molecular weight is obtained by dividing the chains into size
ranges and determining the fraction of chains having molecular weights within that
range. The weight average molecular weight
M
w
is
M
w
¼
X
f
i
M
i
ð16-2Þ
where M
i
is the mean molecular weight of each range and f
i
is the weight fraction of
the polymer having chains within that range.
The number average molecular weight
M
n
is based on the number fraction, rather
than the weight fraction, of the chains within each size range. It is always smaller than
the weight average molecular weight and is given by
M
n
¼
X
x
i
M
i
ð16-3Þ
CHAPTER 16 Polymers504
where M
i
is again the mean molecular weight of each size range, but x
i
is the fraction of
the total number of chains within each range. Either
M
x
or M
n
can be used to calculate
the degree of polymerization.
The two following examples illustrate these concepts.
EXAMPLE 16-4
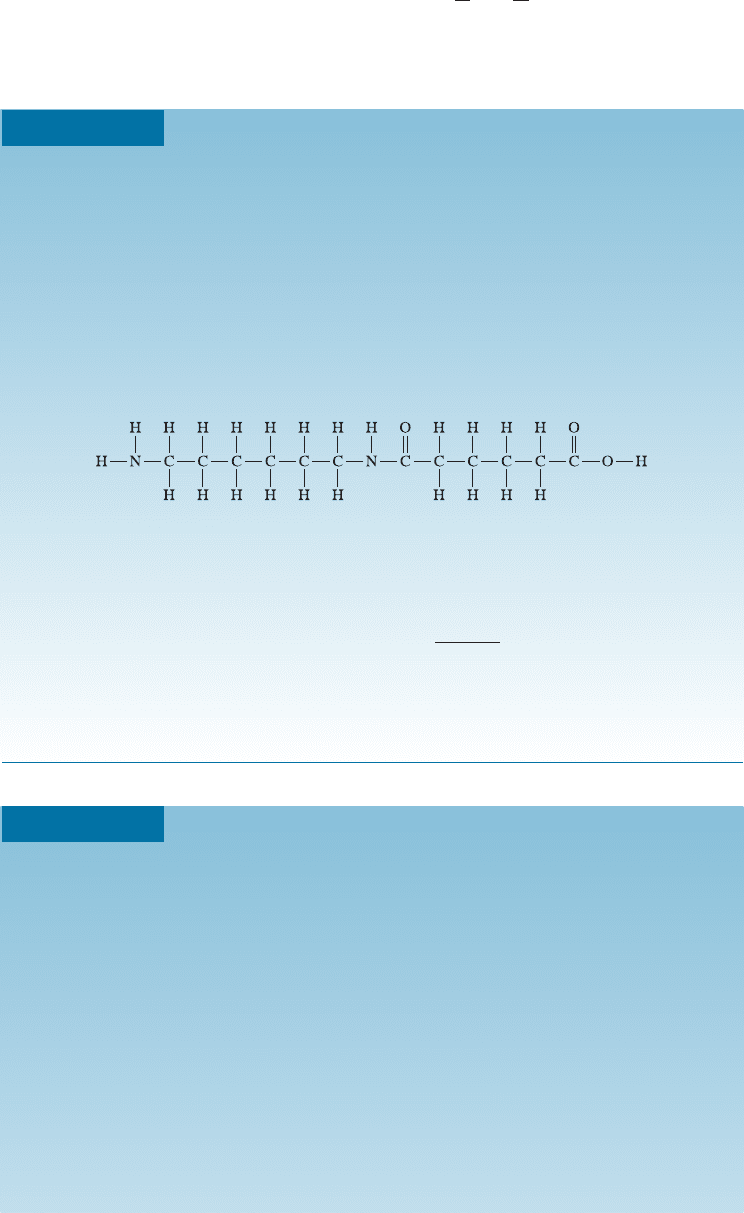
Degree of Polymerization for 6,6-Nylon
Calculate the degree of polymerization if 6,6-nylon has a molecular weight of
120,000 g/mol.
SOLUTION
The reaction by which 6,6-nylon is produced was described in Example 16-3.
Hexamethylene diamine and adipic acid combine and release a molecule of
water. When a long chain forms, there is, on an average, one water molecule
released for each reacting molecule. The molecular weights are 116 g/mol for
hexamethylene diamine, 146 g/mol for adipic acid, and 18 g/mol for water.
The repeat unit for 6,6-nylon is:
The molecular weight of the repeat unit is the sum of the molecular weights
of the two monomers, minus that of the two water molecules that are evolved:
M
repeat unit
¼ 116 þ 146 2ð18Þ¼226 g=mol
Degree of polymerization ¼
120;000
226
¼ 531
The degree of polymerization refers to the total number of repeat units in
the chain. The chain contains 531 hexameth ylene diamine and 531 adipic acid
molecules.
EXAMPLE 16-5
Number and Weight Average Molecular Weights
We have a polyethylene sample containing 4000 chains with molecular weights
between 0 and 5000 g/mol , 8000 chains with molecular weights between 5000
and 10,000 g/mol, 7000 chains with molecular weights between 10,000 and
15,000 g/mol, and 2000 chains with molecular weights between 15,000 and
20,000 g/mol. Determine both the number and weight average molecular
weights.
SOLUTION
First we need to determine the number fraction x
i
and weight fraction f
i
for each
of the four ranges. For calculating x
i
, we simply divide the number in each range
by 21,000, the total number of chains. To find f
i
, we first multiply the number
of chains by the mean molecular weight of the chains in each range, giving the
‘‘weight’’ of each group, then find f
i
by dividing by the total weight of 192:5
10
6
. We can then use Equations 16-2 and 16-3 to find the molecular weights.
16-3 Degree of Polymerization 505

Number of
Chains
Mean M
per Chain x
i
x
i
M
i
Weight f
i
f
i
M
i
4000 2500 0.191 477.5 10 10
6
0.0519 129.75
8000 7500 0.381 2857.5 60 10
6
0.3118 2338.50
7000 12,500 0.333 4162.5 87:5 10
6
0.4545 5681.25
2000 17,500 0.095 1662.5 35 10
6
0.1818 3181.50
P
¼ 21;000
P
¼ 1:00
P
¼ 9160
P
¼ 192:5 10
6
P
¼ 1
P
¼ 11;331
M
n
¼
X
x
i
M
i
¼ 9160 g=mol
M
w
¼
X
f
i
M
i
¼ 11;331 g=mol
The weight average molecular weight is larger than the number average
molecular weight.
16-4 Typical Thermoplastics
Some of the typical mechanical properties of typical thermoplastics are shown in Table
16-2.
TABLE 16-2 9 Properties of selected thermoplastics
Tensile
Strength
(MPa)
%
Elongation
Elastic
Modulus
(MPa)
Density
(g/cm
3
)
Izod
Impact
(J/cm)
Polyethylene (PE):
Low-density 21 800 276 0.92 4.9
High-density 38 130 1241 0.96 2.2
Ultrahigh molecular weight 48 350 690 0.934 16.2
Polyvinyl chloride (PVC) 62 100 4140 1.40
Polypropylene (PP) 41 700 1517 0.90 0.5
Polystyrene (PS) 55 60 3103 1.06 0.2
Polyacrylonitrile (PAN) 62 4 4000 1.15 2.6
Polymethyl methacrylate (PMMA) 83 5 3100 1.22 0.3
(acrylic, Plexiglas)
Polychlorotrifluoroethylene 41 250 2070 2.15 1.4
Polytetrafluoroethylene 48 400 550 2.17 1.6
(PTFE, Teflon)
Polyoxymethylene (POM) 83 75 3590 1.42 1.2
(acetal)
Polyamide (PA) 83 300 3450 1.14 1.1
(nylon)
Polyester (PET) 72 300 4140 1.36 0.3
Polycarbonate (PC) 76 130 2760 1.20 8.6
Polyimide (PI) 117 10 2070 1.39 0.8
Polyetheretherketone (PEEK) 70 150 3790 1.31 0.9
Polyphenylene sulfide (PPS) 66 2 3310 1.30 0.3
Polyether sulfone (PES) 84 80 2410 1.37 0.9
Polyamide-imide (PAI) 186 15 5030 1.39 2.2
CHAPTER 16 Polymers506
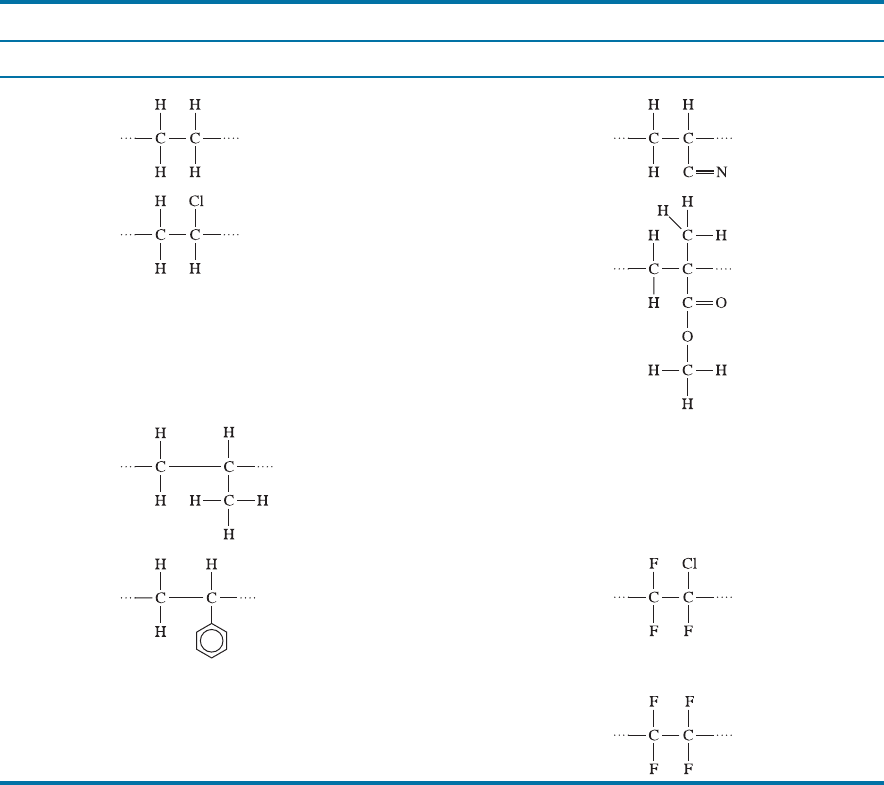
Because bonding within the chains is stronger, rotation and sliding of the chains is
more di‰cult, leading to higher strengths, higher sti¤nesses, and higher melting points
than the simpler addition polymers (Table 16-3). In some cases, good impact properties
can be gained from these complex chains, with polycarbonates being particularly
remarkable. Polycarbonates (Lexan
TM
, Merlon
TM
, and Sparlux
TM
) are used to make
bulletproof windows, compact disks for data storage, and in many other applications.
Thermoplastics with Complex Structures A large number of polymers, that typically
are used for special applications and in relatively small quantities, are formed from
complex monomers, often by the condensation mechanism. Oxygen, nitrogen, sulfur,
and benzene rings (or aromatic groups) may be incorporated into the chain. Table 16-4
shows the repeat units and typical applications for a number of these complex poly-
mers. Polyoxymethyl ene, or acetal, is a simple example in which the backbone of the
polymer chain contains alternating carbon and oxygen atoms. A number of these pol-
ymers, including polyimides and polyetheretherketone (PEEK), are importan t aero-
space materials.
TABLE 16-3 9 Repeat units and applications for selected addition thermoplastics
Polymer Repeat Unit Application Polymer Repeat Unit Application
Polyethylene
(PE)
Packing films, wire
insulation, squeeze
bottles, tubing,
household items
Polyacrylonitrile
(PAN)
Textile fibers,
precursor for
carbon fibers,
food container
Polyvinyl
chloride
(PVC)
Pipe, valves, fittings,
floor tile, wire
insulation, vinyl
automobile roofs
Polymethyl
methacrylate
(PMMA)
(acrylic-
Plexiglas)
Windows,
windshields,
coatings, hard
contact lenses,
lighted signs
Polypropylene
(PP)
Tanks, carpet fibers,
rope, packaging
Polystyrene
(PS)
Packaging and
insulation foams,
lighting panels,
appliance
components, egg
cartons
Polychlorotri-
fluoroethylene
Valve components,
gaskets, tubing,
electrical
insulation
Polytetrafluoro-
ethylene
(Teflon)
(PTFE)
Seals, valves,
nonstick
coatings
16-4 Typical Thermoplastics 507
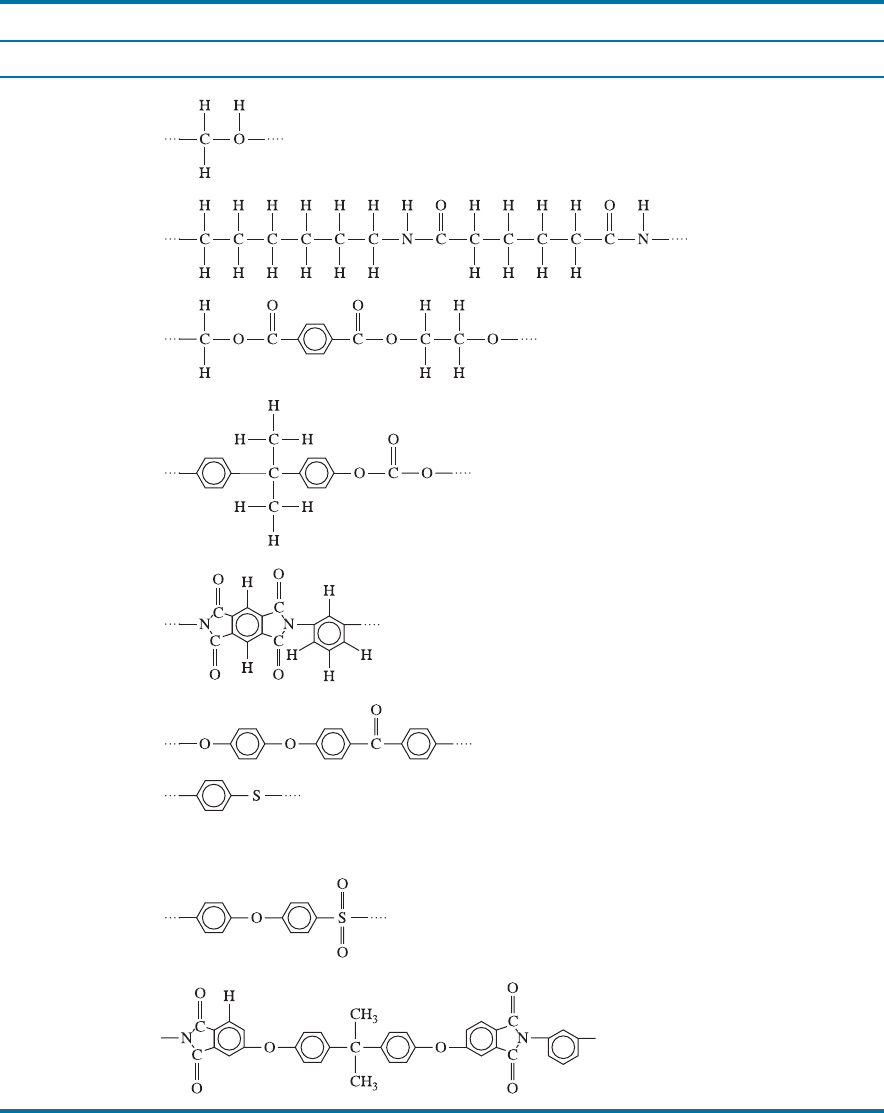
TABLE 16-4 9 Repeat units and applications for complex thermoplastics
Polymer Repeat Unit Applications
Polyoxymethylene
(acetal)(POM)
Plumbing fixtures, pens,
bearings, gears, fan
blades
Polyamide (nylon)
(PA)
Bearings, gears, fibers,
rope, automotive
components, electrical
components
Polyester (PET)
Fibers, photographic film,
recording tape, boil-in-
bag containers,
beverage containers
Polycarbonate (PC)
Electrical and appliance
housings, automotive
components, football
helmets, returnable
bottles
Polyimide (PI)
Adhesives, circuit boards,
fibers for space shuttle
Polyetheretherketone
(PEEK)
High-temperature
electrical insulation
and coatings
Polyphenylene
sulfide (PPS)
Coatings, fluid-handling
components, electronic
components, hair dryer
components
Polyether sulfone
(PES)
Electrical components,
coffeemakers, hair
dryers, microwave oven
components
Polyamide-imide
(PAI)
Electronic components,
aerospace and
automotive
applications
CHAPTER 16 Polymers508
16-5 Structure–Property Relationships in Thermoplastics
Degree of Polymerization In general, for a given type of thermoplastic (e.g., poly-
ethylene) the tensile strength, creep resistance, impact toughness, wear resistance, and
melting temperature all increase with increasing average molecular weight or degree of
polymerization. The increases in these properties are not linear. As the average mole-
cular weight increases, the melting temperature increases and this makes the processing
more di‰cult. In fact, we can make use of a bimodal molecular weight distribution in
polymer processing. One component has lower molecular weight and helps melting. In
certain applications (e.g., polystyrene for co¤ee cups), it is important to ensure that the
residual monomer concentration is extremely small (<1 ppm) because of the toxicity
concerns.
Effect of Side Groups In polyethylene, the linear chains easily rotate and slide when
stress is appli ed, and no strong polar bonds are formed between the chains; thus, poly-
ethylene has a low strength.
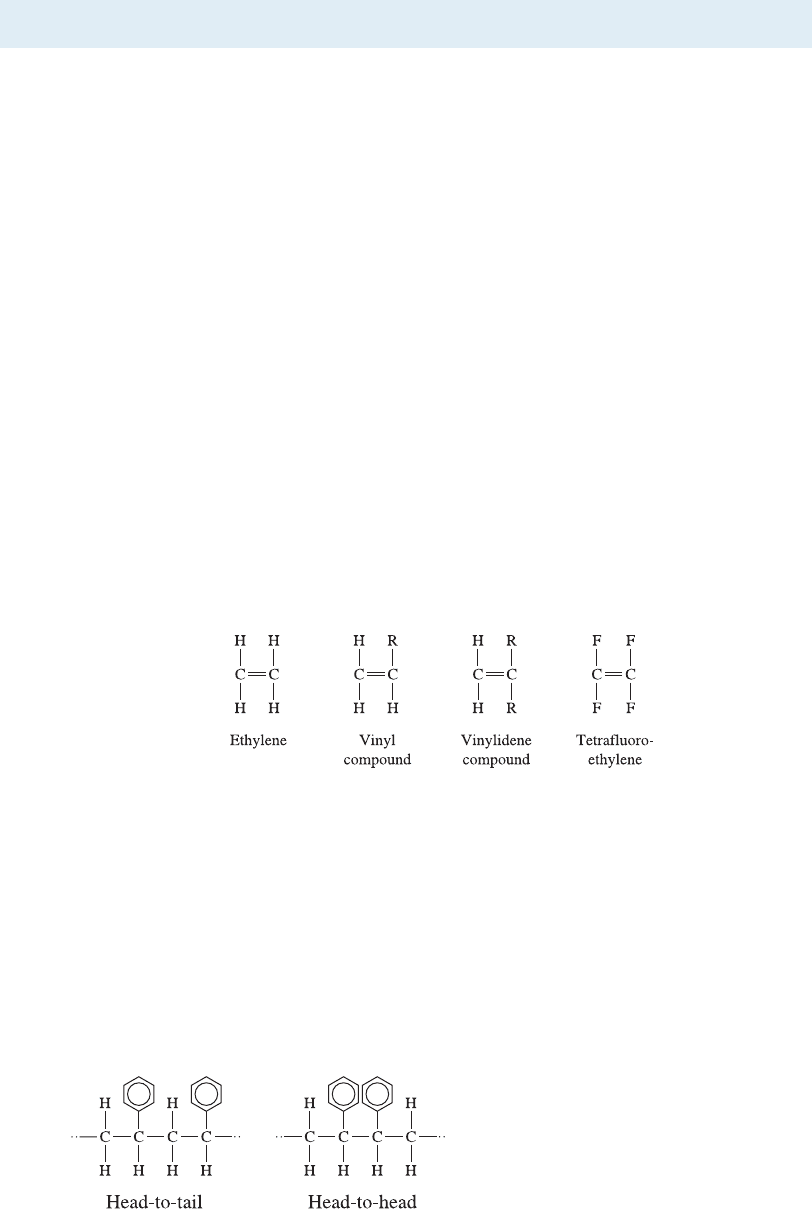
Vinyl compounds have one of the hydrogen atoms replaced with a di¤erent atom or
atom group. When R in the side group is chlorine, we produce polyvinyl chloride
(PVC); when the side group is CH
3
, we produce polypropylene (PP); addition of a
benzene ring as a side group gives polystyrene (PS); and a CN group produces poly-
acrylonitrile (PAN). Generally, a head-to-tail arrangement of the repeat units in the
polymers is obtained (Figure 16-6). When two of the hydrogen atoms are replaced, the
monomer is a vinylidene compound, importan t examples of which include poly-
vinylidene chloride (the basis for Saran Wrap
TM
) and polymethyl methacrylate (acrylics
such as Lucite
TM
and Plexiglas
TM
).
The e¤ects of adding other atom s or atom groups to the carbon backbone in place
of hydrogen atoms are illu strated by the typical properties given in Table 16-3. Larger
atoms such as chlorine or groups of atoms such as methyl (CH
3
) and benzene make
it more di‰cult for the chains to rotate, uncoil, disentangle, and deform by viscous
flow when a stress is applied or when the temperature is increased. This condition leads
to higher strength, sti¤ness, and melting temperature than those for polyethylene. The
chlorine atom in PVC and the carbon-nitrogen group in PAN are strongly attracted
by hydrogen bonding to hydrogen atoms on adjacent chains. This, for example, is the
reason why PVC is more rigid than many other thermoplastics. The way to get around
the rigidity of PVC is to add low molecular weight compounds such as phthalate esters,
Figure 16-6
Head-to-tail versus head-to-head
arrangement of repeat units. The head-to-
tail arrangement is most typical.
16-5 Structure–Property Relationships in Thermoplastics 509
