Askeland D.R., Fulay P.P. Essentials of Materials Science & Engineering
Подождите немного. Документ загружается.

known as plasticizers. When PVC contains these compounds, the glass temperature is
lowered. This makes PVC more ductile and workable and is known as vinyl (not to be
confused with the vinyl group mentioned here and in other places). PVC is used to
make three-ring binders, pipes, tiles, and clear Tygon
TM
tubing.
In polytetrafluoroethylene (PTFE or Teflon
TM
), all four hydrogen atoms in the
polyethylene structure are replaced by fluorine. The monomer again is symmetrical, and
the strength of the polymer is not much greater than that of polyethylene. However, the
C-F bond permits PTFE to have a high melting point with the added benefit of low
friction, nonstick characteristics that make the polymer useful for bearings and cook-
ware. Teflon
TM
was invented by accident by Roy Plunkett, who was working with
tetrafluoroethylene gas. He found a tetrafluoroethylene gas cylinder that had no pres-
sure (and, thus, seemed empty) but was still heavy. The gas inside had polymerized into
solid Teflon
TM
!
Branching prevents dense packing of the chains, thereby reducing the density, sti¤-
ness, and strength of the polymer. Low-density (LD) polyethylene, which has many
branches, is weaker than high-density (HD) polyethylene, which has virtually no
branching (Table 16-2).
Crystallization and Deformation Crystallinity is important in polymers since it a¤ects
mechanical and optical properties. Crystallinity evolves in the processing of polymers
as a result of temperature changes and applied stress (e.g., formation of PET bottles). If
crystalline regions become too large, they begin to scatter light and make the plastic
translucent. Of course, in certain special polymers, localized regions crystallize in re-
sponse to an applied electric field and this is the princi ple by which the liquid crystal
displays work. As we have discussed previously, encouraging crystallization of the pol-
ymer also helps to increase density, resistance to chemical attack, and mechanical
properties—even at higher temperatures—because of the stronger bonding between the
chains. In additi on, deformation straightens and aligns the chains, producing a pre-
ferred orientation. Deformation of a polymer is often used in producing fibers having
mechanical properties in the direction of the fiber that exceed those of many metals and
ceramics. This texture strengthening (Chapter 8), in fact, played a key role in the dis-
covery of nylon fibers. During their processing, PET bottles develop a biaxial texture
and strength along the radial and length direction.
Tacticity When a polymer is formed from nonsymmetrical repeat units, the structure
and properties are determined by the location of the nonsymmetrical atoms or atom
groups. This condition is called tacticity, or stereoisomerism. In the syndiotactic ar-
rangement, the atoms or atom groups alternatively occupy positions on opposite sides
of the linear chain. The atoms are all on the same side of the chain in isotactic polymers,
whereas the arrangement of the atoms is random in atactic polymers (Figure 16-7).
The atactic structure, which is the least regular and least predictable , tends to give
poor packing, low density, low strength and sti¤ness, and poor resistance to heat or
chemical attack. Atactic polymers are more likely to have an amorphous structure with
a relatively high glass temperature. An important example of the importance of tactic-
ity occurs in polypropylene. Atactic polypropylene is an amorphous wax-like polymer
with poor mechanical properties, whereas isotactic polypropylene may crystallize and is
one of the most widely used commercial polymers.
Copolymers Similar to the concept of solid solutions or the idea of composites, linear
addition chains composed of two or more types of molecules can be arranged to form
copolymers. This is a very powerful way to blend properties of di¤erent polymers. The
arrangement of the monomers in a copolymer may take several forms (Figure 16-8).
CHAPTER 16 Polymers510
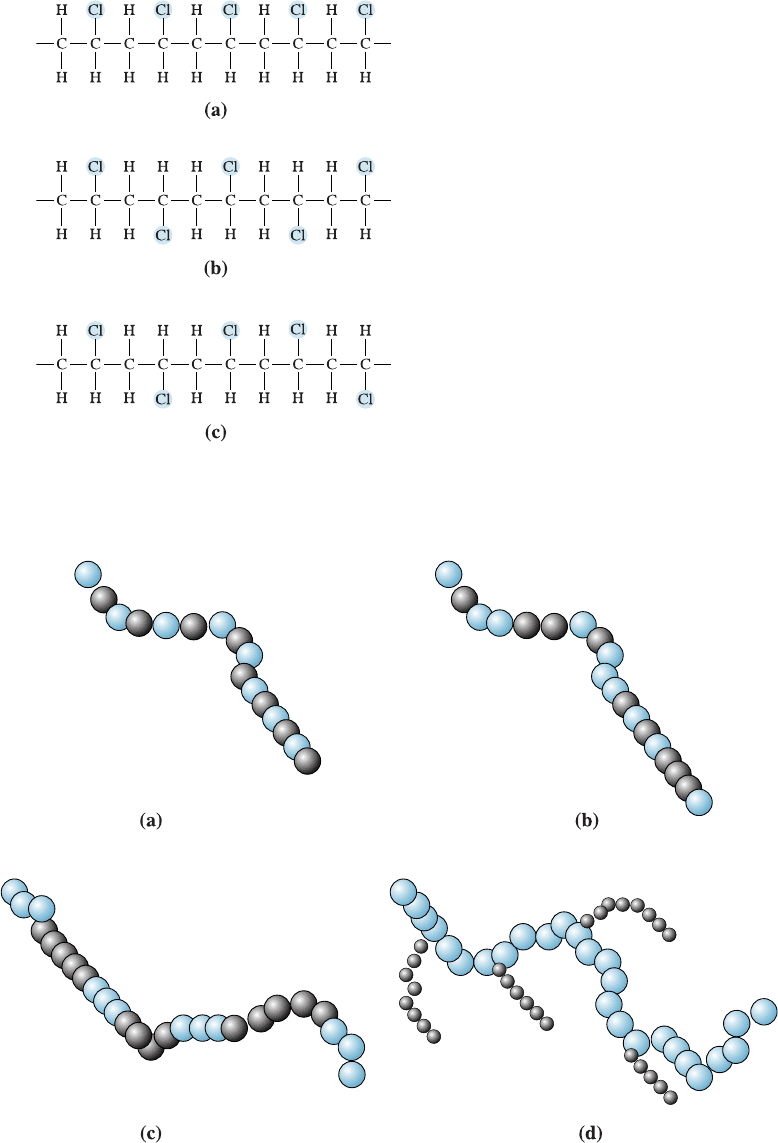
Figure 16-7
Three possible arrangements of
nonsymmetrical monomers: (a) isotactic,
(b) syndiotactic, and (c) atactic.
Figure 16-8 Four types of copolymers: (a) alternating monomers, (b) random monomers,
(c) block copolymers, and (d) grafted copolymers. Circles of different colors or sizes represent
different monomers.
16-5 Structure–Property Relationships in Thermoplastics 511

These include alternating, random, block, and grafted copolymers. A BS, composed of
acrylonitrile, butadiene (a synthetic elastomer), and styrene, is one of the most common
polymer materials (Figure 16-9). Styrene and acrylonitrile form a linear copolymer
(SAN) that serves as a matrix. Styrene and butadiene also form a linear copolymer, BS
rubber, which acts as the filler material. The combination of the two copolymers gives
ABS an excellent combination of strength, rigidity, and toughness. Another common
copolymer contains repeat units of ethylene and propylene. Whereas polyethylene and
polypropylene are both easily crystallized, the copolymer remains amorphous. When
this copolymer is cross-linked, it behaves as an elastomer. Dylark
TM
is a copolymer of
maleic anhydride and styrene. Styrene provides toughness, while maleic anhydride pro-
vides high-temperature properties. Carbon black (for protection from ultraviolet rays
and enhancing sti¤ness), rubber (for toughness), and glass fibers (for sti¤ness) are added
to the Dylark
TM
copolymer. It is used to make instrument panels for car dashboards.
The Dylark
TM
plastic is then coated with vinyl that provides a smooth and soft finish.
Blending and Alloying We can improve the mechanical properties of many of the
thermoplastics by blending or alloying. By mixing an immiscible elastomer with the
thermoplastic, we produce a two-phase polymer, as we found in ABS. The elastomer
does not enter the structure as a copolymer but, instead, helps to absorb energy and
improve toughness. Polycarbonates used to produce transparent aircraft canopies are
also toughened by elastomers in this manner.
Liquid Crystalline Polymers Some of the complex thermoplastic chains become so sti¤
that they act as rigid rods, even when heated above the melting point. These materials
are liquid crystalline polymers (LCPs). Some aromatic polyesters and aromati c poly-
amides (or aramids) are examples of liquid crystalline polymers and are used as high-
strength fibers (as to be discussed in Chapter 17). Kevlar
TM
, an aromatic polyamide, is
the most familiar of the LCPs and is used as a reinforcing fiber for aerospace applica-
tions and for bulletproof vests. Liquid crystal polymers are, of course , used to make
electronic displays.
16-6 Effect of Temperature on Thermoplastics
Properties of thermoplastics change depending upon temperature. We need to know
how these changes occur because this can help us (a) better design components, and (b)
guide the type of processing techniques that need to be used. Several critical temper-
atures and structures, summarized in Figures 16-10 and 16-11, may be observed.
Thermoplastics can be amorphous or crystalline once they cool below the melting
temperature, (Figure 16-10). Most often, engineered thermoplastics consist of regions
Figure 16-9 Copolymerization produces the polymer ABS, which is really made up of two
copolymers, SAN and BS, grafted together.
CHAPTER 16 Polymers512
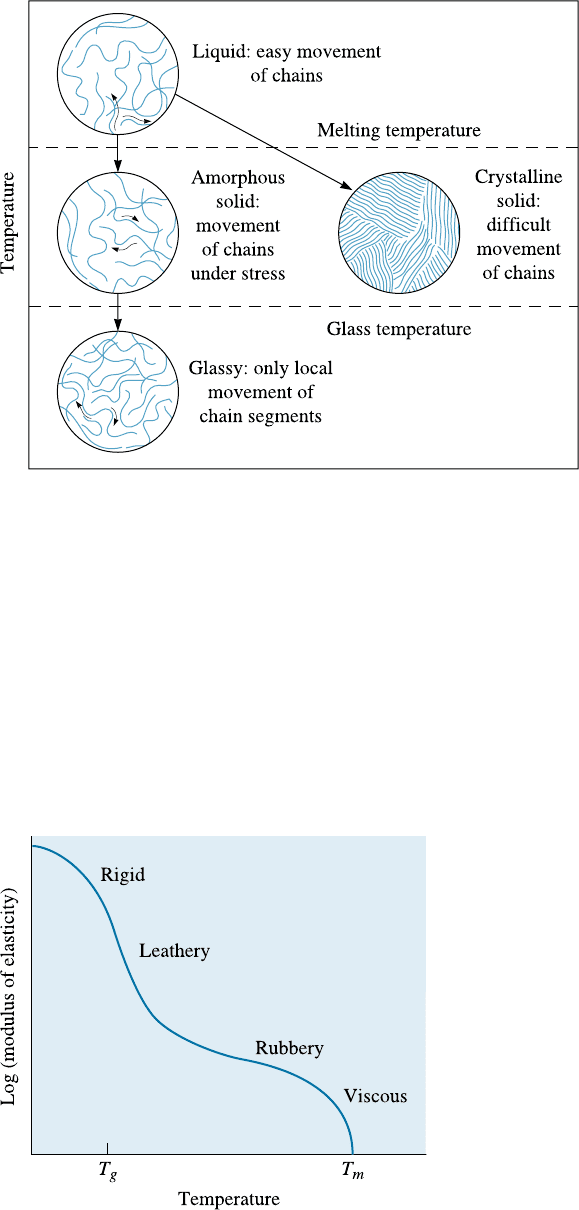
that are amorphous and crystalline. The crystallinity in thermoplastics can be in-
troduced by temperature (slow cooling) or by stress-induced crystallization (the appli-
cation of stress that can untangle chains, Chapter 7). Similar to dispersion strengthen-
ing of metallic materials, the formation of crystalline regions in an otherwise
amorphous matrix helps increase the strength of thermoplastics. In typical thermo-
plastics, bonding within the chains is covalent, but the long coiled chains are held to
one another by weak van der Waals bonds and by entanglement. When a tensile stress
is applied to the thermoplastic, the weak bonding between the chains can be overcome
and the chains can rotate and slide relative to one another. The ease with which the
chains slide depends on both temperature and the polymer structure.
Figure 16-10 The effect of temperature on the structure and behavior of thermoplastics.
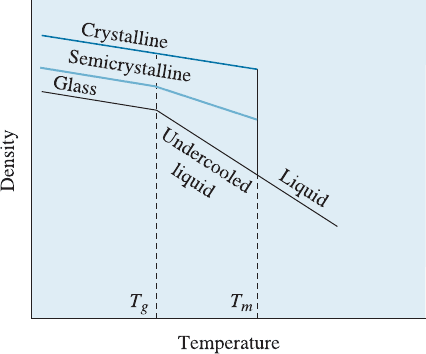
Figure 16-11
The effect of temperature on the
modulus of elasticity for an
amorphous thermoplastic. Note that
T
g
and T
m
are not fixed.
16-6 Effect of Temperature on Thermoplastics 513

Degradation Temperature At very high temperatures, the covalent bonds between the
atoms in the linear chain may be destroyed, and the polymer may burn or char. In
thermoplastics decompositio n occurs in the liquid state, in thermosets the decom-
position occurs in the solid state. This temperature T
d
(not shown in Figure 16-11), is
the degradation (or decomposition) temperature. When plastics burn, they create smoke,
and that is dangerous. Some materials (such as limestone, talc, alumina etc.) added to
thermoplastics are thermal or heat stabilizers. They absorb heat and protect the poly-
mer matrix. Fire retardant additives, such as hydrated alumina, antimony compounds,
or halogen compounds (e.g., MgBr, PCl
5
) are added to retard the flammability of poly-
mers. Some additives retard fire by excluding oxygen but generate dangerous gases and
are not appropriate for certain applications.
Exposure to other forms of chemicals or energy (e.g., oxygen, ultraviolet radiation,
and attack by bacteria) also cause a polymer to degrade or age slowly, even at low
temperatures. Carbon black (up to @3%) is one of the commonly used additives that
helps improve the resistance of plastics to ultraviolet degradation.
Liquid Polymers Thermop lastics usually do not melt at a precise temperature. Instead
there is usually a range of temperatures over which melting occurs. The approximate
melting ranges of typical polymers are included in Table 16-5. At or above the melting
temperature T
m
, bonding between the twisted and intertwined chains is weak. If a force
is applied, the cha ins slide past one another and the polymer flows with virtually no
TABLE 16-5 9 Melting, glass, and processing temperature ranges (
˚
C) for selected thermoplastics
and elastomers
Polymer
Melting
Temperature
Range
Glass
Temperature
Range (T
g
)
Processing
Temperature
Range
Addition polymers
Low-density (LD) polyethylene 98–115 90 to 25 149–232
High-density (HD) polyethylene 130–137 110 177–260
Polyvinyl chloride 175–212 87
Polypropylene 160–180 25 to 20 190–288
Polystyrene 240 85–125
Polyacrylonitrile 320 107
Polytetrafluoroethylene (Teflon) 327
Polychlorotrifluoroethylene 220
Polymethyl methacrylate (acrylic) 90–105
Acrylonitrile butadiene styrene (ABS) 110–125 100 177–260
Condensation polymers
Acetal 181 85
6,6-nylon 243–260 49 260–327
Cellulose acetate 230
Polycarbonate 230 149 271–300
Polyester 255 75
Polyethylene terephthalate (PET) 212–265 66–80 227–349
Elastomers
Silicone 123
Polybutadiene 120 90
Polychloroprene 80 50
Polyisoprene 30 73
CHAPTER 16 Polymers514
elastic strain. The strength and modulus of elasticity are nearly zero and the polymer is
suitable for casting and many forming processes. Most thermoplastic melts are shear
thinning (i.e., their apparent viscosity decreases within an increase in the steady-state
shear rate).
Rubbery and Leathery States Below the melting temperature, the polymer chains are
still twisted and intertwined. These polymers hav e an amorphous structure. Just below
the melting temperature, the polymer behaves in a rubbery manner. When stress is
applied, both elastic and plastic deformation of the polymer occurs. When the stress is
removed, the elastic deformation is quickly recover ed, but the polymer is permanently
deformed due to the movement of the chains. Some of this deformation is recovered
over a period of time. Thus, many polymers exhibit a viscoelastic behavior (Chapter 6).
Large permanent elongations can be achieved, permitting the polymer to be formed
into useful shapes by molding and extrusion.
At lower temperatures, bonding between the chains is stronger, the polymer
becomes sti¤er and stronger, and a leathery behavior is observed. Many of the com-
mercial polymers, including polyethylene, have a useable strength in this condition.
Glassy State Below the glass temperature T
g
, the linear amorphous polymer becomes
hard, brittle, and glass-like. This is again not a fixed temperature but a range of tem-
peratures. When the polymer cools below the glass temperature, certain properties—
such as density or modulus of elasticity—change at a di¤erent rate (Figure 16-12).
Although glassy polymers have poor ductility and formability, they do have good
strength, sti¤ness, and creep resistance. A number of important polymers, including
polystyrene and polyvinyl chloride, have glass temperatures above room temperature
(Table 16-5).
The glass temperature is typically about 0.5 to 0.75 times the absolute melting
temperature T
m
. Polymers such as polyethylene, which have no complicated side groups
attached to the carbon backbone, have low glass temperatures (even below room tem-
perature) compared with polymers such as polystyrene, which have more complicated
side groups.
As pointed out in Chapter 6, many thermoplasti cs become brittle at lower temper-
atures. The brittleness of the polymer used for some of the O-rings ultimately cau sed
the 1986 Challenger disaster. The lower temperatures that existed during the launch
time caused the embrittlement of the rubber O-rings used for the booster rockets.
Figure 16-12
The relationship between the density
and the temperature of the polymer
shows the melting and glass
temperatures. Note that T
g
and T
m
are not fixed; rather, they are ranges
of temperatures.
16-6 Effect of Temperature on Thermoplastics 515
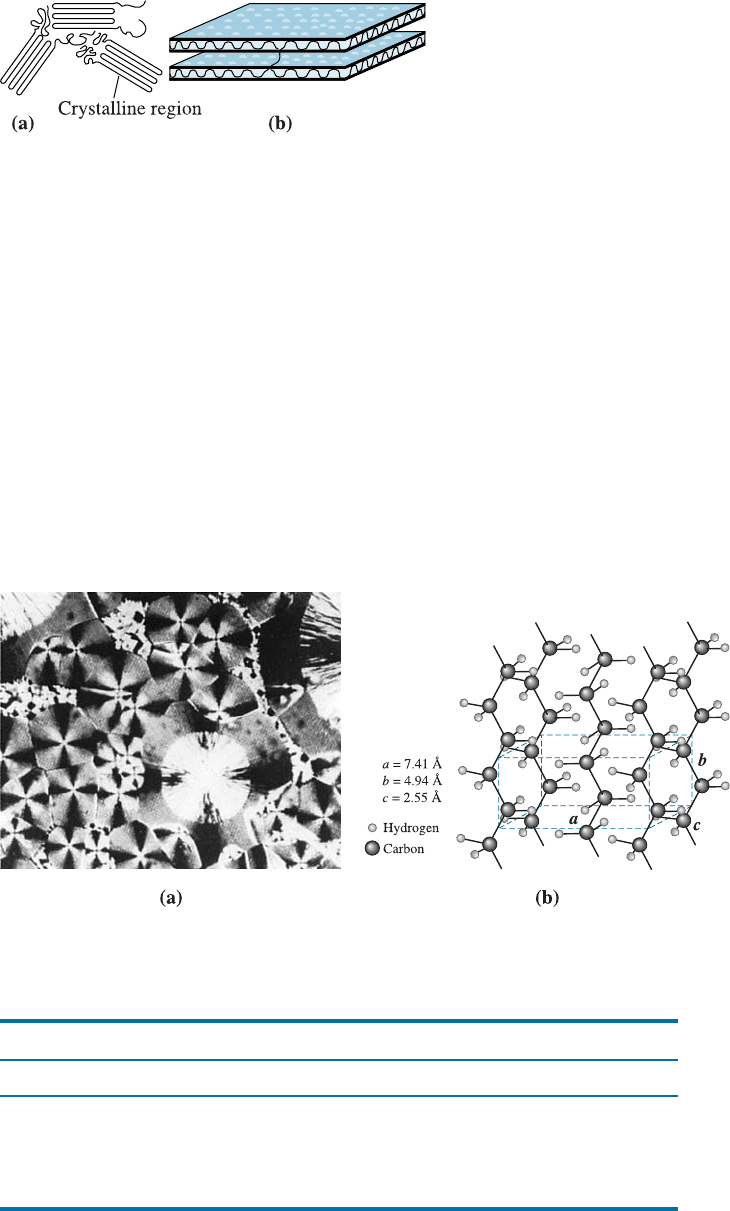
Observing and Measuring Crystallinity in Polymers Many thermoplastics partially
crystallize when cooled below the melting temperature, with the chains becoming
closely aligned over appreciable distances. A sharp increase in the density occurs as the
coiled and intertwined chains in the liquid are rearranged into a more orderly, close-
packed structure (Figure 16-12).
One model describing the arrangement of the chains in a crystalline polymer is
shown in Figure 16-13. In this folded chain model, the chains loop back on themselves,
with each loop being approximately 100 carbon atoms long. The folded chain extends
in three dimensions, producing thin plates or lamellae. The crystals can take various
forms, with the spherulitic shape shown in Figure 16-14(a) being particularly common.
The crystals have a unit cell that describes the regular packing of the chains. The crystal
structure for polyethylene, shown in Figure 16-14(b), describes one such unit cell.
Crystal structures for several polymers are described in Table 16-6. Some polymers are
polymorphic, having more than one crystal structure.
Even in crystalline polymers, there are always thin regions between the lamellae, as
well as between spherulites, that are amorphous transition zones. The weight percentage
Figure 16-13
The folded chain model for
crystallinity in polymers, shown in
(a) two dimensions and (b) three
dimensions.
Figure 16-14 (a) Photograph of spherulitic crystals in an amorphous matrix of nylon (200).
(Source: From R. Brick, A. Pense and R. Gordon , Structure and Properties of Engineering
Materials, 4th Ed., McGraw-Hill, 1997.) (b) The unit cell of crystalline polyethylene.
TABLE 16-6 9 Crystal structures of several polymers
Polymer Crystal Structure Lattice Parameters (nm)
Polyethylene Orthorhombic a ¼ 0:742 b ¼ 0:495 c ¼ 0:255
Polypropylene Orthorhombic a ¼ 1:450 b ¼ 0:569 c ¼ 0:740
Polyvinyl chloride Orthorhombic a ¼ 1:040 b ¼ 0:530 c ¼ 0:510
Polyisoprene (cis) Orthorhombic a ¼ 1:246 b ¼ 0 :886 c ¼ 0:810
CHAPTER 16 Polymers516
of the structure that is crystalline can be calculated from the density of the polymer:
% Crystalline ¼
r
c
r
ðr r
a
Þ
ðr
c
r
a
Þ
100 ð16-4Þ
where r is the measured density of the polymer, r
a
is the density of amorphous poly-
mer, and r
c
is the density of completely crystalline polymer. Similarly, x-ray di¤raction
(XRD) can be used to measure the level of crystallinity and dete rmination of lattice
constants for single crystal polymers.
As the side groups get more complex, it becomes harder to crystallize thermo-
plastics. For example, polyethylene (H as side group) can be crystallized more easily
than polystyrene (benzene ring as side group). High-density polyethylene (HDPE) has a
higher level of crystallinty and, therefore, a higher density (0.97 g/cc). Low-density
polyethylene (LDPE) has a density of 0.92 g/cc. The crystallinity and, hence, the den-
sity in LDPE is lower, since the polymer is branched. Thus, branched polymers show
lower levels of crystallinity. A completely crystalline polymer would not display a glass
temperature; however, the amorphous regions in semicrystalline polymers do change to
a glassy material below the glass temperature (Figure 16-12). Such polymers as acetal,
nylon, HDPE, and polypropylene are referred to as crystalline even though the level of
crystallinity may be moderate. Sometimes, the trade names can be confusing. For ex-
ample, what is described as a ‘‘crystal polystyrene’’ is actually an amorphous material.
It looks transparent and shiny though, and hence, the trade name crystal polystyrene.
The following examples show how properties of plastics can be accounted for in di¤er-
ent applications.
EXAMPLE 16-6
Design of a Polymer Insulation Material
A storage tank for liquid hydrogen will be made of metal, but we wish to coat
the metal with a 3-mm thickness of a polymer as an intermediate layer between
the metal and additional insulation layers. The temperature of the intermediate
layer may drop to 80
C. Design a material for this layer.
SOLUTION
We want the material to have reasonable ductility. As the temperature of the
tank changes, stresses develop in the coating due to di¤erences in thermal
expansion, and we do not want the polymer to fail due to these stresses.
A material that has good ductility and/or can undergo large elastic strains is
needed. We therefore would prefer either a thermoplastic that has a glass tem-
perature below 80
C or an elastomer, also with a glass temperature below
80
C. Of the polymers listed in Table 16-2, thermoplastics such as poly-
ethylene and acetal are satisfactory. Suitable elastomers include silicone and
polybutadiene.
We might prefer one of the elastomers, for they can accommodate thermal
stress by elastic, rather than plastic, deformation.
EXAMPLE 16-7 Impact-Resistant Polyethy lene
A new grade of flexible, impact-resistant polyethylene for use as a thin film
requires a density of 0.88 to 0.915 g/cm
3
. Design the polyethylene required
to produce these properties. The density of amorphous polyethylene is about
0.87 g/cm
3
.
16-6 Effect of Temperature on Thermoplastics 517
SOLUTION
To produce the required properties and density, we must control the percent
crystallinity of the polyethylene. We can use Equation 16-4 to determine the
crystallinity that corresponds to the required density range. To do so, however,
we must know the density of completely crystalline polyethylene. We can use
the data in Table 16-3 to calculate this density if we recognize that there are
two polyethylene repeat units in each unit cell:
r
c
¼
ð4CÞð12Þþð8HÞð1Þ
ð7:42Þð4:95Þð2:55Þð10
24
Þð6:02 10
23
Þ
¼ 0:9932 g=cm
3
We know that r
a
¼ 0:87 g/cm
3
and that r varies from 0.88 to 0.915 g/cm
3
. The
required crystallinity then varies from:
% crystalline ¼
ð0:9932Þð0:88 0:87Þ
ð0:88Þð0:9932 0:87Þ
100 ¼ 9: 2
% crystalline ¼
ð0:9932Þð0:915 0:87Þ
ð0:915Þð0:9932 0:87Þ
100 ¼ 39:6
Therefore, we must be able to process the polyethylene to produce a range of
crystallinity between 9.2 and 39.6%.
16-7 Mechanical Properties of Thermoplastics
Most thermopl astics (molten and solid) exhibit a non-Newtonian and viscoelastic
behavior. The behavior is non-Newtonian (i.e., the stress and strain are not linearly re-
lated for most parts of the stress-strain curve). The viscoelastic behavior means when an
external force is applied to a thermoplastic polymer, both elastic and plastic (or viscous)
deformation occurs. The mechanical behavio r is closely tied to the manner in which the
polymer chains move relative to one another under load. Deformation is more compli-
cated in thermoplastics. The deformation process depends on both time and the rate at
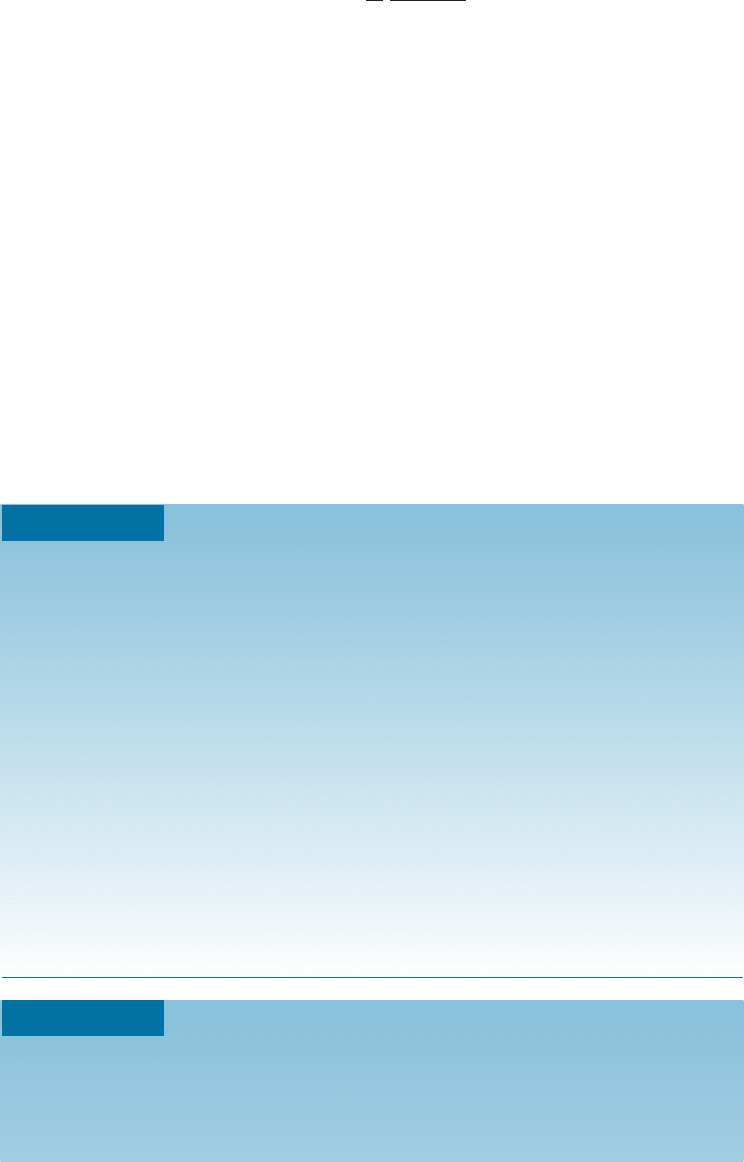
which the load is applied. Figure 16-15 shows a stress-strain curve for 6,6-nylon.
Figure 16-15
The stress-strain curve for 6,6-nylon,
a typical thermoplastic polymer.
CHAPTER 16 Polymers518
Elastic Behavior Elastic deformation in thermoplastics is the result of two mecha-
nisms. An applied stress cau ses the covalent bonds within the chain to stretch and dis-
tort, allowing the chains to elongate el astically. When the stress is removed, recovery
from this distortion is almost instantaneous. This behavior is similar to that in metals
and ceramics, which also deform elastically by the stretching of metallic, ionic, or co-
valent bonds. But in addition, entire segments of the polymer chains may be distorted;
when the stress is removed, the segments move back to their original positions only over
a period of time—often hours or even months. This time-dependent, or viscoelastic,
behavior may contribute to some nonlinear elastic behavior.
Plastic Behavior of Amorphous Thermoplastics These polymers deform plastically
when the stress exceeds the yield strength. Unlike deformation in the case of metals,
however, plastic deformation is not a consequence of dislocation movement. Instead,
chains stretch, rotate, slide, and disentangle under load to cause permanent deforma-
tion. The drop in the stress beyond the yield point can be explained by this phenom-
enon. Initially, the chains may be highly tangled and intertwined. When the stress is
su‰ciently high, the chains begin to untangle and straighten. Necking also occurs, per-
mitting continued sliding of the chains at a lesser stress. Eventually, however, the chains
become almost parallel and close together; stronger van der Waals bonding between the
more closely aligned chains requires higher stresses to complete the deformation and
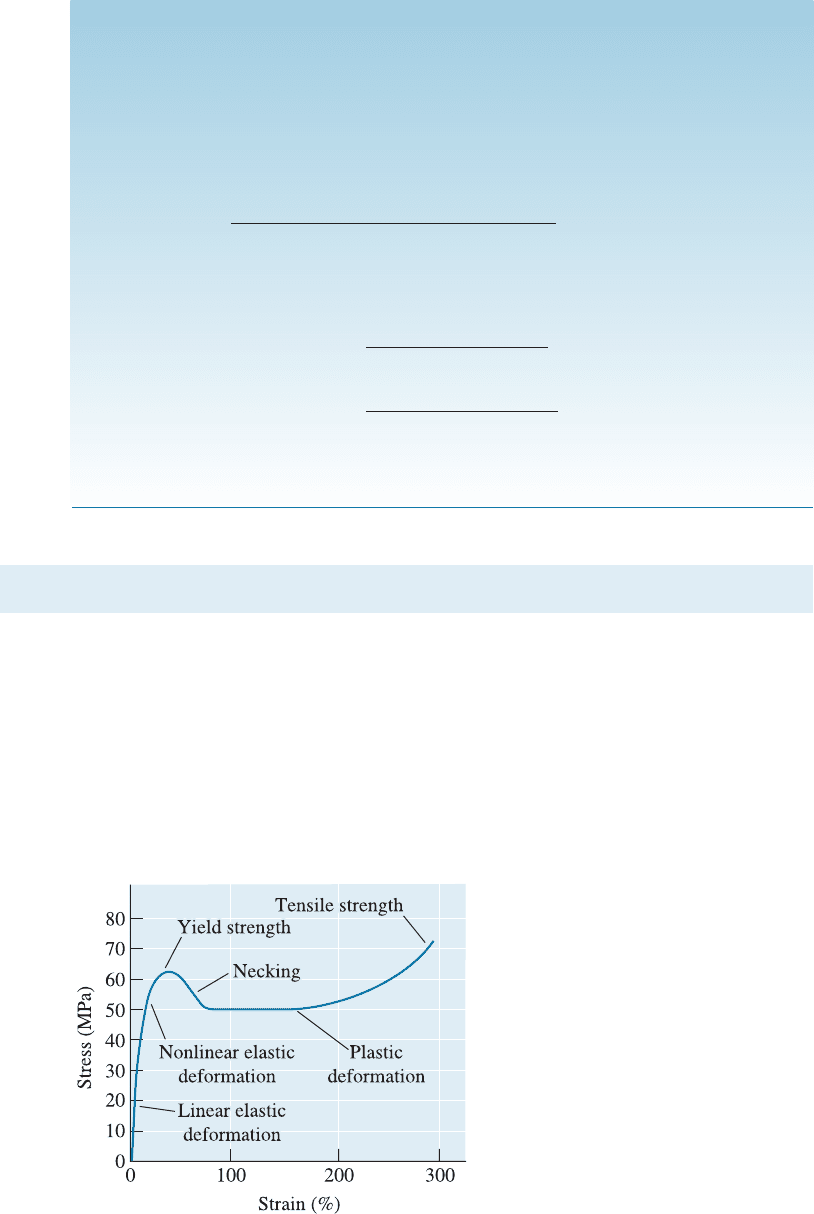
fracture process (Figure 16-16). This type of crystallization due to orientation played an
important role in the discovery of nylon as a material to make strong fibers.
Figure 16-16 Necks are not stable in amorphous polymers, because local alignment
strengthens the necked region and reduces its rate of deformation.
EXAMPLE 16-8
Comparing Mechanical Properties of Thermoplastics
Compare the mechan ical propertie s of LD polyethylene, HD polyethy lene,
polyvinyl chloride, polypropylene, and polystyrene, and explain their di¤er-
ences in terms of their structures.
16-7 Mechanical Properties of Thermoplastics 519
