Askeland D.R., Fulay P.P. Essentials of Materials Science & Engineering
Подождите немного. Документ загружается.


Phenolics Phenolics, the most commonly used thermosets, are often used as adhe-
sives, coatings, laminates, and molded components for electrical or motor applications.
Bakelite
TM
is one of the common phenolic thermosets. A condensation reaction
joining phenol and formaldehyde molecules produces the initial linear phenolic resin.
This process continues until a linear phenol-formaldehyde chain is formed. How-
ever, phenol is trifunctional. After the chain has formed, there is a third location on
each phenol ring that provides a site for cross-linking with the adjacent chains.
Amines Amino resins, produced by combining urea or melamine monomers with
formaldehyde, are similar to the phenolics. The monomers are joined by a form-
aldehyde link to produce linear chains. Excess formaldehyde provides the cross-linking
needed to give strong, rigid polymers suitable for adhesives, laminates, molding mate-
rials for cookware, and electrical hardware such as circuit breakers, switches, outlets,
and wall plates.
Urethanes Dependi ng on the degree of cross-linking, the urethanes behave as ther-
mosetting polymers, thermoplastics, or elastomers. These polymers find application as
fibers, coatings, and foams for furniture, mattresses, and insulation.
Polyesters Polyester s form chains from acid and alcohol molecules by a conde nsation
reaction, giving water as a byproduct. When these chains contain unsaturated bonds, a
styrene molecule may provide cross-linking. Polyesters are used as molding or casting
materials for a variety of electrical applications, decorative la minates, boats and other
marine equipment, and as a matrix for composites such as fiberglass.
Epoxies Epoxies are thermosetting polymers formed from molecules containing a
tight CaaOaaC ring. During polymerization, the CaaOaaC rings are opened and the
bonds are rearranged to join the molecules. The most common of the commercial ep-
oxies is based on bisphenol A, to which have been added two epoxide units. These
molecules are polymerized to produce chains and then co-reacted with curing agents
that provide cross-linking. Epoxies are used as adhesives, rigid molded parts for elec-
trical applications, automotive components, circuit boards, sporting goods and a matrix
for high-performance fiber-reinforced composite materials for aerospace.
Polyimides Polyimides display a ring structure that contains a nitrogen atom. One
special group, the bismaleimides (BMI), is important in the aircraft and aerospace
industry. They can operate continuously at temperatures of 175
C and do not decom-
pose until reaching 460
C.
Interpenetrating Polymer Networks Some special polymer materials can be produced
when linear thermoplastic chains are intertwined through a thermosetting framework,
forming interpenetrating polymer networks. For example, nylon, acetal, and poly-
propylene chains can penetrate into a cross-linked silicone thermoset. In more advanced
systems, two interpenetrating thermosetting framework structures can be produced.
16-10 Adhesives
Adhesives are polymers used to join other polymers, metals, ceramics, composites, or
combinations of these materials. The adhesives are used for a variety of applications.
The most critical of these are the ‘‘structural adhesives,’’ which find use in the auto-
motive, aerospace, appliance, electronics, construction, and sporting equipme nt areas.
CHAPTER 16 Polymers530

Chemically Reactive Adhesives These adhesives include polyurethane, epoxy, sili-
cone, phenolics, anaerobics, and polyimides. One-component systems consist of a single
polymer resin cured by exposure to moisture, heat, or—in the case of anaerobics—the
absence of oxygen. Two-component systems (such as epoxies) cure when two resins are
combined.
Evaporation or Diffusion Adhesives The adhesive is dissolved in either an organic
solvent or water and is applied to the surfaces to be joined. When the carrier evapo-
rates, the remaining polymer provides the bond. Water-base adhesives are preferred
from the standpoint of environmental and safety considerations. The polymer may be
completely dissolved in water or may consist of latex, or a stable dispersion of polymer
in water. A number of elastomers, vinyls, and acrylics are used.
Hot-Melt Adhesives These thermoplastics and thermoplastic elastomers melt when
heated. On cooling, the polymer solidifies and joins the materials. Typical melting
temperatures of commercial hot-melts are about 80
C to 110
C, which limits the ele-
vated-temperature use of these adhesives. High-performance hot-melts, such as poly-
amides and polyesters, can be used up to 200
C.
Pressure-Sensitive Adhesives These adhesives are primarily elastomers or elastomer
copolymers produced as films or coatings. Pressure is required to cause the polymer to
stick to the substrate. They are used to produce electrical and packaging tapes, labels,
floor tiles, wall coverings, and wood-grained textured films.
Conductive Adhesives A polymer adhesive may contain a filler material such as silver,
copper, or aluminum flakes or powders to provide electrical and thermal conductivity.
In some cases, thermal conductivity is desired but electrical conductivity is not wanted;
alumina, boron nitride, and silica may be used as fillers to provide this combination of
properties.
16-11 Polymer Processing and Recycling
There are a number of methods for producing polymer shapes, including molding,
extrusion, and manufacture of films and fibers. The techniques used to form the poly-
mers depend to a large extent on the nature of the polymer—in particular, whether it is
thermoplastic or thermosetting. The greatest variety of techniques are used to form the
thermoplastics. The polymer is heated to near or above the melting temperature so that
it becomes rubbery or liquid. The polymer is then formed in a mold or die to produce
the required shape. Thermoplastic elastomers can be formed in the same manner. In
these processes, scrap can be easily recycled and waste is minimized. Fewer forming
techniques are used for the thermosetting polymers because, once cross-linking has
occurred, the thermosetting polymers are no longer capable of being form ed. Elas-
tomers are processed in high-shear equipment such as a Banbury mixer. Carbon black
and other additives are added. The heating from viscoelastic deformation can begin to
cross-link the material prematurely. After the mixing step, a curing agent (e.g., zinc
oxide) is added. The material discharged from the mixer is pliable and is processed
using a short extruder, molded using a two-roll mill, or applied on parts by dip coating.
This processing of elastomers is known as compounding of rubber.
The following are some of the techniques mainly used for processing of polymers;
most of these, you will note, apply only to thermoplastics.
16-11 Polymer Processing and Recycling 531
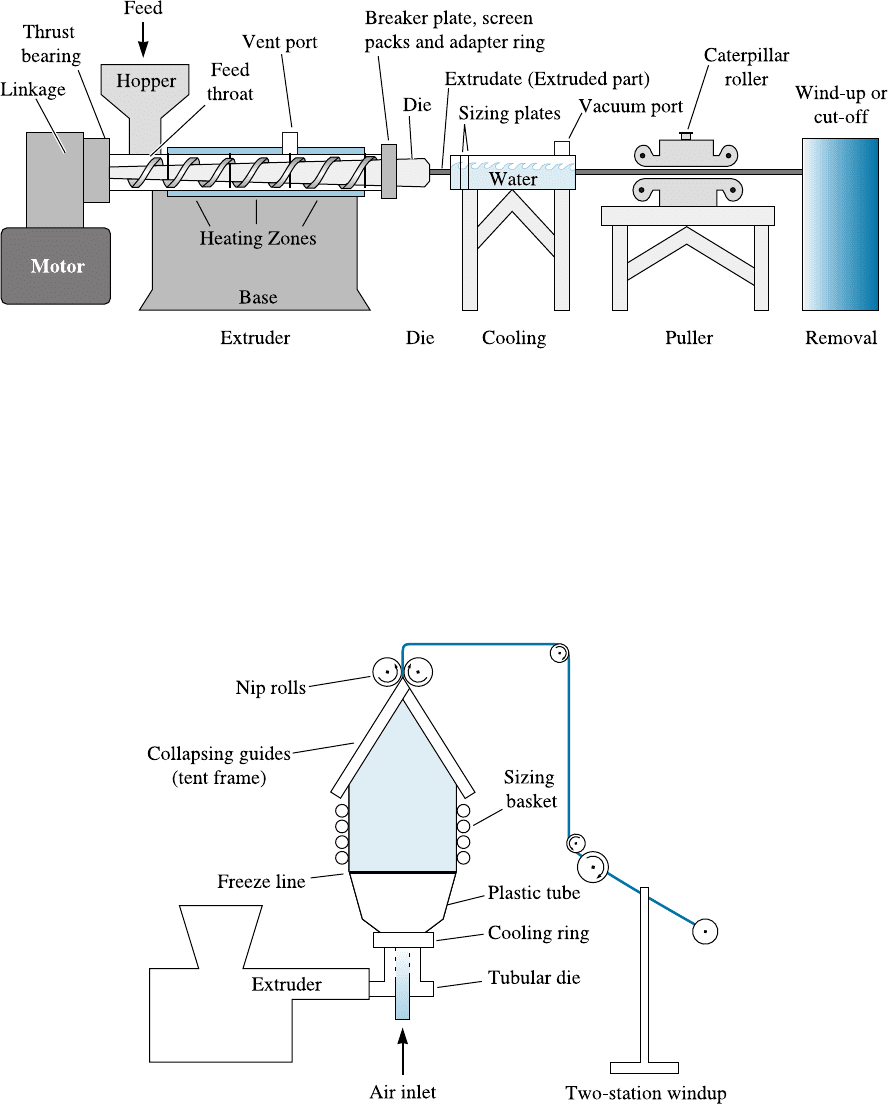
Extrusion This is the most widely used technique for processing thermoplastics.
Extrusion can serve two purposes. First, it provides a way to form certain simple shapes
continuously (Figure 16-24). Second, extrusion provides an excellent mixer for additives
(e.g., carbon black, fillers, etc.) when processing polymers (Figure 16-25) that ultimately
may be processed using some other process. A screw mechanism consisting of one or
Figure 16-24 Schematic of an extruder used for polymer processing. (Source: Strong, A.
Brent, Plastics: Materials and Processing, 2nd, > 2000. Electronically reproduced by
permission of Pearson Education, Inc., Upper Saddle River, New Jersey. )
Figure 16-25 One technique by which polymer films (used in the manufacture of garbage
bags, for example) can be produced. The film is extruded in the form of a bag, which is
separated by air pressure until the polymer cools. (Source: Strong, A. Brent, Plastics: Materials
and Processing, 2nd, > 2000. Electronically reproduced by permission of Pearson Education,
Inc., Upper Saddle River, New Jersey.)
CHAPTER 16 Polymers532
a pair of screws (twin screw) forces heated thermoplastic (either solid or liquid) and
additives through a die opening to produce solid shapes, films, sheets, tubes, pipes, and
even plastic bags (Figure 16-24). An industrial extruder can be up to 18 m to 21 m long,
60 cm in diameter, and consist of di¤erent heating or cooling zones. Since thermo-
plastics show shear thinning behavior and are viscoelastic, the control of both temper-
ature and viscosity is critical in polymer extrusion. One special extrusion process for
producing films is illustrated in Figure 16-25. Extrusion also can be used to coat wires
and cables with either thermoplastics or elastomers.
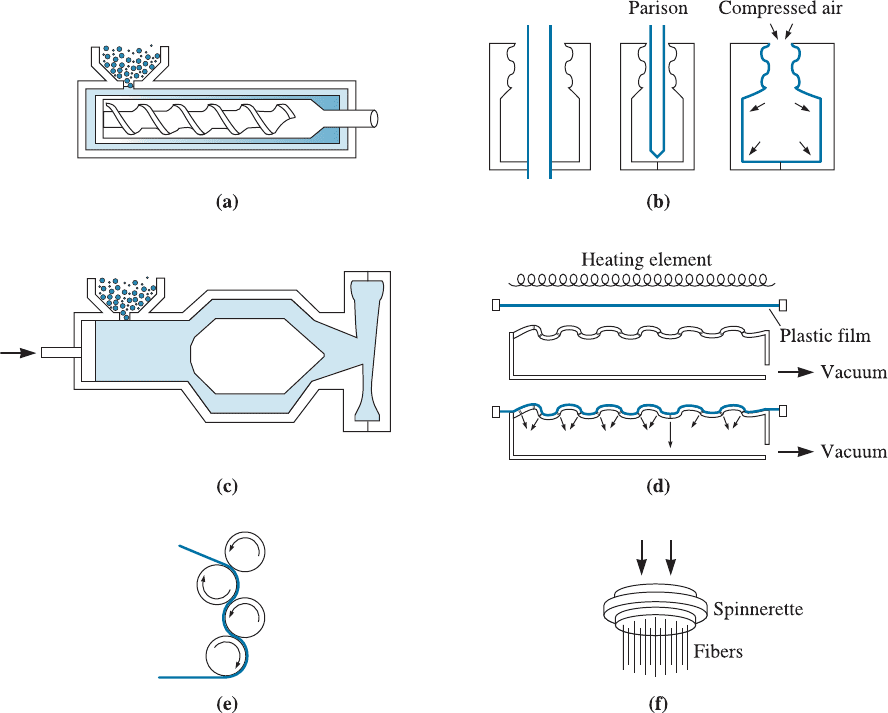
Blow Molding A hollow preform of a thermoplastic called a parison is introduced
into a die by gas pressure and expanded against the walls of the die (Figure 16-26). This
process is used to produce plastic bottles, containers, automotive fuel tanks, and other
hollow shapes.
Injection Molding Thermoplastics heated above the melting temperature using an
extruder are forced into a closed die to produce a molding. This process is similar to
die casting of molten metals. A plunger or a special screw mechanism applies pressure
Figure 16-26 Typical forming processes for thermoplastic: (a) extrusion, (b) blow molding,
(c) injection molding, (d) thermoforming, (e) calendaring, and (f ) spinning.
16-11 Polymer Processing and Recycling 533
to force the hot polymer into the die. A wide variety of products, ranging from cups,
combs, and gears to garbage cans, can be produced in this manner.
Thermoforming Thermoplastic polymer sheets heated to the plastic region can be
formed over a die to produce such diverse products as egg cartons and decorative
panels. The forming can be done using matching dies, a vacuum, or air pressure.
Calendaring In a calendar, molten plastic is poured into a set of rolls with a small
opening. The rolls, which may be embossed with a pattern, squeeze out a thin sheet of
the polymer—often, polyvinyl chloride. Typical products include vinyl floor tile and
shower curtains.
Spinning Filaments, fibers, and yarns may be produced by spinning. The molten
thermoplastic polymer is forced through a die containing many tiny holes. The die,
called a spinnerette, can rotate and produce a yarn. For some materials, including
nylon, the fiber may subsequently be stretched to align the chains parallel to the axis of
the fiber; this process increases the strength of the fibers.
Casting Many polymers can be cast into molds and permitted to solidify. The molds
may be plate glass for producing individual thick plastic sheets or moving stainless steel
belts for continuous casting of thinner sheets. Rotational molding is a special casting
process in which molten polymer is poured into a mold rotating about two axes. Cen-
trifugal action forces the polymer against the walls of the mold, producing a thin shape
such as a camper top.
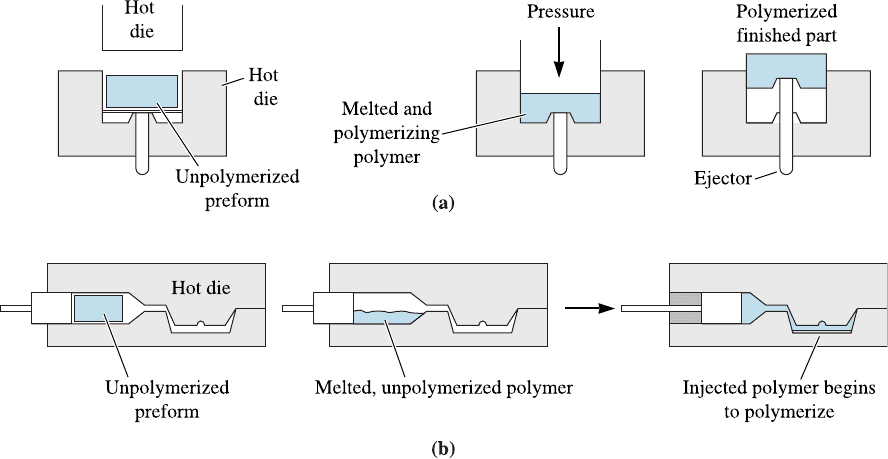
Compression Molding Thermoset moldings are most often formed by placing the solid
material before cross-l inking into a heated die (Figure 16-27a). Application of high
Figure 16-27 Typical forming processes for thermosetting polymers: (a) compression molding
and (b) transfer molding.
CHAPTER 16 Polymers534

pressure and temperature causes the polymer to melt, fill the die, and immediately begin
to harden. Small electrical housings as well as fenders, hoods, and side panels for au-
tomobiles can be produced by this process.
Transfer Molding A double chamber is used in the transfer molding of thermosetting
polymers. The polymer is heated under pressure in one chamber. After melting, the
polymer is injected into the adjoining die cavity. This process permits some of the ad-
vantages of injection molding to be used for thermosetting polymers (Figure 16-27b).
Reaction Injection Molding (RIM) Thermosetting polymers in the form of liquid res-
ins are first injected into a mixer and then directly into a heated mold to produce a
shape. Forming and curing occur simultaneously in the mold. In reinforced-reaction
injection molding (RRIM), a reinforcing material consisting of particles or short fibers
is introduced into the mold cavity and is impregnated by the liquid resins to produce a
composite material. Automotive bumpers, fenders, and furniture parts are made using
this process.
Foams Foamed products can be produced in polystyrene, urethanes, polymethyl
methacrylate, and a number of other polymers. The polymer is produced in the form of
tiny beads, often containing a blowing agent such as pentane. During the pre-expansion
process, the bead increases in diameter by as many as 50 times. The pre-expanded beads
are then injected into a die, with the indiv idual beads fusing together, using steam, to
form exceptionally lightweight products with densities of perhaps only 0.02 g/cm
3
.
Expandable polystyrene (EPS) cups, packaging, and insulation are some of the appli-
cations for foams. Engine blocks for many automobiles are made using a patter n made
from expanded polystyrene beads.
EXAMPLE 16-10
Insulation Boards for Houses
You want to design a material that can be used for making insulation boards
that are approximately 120 cm wide and 240 cm tall. The material must provide
good thermal insulation. What material would you choose?
SOLUTION
Glasses tend to be good insulators of heat. However, they will be heavy, more
expensive, and prone to fracture. Polymers are lightweight, can be produced
inexpensively, and they can be good thermal insulators. We can use foamed
polystyrene since the air contained in the beads adds significantly to their
e¤ectiveness as thermal insulators. For better mechanical properties, we may
want to produce foams that have relatively high density (compared to foams
that are used to make co¤ee cups). Finally, from a safety viewpoint, we want
to be sure that some fire and flame retardants are added to the foams. Such
panels are made using expanded polystyrene beads containing pentane. A
molding process is used to make the foams. The sheets can be cut into required
sizes using a heated metal wire.
16-11 Polymer Processing and Recycling 535
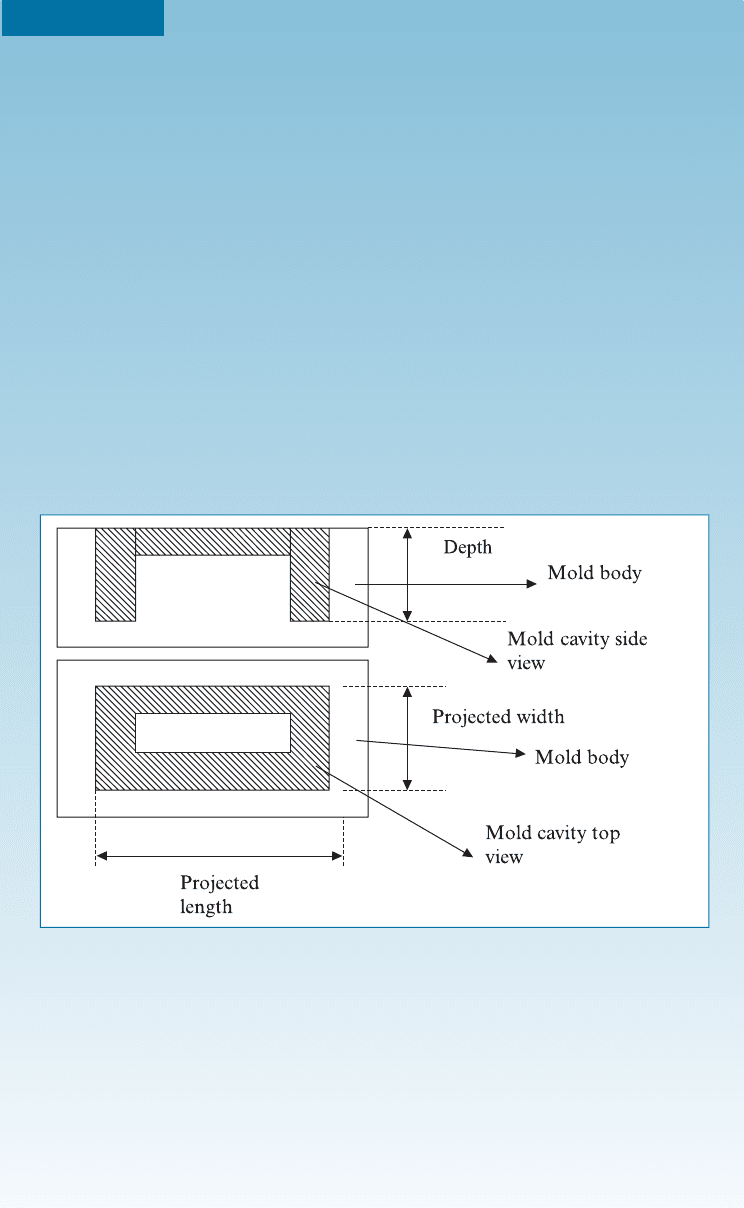
EXAMPLE 16-11 Thermoset Polymers by Compression Molding
Thermosets are commonly processed using a process known as compression
molding. This process is similar to sintering of ceramics in that resin (like ce-
ramic powder) is fed into mold cavities, that depend upon the shape of the
object being made, and then compressed between platens. The resin is then
heated while being pressed. Under the application of heat and pressure the
resin crosslinks (Figure 16-27). Additional pressure is needed if the component
being made is deeper (thicker). The force required to form a part of certain size
by compression molding is given by:
F ¼ðAÞ½ðP
A
Þþðr d
e
Þ
In this, F: force, A: projected area of the part which is exposed to pressure, P
A
is the cavity pressure (usually experimentally developed) necessary for a given
thermoset, r: depth factor, i.e., pressure per unit length needed beyond
a certain depth d
e
(Ref. A.B. Strong, Plastics: Materials and Processing, Second
Edn., Publisher Prentice Hall). Calculate the force needed to form a thermoset
shown below. Assume that for a thin part (i.e., less than 3 cm thick part) the
value of P
A
is 25 MPa, r is 2 MPa/cm beyond a depth of 3 cm. Assume the
projected width and length are 10 cm and 20 cm, respectively. Assume that
the depth of the part is 7 cm.
SOLUTION
The total projected area for the cavity is 20 10 cm ¼ 200 cm
2
. In this case,
there is only one cavity that is in the mold that will be filled with the resin. The
depth of the part is 7 cm; this is 4 cm beyond what is considered thin here.
Thus, the total force necessary will be:
F ¼ðAÞ½ðP
A
Þþðr d
e
Þ
F ¼ð200 cm
2
Þ½25 MPa þð2 MPa=cm ð7 3ÞcmÞ
F ¼ 200 cm
2
½33 MPa
F ¼ð200 10
4
m
2
Þ33 MN=m
2
¼ 0:66 MN ¼ 660 kN
CHAPTER 16 Polymers536

In practice, it can be assumed that a factor of safety of 1.3 is necessary. This
means, we should have equipment that can deliver a necessary force of 660 þ
198 kN. Thus, a force of 858 kN or approximately 900 kN will be required.
Recycling of Plastics Recycling is a very important issue and a full discussion of the
entire process is outside the scope of this book. But recycling plays an important role in
our everyday lives. Material is recycled in many ways. For example, part of the poly-
mer that is scrap from a manufacturing process (known as regrind) is used by recycling
plants. The recycling of thermoplastics is relatively easy and practiced widely. Note that
many of the everyday plastic products you encounter (bags, soda bottles, yogurt con-
tainers, etc.) have numbers stamped on them. For PET products (recycling symbol
‘‘PETE’’, because of trademark issues), the number is 1. For HDPE vinyl (recycling
symbol V), LDPE, PP, and PS the numbers are 2, 3, 4, 5 and 6, respectively. Other
plastics are marked number 7.
Thermosets and elastomers are more di‰cult to recycle, although they can still be
used. For example, tires can be shredded and used to make safer playground surfaces or
roads.
Despite enormous recycling e¤orts, a large portion of the materials in a landfill to-
day are plastics (the largest is that of paper). Given the limited amount of petroleum,
the threat of global warming, and a need for a cleaner and safer environment, careful
use and recycling makes sense for all materials.
SUMMARY
V Polymers are made from large macromolecules produced by the joining of smaller
molecules, called monomers, using addition or condensation polymerization re-
actions. Plastics are materials that are based on polymeric compounds, and they
contain many other additives that improve their prop erties. Compared with most
metals and ceramics, plastics have low strength, sti¤ness, and melting temperatures;
however, they also have a low density and good chemical resistance. Plastics are
used in a most diverse number of technologies.
V Thermoplastics have chains that are not chemically bonded to each other, permit-
ting the material to be easily formed into useful shapes, to have good ductility, and
to be economically recycled. Thermoplastics can have an amorphous structure,
which provides low stren gth and good ductility when the ambient temperature is
above the glass temperature. The polymers are more rigid and brittle when the tem-
perature falls below the glass temperature. Many thermoplastics can partially crys-
tallize during cooling or by application of a stress. This increases their strength.
V The thermoplastic chains can be made more rigid and stronger by using nonsym-
metrical monomers that increase the bonding strength between the chains and
make it more di‰cult for the chains to disentangle when stress is applied. In addi-
tion, many monomers produce more rigid chains containing atoms or groups of
atoms other than carbon; this structure also produces high-strength thermoplastics.
V Elastomers are thermoplastics or lightly cross-linked thermosets that exhibit greater
than 200% elastic deformation. Chains are eventually cross-linked using vulcan-
Summary 537

ization. The cross-linking makes it possible to obtain very large elastic deformatio ns
without permanent plastic deformation. Increasing the number of cross-links in-
creases the sti¤ness and reduces the amount of elastic deformation of the elastomers.
V Thermoplastic elastomers combine features of both thermoplastics and elastomers.
At high temperatures, these polymers behave as thermoplastics and are plastically
formed into shapes; at low temperatures, they behave as elastomers.
V Thermosetting polymers are highly cross-linked into a three-dimensional network
structure. Typically, high glass temperatures, good strength, and brittle behavior
are found. Once cross-linking occurs, these polymers cannot be easily recycled.
V Manufacturing processes used for polymers depend on their behavior. Processes
such as extrusion, injection molding, thermoforming, casting, drawing, and spin-
ning are made possible by the viscoelastic behavior of the thermoplastics. The non-
reversible behavior of bonding in thermosetting polymers limits their processing to
fewer techniques, such as compression molding, transfer molding and reaction-
injection molding.
GLOSSARY Addition polymerization Process by which polymer chains are built up by adding monomers
together without creating a byproduct.
Aging Slow degradation of polymers as a result of exposure to low levels of heat, oxygen, bac-
teria, or ultraviolet rays.
Aramids Polyamide polymers containing aromatic groups of atoms in the linear chain.
Blushing A thermoplastic bent repeatedly leads to small volumes of the material crystallizing;
this leads to voids that ultimately cause the material to fail.
Branched polymer Any polymer consisting of chains that consist of a main chain and secondary
chains that branch o¤ from the main chain.
Compounding Processing of elastomers in device known as a Banbury mixer followed by form-
ing using extrusion, molding, or dip coating.
Condensation polymerization A polymerization mechanism in which a small molecule (e.g.,
water, methanol, etc.) is condensed out as a byproduct.
Copolymer An addition polymer produced by joining more than one type of monomer.
Crazing Localized plastic deformation in a polymer. A craze may lead to the formation of
cracks in the material.
Cross-linking Attaching chains of polymers together to produce a three-dimensional network
polymer.
Degree of polymerization The average molecular weight of the polymer divided by the mole-
cular weight of the monomer.
Diene A group of monomers that contain two double-covalent bonds. These monomers are of-
ten used in producing elastomers.
Elastomers These are polymers (thermoplastics or lightly cross-linked thermosets) that have an
elastic deformation > 200%.
CHAPTER 16 Polymers538
Geometric isomer A molecule that has the same composition as, but a structure di¤erent from,
a second molecule.
Glass temperature (T
g
) The temperature range below which the amorphous polymer assumes a
rigid glassy structure.
Heat-deflection temperature The temperature at which a polymer will deform a given amount
under a standard load (also called distortion temperature).
Heat-degradation temperature The temperature above which a polymer burns, chars, or de-
composes.
Interpenetrating polymer networks Polymer structures produced by intertwining two separate
polymer structures or networks.
Linear polymer Any polymer in which molecules are in the form of spaghetti-like chains.
Liquid-crystalline polymers Exceptionally sti¤ polymer chains that act as rigid rods, even above
their melting point.
Mer A unit group of atoms and molecules that defines a characteristic arrangement for a poly-
mer. A polymer can be thought o¤ as a material made by combining several mers or units.
Monomer The molecule from which a polymer is produced.
Oligomer Low molecular weight molecules, these may contain two (dimers) or three (trimers)
mers.
Parison A hot glob of soft or molten polymer that is blown or formed into a useful shape.
Plastic A predominantly polymeric material made with some additives.
Polymer Polymers are materials made from giant (or macromolecular), chain-like molecules
having average molecular weights from 10,000 to more than 1,000,000 g/mol built by the joining
of many mers or units by chemical bonds. Polymers are usually, but not always, carbon based.
Relaxation time A property of a polymer that is related to the rate at which stress relaxation
occurs.
Repeat unit The repeating structural unit from which a polymer is built. Also called a mer.
Spinnerette An extrusion die containing many small openings through which hot or molten
polymer is forced to produce filaments. Rotation of the spinnerette twists the filaments into a yarn.
Stress-induced crystallization The process of forming crystals by the application of an external
stress. Typically, a significant fraction of many amorphous plastics can be crystallized in this
fashion, making them stronger.
Stress relaxation A reduction of the stress acting on a material over a period of time at a con-
stant strain due to viscoelastic deformation.
Tacticity Describes the location in the polymer chain of atoms or atom groups in nonsym-
metrical monomers.
Thermoplastic elastomers Polymers that behave as thermoplastics at high temperatures, but as
elastomers at lower temperatures.
Thermoplastics Linear or branched polymers in which chains of molecules are not inter-
connected to one another.
Thermosetting polymers Polymers that are heavily cross-linked to produce a strong three
dimensional network structure.
Glossary 539
