Askeland D.R., Fulay P.P. Essentials of Materials Science & Engineering
Подождите немного. Документ загружается.


SOLUTION
Let us look at the maximum tensile strength and modulus of elasticity for each
polymer.
Polymer
Tensile
Strength
(MPa)
Modulus of
Elasticity
(MPa) Structure
LD polyethylene 21 276 Highly branched, amorphous structure with
symmetrical monomers
HD polyethylene 38 1241 Amorphous structure with symmetrical monomers
but little branching
Polypropylene 41 1517 Amorphous structure with small methyl side groups
Polystyrene 55 3103 Amorphous structure with benzene side groups
Polyvinyl chloride 62 4137 Amorphous structure with large chlorine atoms as
side groups
We can conclude that:
1. Branching, which reduces the density and close packing of chains,
reduces the mechanical properties of polyethylene.
2. Adding atoms or atom groups other than hydrogen to the chain
increases strength and sti¤ness. The methyl group in polypropylene provides
some improvement, the benzene ring of styrene provides higher properties, and
the chlorine atom in polyvinyl chloride provides a large increase in properties.
Creep and Stress Relaxation Thermoplastics also exhibit creep, a time-dependent
permanent deformation with constant stress or load (Figures 16-17 and 16-18).
They also show stress relax ation (i.e., under a constant strain the stress level de-
creases with time) (Chapter 6). Stress relaxation, like creep, is a consequence of the vis-
coelastic behavior of the polymer. Perhaps the most familiar example of this behavior is
a rubber band (an elastomer) stretched around a pile of books. Initially, the tension in
the rubber band is high, when the rubber band is taut. After several weeks, the strain in
the rubber band is unchanged (it still completely encircles the books), but the stress will
have decreased—that is, the band is no longer taut. Similarly, the nylon strings in tennis
rackets are pulled at a higher tension initially since this tension (i.e., stress) decreases
with time.
Applied stress (MPa)
1.4
2.8
4.2
6.9
13.8
27.6
Figure 16-17
The effect of temperature on the
stress-rupture behavior of high-
density polyethylene.
CHAPTER 16 Polymers520
In a simple model, the rate at which stress relaxation occurs is related to the relax-
ation time l, which is considered a property of the polymer (more complex models
consider a distribution of relaxation times). The stress after time t is given by
s ¼ s
0
expðt=lÞð16-5Þ
where s
0
is the original stress. The relaxation time, in turn, depends on the viscosity
and, thus, the temperature:
l ¼ l
0
expðQ=RTÞð16-6Þ
where s
0
is a constant and Q is the activation energy related to the ease with which
polymer chains slide past each other. Stress relaxation occurs more rapidly at higher
temperatures and for polymers with a low viscosity.
The following example shows how stress relaxation can be accounted for while
designing with polymers.
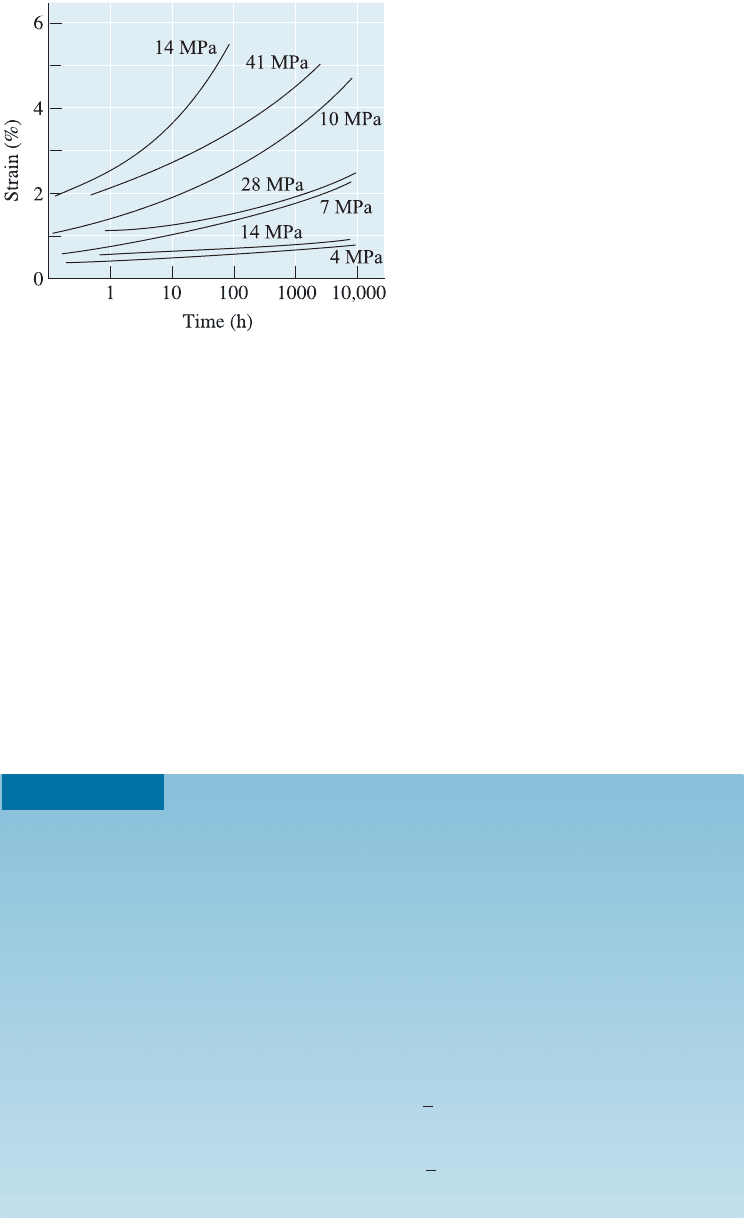
Figure 16-18
Creep curves for acrylic (PMMA) (colored
lines) and polypropylene (black lines) at
20
C and several applied stresses.
EXAMPLE 16-9
Design of Initial Stress in a Polymer
A band of polyisoprene is to hold together a bundle of steel rods for up to one
year. If the stress on the band is less than 10 MPa, the band will not hold the
rods tightly. Design the initi al stress that must be applied to a polyisoprene
band when it is slipped over the steel. A series of tests showed that an initial
stress of 7 MPa decreased to 6.8 MPa after six weeks.
SOLUTION
Although the strain of the elastomer band may be constant, the stress will
decrease over time due to stress relaxation. We can use Equation 16-5 and our
initial tests to determine the relaxation time for the polymer:
s ¼ s
0
exp
t
l
6:8 ¼ 7 exp
6
l
16-7 Mechanical Properties of Thermoplastics 521

6
l
¼ ln
6:8
7
¼ lnð0:97Þ¼0:0305
l ¼
6
0:0305
¼ 197 weeks
Now that we know the relaxation time, we can determine the stress that must
be initially placed onto the band in order that it still be stressed to 10 MPa
after 1 year (52 weeks).
10 ¼ s
0
expð52=197Þ¼s
0
expð0:264Þ¼0:768s
0
s
0
¼
10
0:768
¼ 13 MPa
The polyisoprene band must be made significantly undersized so it can slip
over the materials it is holding together with a tension of 13 MPa. After one
year, the stress will still be 10 MPa.
One more practical measure for high temperature and creep properties of a poly-
mer is the heat deflection temperature or heat distortion temperature under load, which is
the temperature at which a given deformation of a beam occurs for a standard load. A
high-heat deflection temperature indicates good resistance to creep and permits us to
compare various polymers. The deflection temperatures for several polymers are shown
in Table 16-7, which gives the temperature required to cause a 0.025 cm deflection for a
1.8 MPa load at the center of a bar resting on supports 10 cm apart. A polymer is
‘‘dishwasher safe’’ if it has a heat distortion temperature greater than @50
C.
TABLE 16-7 9 Deflection temperatures for selected polymers for a
1.8 MPa load
Polymer Deflection Temperature (
˚
C)
Polyester 40
Polyethylene (ultra-high density) 40
Polypropylene 60
Phenolic 80
Polyamide (6,6-nylon) 90
Polystyrene 100
Polyoxymethylene (acetal) 130
Polyamide-imide 280
Epoxy 290
Impact Behavior Viscoelastic behavior also helps us understand the impact properties
of polymers. At very high rates of strain, as in an impact test, there is insu‰cient time
for the chains to slide and cause plastic deformation. For these conditions, the thermo-
plastics behave in a brittle manner and have poor impact values. Polymers may have a
transition temperature. At low temperatures, brittle behavior is observed in an impact
test, whereas more ductile behavior is observed at high temperatures, where the chains
move more easily. These e¤ects of temperature and strain rate are similar to those seen
CHAPTER 16 Polymers522

in metals that exhibit a ductile-to-brittle transition temperature; however, the mecha-
nisms by which ductile to brittle transition occurs are di¤erent.
Deformation of Crystalline Polymers A number of polymers are used in the crys-
talline state. As we discussed earlier, however, the polymers are never completely crys-
talline. Instead, small regions—between crystalline lamellae and between crystalline
spherulites—are amorphous transition regions. Polymer chains in the crystalline region
extend into these amorphous regions as tie chains. When a tensile load is applied to the
polymer, the crystalline lamellae within the spherulites slide past one another and begin
to separate as the tie chains are stretched. The folds in the lamellae tilt and become
aligned with the direction of the tensile load. The crystalline lamellae break into smaller
units and slide past one another, until eventually the polymer is composed of small
aligned crystals joined by tie chains and oriented parallel to the tensile load. The
spherulites also change shape and become elongated in the direction of the applied
stress. With continued stress, the tie chains disentangle or break, causing the polymer to
fail.
Crazing Crazing occurs in thermoplastics when localized regions of plastic deforma-
tion occur in a direction perpendicular to that of the applied stress. In transparent
thermoplastics, such as some of the glassy polymers, the craze produces a translucent or
opaque region that looks like a crack. The craze can grow until it extends across the
entire cross-section of the polymer part. However, the craze is not a crack, and, in fact,
it can continue to support an applied stress. The process is similar to that for the plastic
deformation of the polymer, but the process can proceed even at a low stress over an
extended length of time. Crazing can lead to brittle fracture of the polymer and is often
assisted by the presence of a solvent (known as solvent crazing).
Blushing Blushing or whitening refers to failure of a plastic because of localized crys-
tallization (due to repeated bending, for example) that ultimately causes voids to form.
16-8 Elastomers (Rubbers)
A number of natural and synthetic polymers called elastomers display a large amount
(>200%) of elastic deformation when a force is applied. Rubber bands, automobile
tires, O-rings, hoses, and insulation for electrical wires are common uses for these ma-
terials. Crude natural rubber could erase pencil marks; hence, elastomers got the name
rubber.
Geometric Isomers Some monomers that have di¤erent structures, even though they
have the same composition, are called geometric isomers. Isoprene, or natural rubber,
is an important example (Figure 16-19). The monomer includes two double bonds
between carbon atoms; this type of monomer is called a diene. Polymerization occurs
by breaking both double bonds, creating a new double bond at the center of the mole-
cule and active sites at both ends.
In the trans form of isoprene, the hydrogen atom and the methyl group at the cen-
ter of the repeat unit are located on opposite sides of the newly formed double bond.
This arrangement leads to relatively straight chains; the polymer crystallizes and forms
a hard rigid polymer called gutta percha. This is used to make golf balls and shoe soles.
16-8 Elastomers (Rubbers) 523
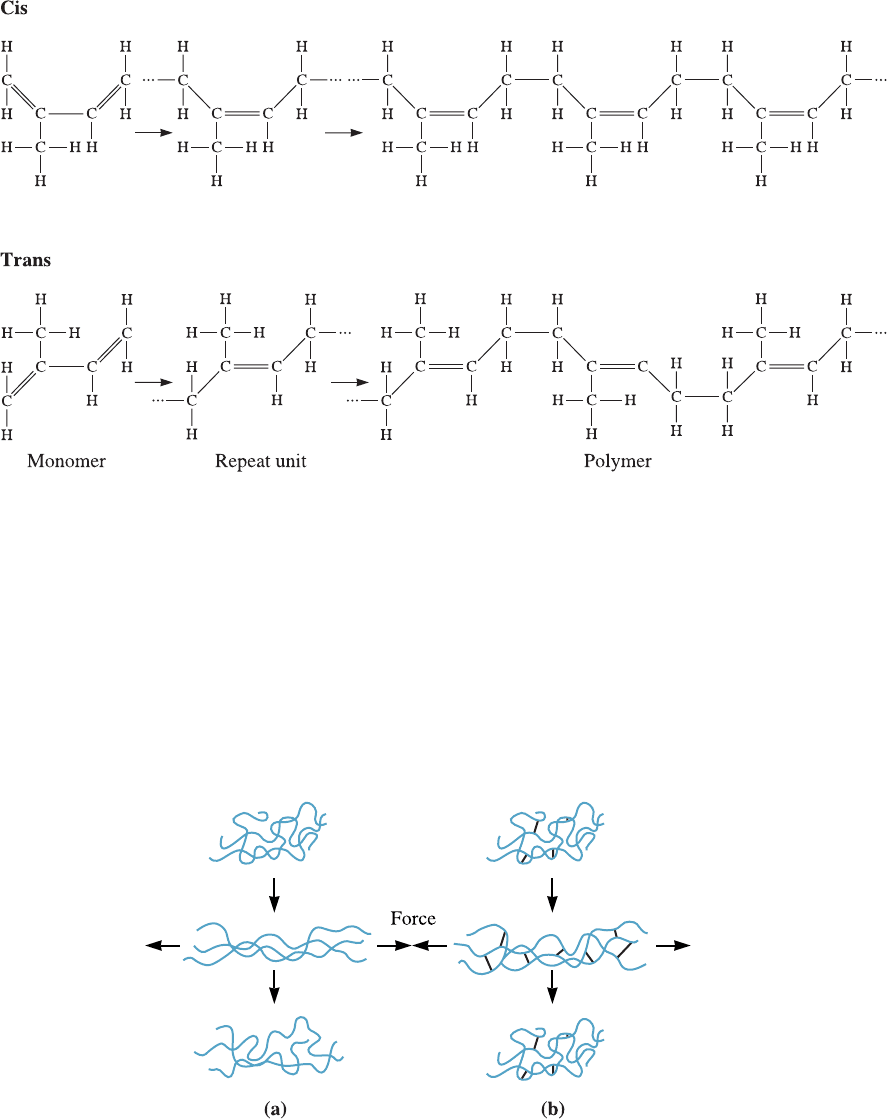
In the cis form, however, the hydrogen atom and the methyl group are located on the
same side of the double bond. This di¤erent geometry causes the polymer chains to de-
velop a highly coiled structure, preventing close packing and leading to an amorphous,
rubbery polymer. If a stress is applied to the cis-isoprene, the polymer behaves in a vis-
coelastic manner. The chains uncoil and bonds stretch, producing elastic deforma tion,
but the chains also slide past one another, producing nonrecoverable plastic deforma-
tion. The polymer behaves as a thermoplastic rather than an elastomer (Figure 16-20).
Figure 16-19 The cis and trans structures of isoprene. The cis form is useful for producing the
isoprene elastomer.
Figure 16-20 (a) When the elastomer contains no cross-links, the application of a force causes
both elastic and plastic deformation; after the load is removed, the elastomer is permanently
deformed. (b) When cross-linking occurs, the elastomer still may undergo large elastic
deformation; however, when the load is removed, the elastomer returns to its original shape.
CHAPTER 16 Polymers524
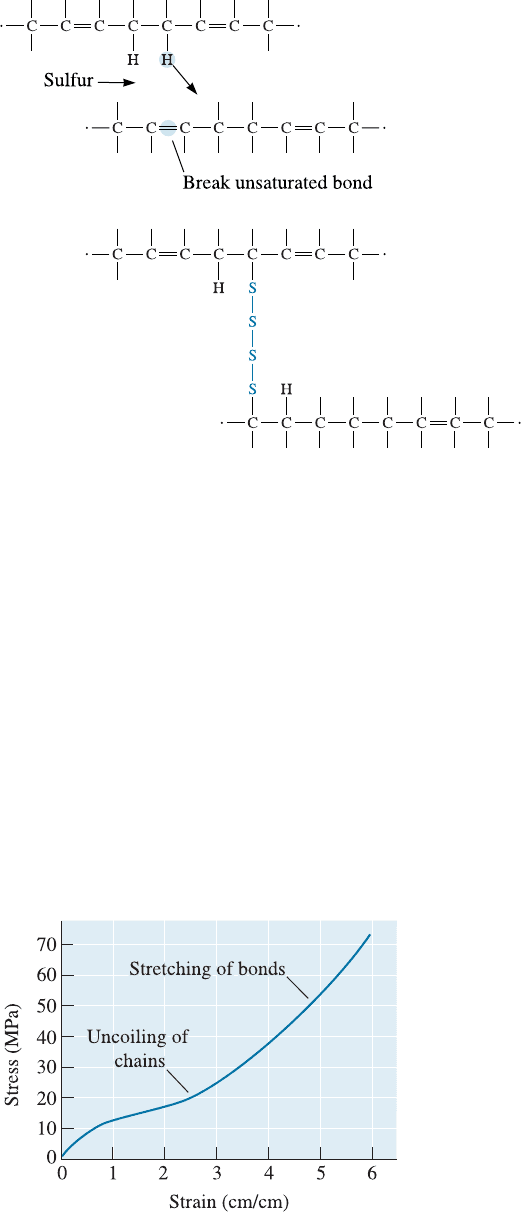
Cross-Linking We prevent viscous plastic deformation while retaining large elastic
deformation by cross-linking the chains. Vulcanization, which uses sulfur atoms, is a
common method for cross-linking. Vulcanization describes how strands of sulfur atoms
can link the polymer chains as the polymer is processed and shaped at temperatures of
about 120 to 180
C (Figure 16-21). The cross-linking steps may include rearranging
a hydrogen atom and replacing one or more of the double bonds with single bonds.
The cross-linking process is not reversible; consequently, the elastomer cannot be re-
cycled easily.
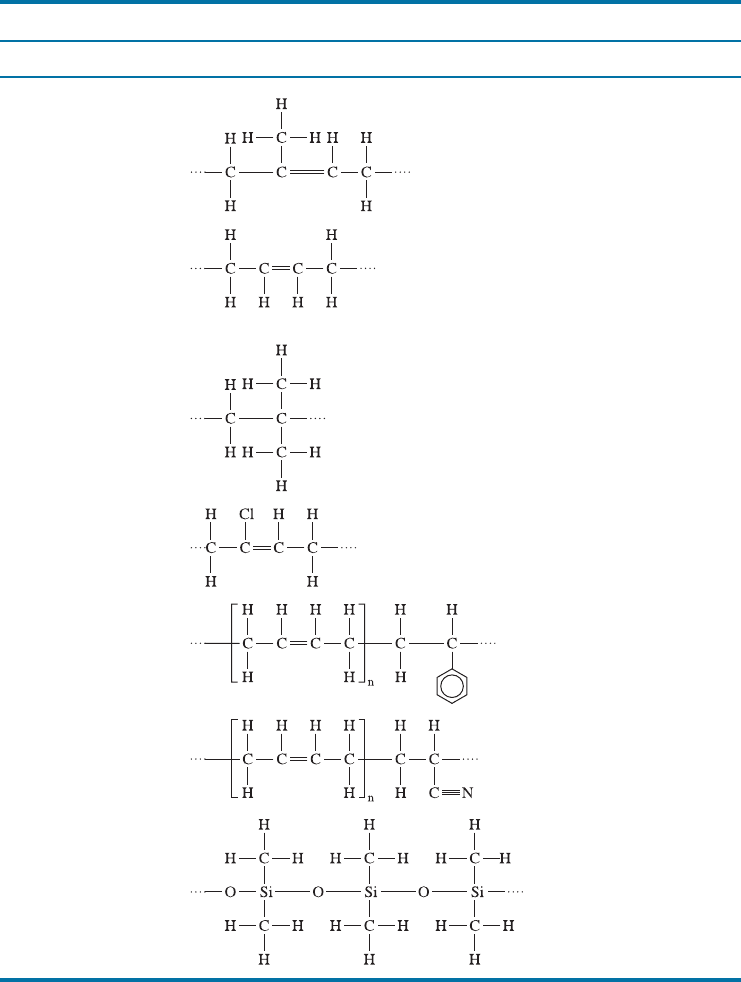
The stress-strain curve for an elastomer is shown in Figure 16-22. Virtually all of
the curve represents elastic deformation; thus, elastomers display a nonlinear elastic
behavior. Initially, the modulus of elasticity decreases because of the uncoiling of the
chains. However, after the chains have been extended, further elastic deformation
occurs by the stretching of the bonds, leading to a higher modulus of elasticity.
The number of cross-links determines the elasticity of the rubber or the amount of
sulfur added to the material. Low sulfur additions leave the rubber soft and flexible, as
in elastic bands or rubber gloves. Increasing the sulfur content restricts the uncoiling of
Figure 16-21
Cross-linking of
polyisoprene chains may
occur by introducing
strands of sulfur atoms.
Sites for attachment of
the sulfur strands occur
by rearrangement or loss
of a hydrogen atom and
the breaking of an
unsaturated bond.
Figure 16-22
The stress-strain curve for an
elastomer. Virtually all of the
deformation is elastic; therefore, the
modulus of elasticity varies as the
strain changes.
16-8 Elastomers (Rubbers) 525
the chains and the rubber becomes harder, more rigid, and brittle (as in rubber used for
motor mounts). Typically, 0.5 to 5% sulfur is added to provide cross-linking in elas-
tomers. Many more e‰cient vulcanizing (EV) systems, which are sulfur free, also have
been developed and used in recent years.
Typical Elastomers Elastomers, which are amorphous polymers, do not easily crys-
tallize during processing. They have a low glass temperature, and chains can easily be
deformed elastically when a force is applied. The typical elastomers (Tables 16-8 and
16-9) meet these requirements. Polyisoprene is a natural rubber. Polychloroprene, or
TABLE 16-8 9 Repeat units and applications for selected elastomers
Polymer Repeat Unit Applications
Polyisoprene Tires, golf balls, shoe
soles
Polybutadiene
(or butadiene
rubber or Buna-S)
Industrial tires, toughening
other elastomers, inner
tubes of tires,
weatherstripping, steam
hoses
Polyisobutylene
(or butyl rubber)
Polychloroprene
(Neoprene)
Hoses, cable sheathing
Butadiene-styrene
(BS or SBR rubber)
Tires
Butadiene-acrylonitrile
(Buna-N)
Gaskets, fuel hoses
Silicones
Gaskets, seals
CHAPTER 16 Polymers526
Neoprene, is a common material for hoses and electrical insulation. Many of the
important synthetic elastomers are copolymers. Polybutadiene (butadiene rubber or
Buna-S) is similar to polyisoprene, but the repeat unit has four carbon atoms consisting
of one double bond. This is a relatively low-cost rubber, though the resistance to sol-
vents is poor. As a result, it is used as a toughening material to make other elastomers.
Butadiene-styrene rubber (BSR or BS), which is also one of the components of ABS
(Figure 16-9), is used for automobile tires. Butyl rubber is di¤erent from polybutadiene
(butadiene rubber). Butyl rubber, or polyisobutadiene, is used to make inner tubes for
tires, vibration mounts, and as weather-stripping material. Silicones are another im-
portant elastomer based on chains composed of silicon and oxygen atoms. Silly Putty
8
was invented by James Wright of General Electric. It is made using hydroxyl termi-
nated polydimethyl siloxane, boric oxide, and some other additives. At slow strain rates
you can stretch it significantly, while if you pull it fast it snaps. The silicone rubbers
(also known as polysiloxanes) provide high-temperature resistance, permitting use of
the elastomer at temperatures as high as 315
C. Low molecular weight silicones form
liquids and are known as silicon oils. Silicones can also be purchased as a two-part
system that can be molded and cured. Chewing gum contains a base that is made from
natural rubber, styrene butadiene, or polyvinyl acetate (PVA).
Thermoplastic Elastomers (TPEs) This is a special group of polymers that do not rely
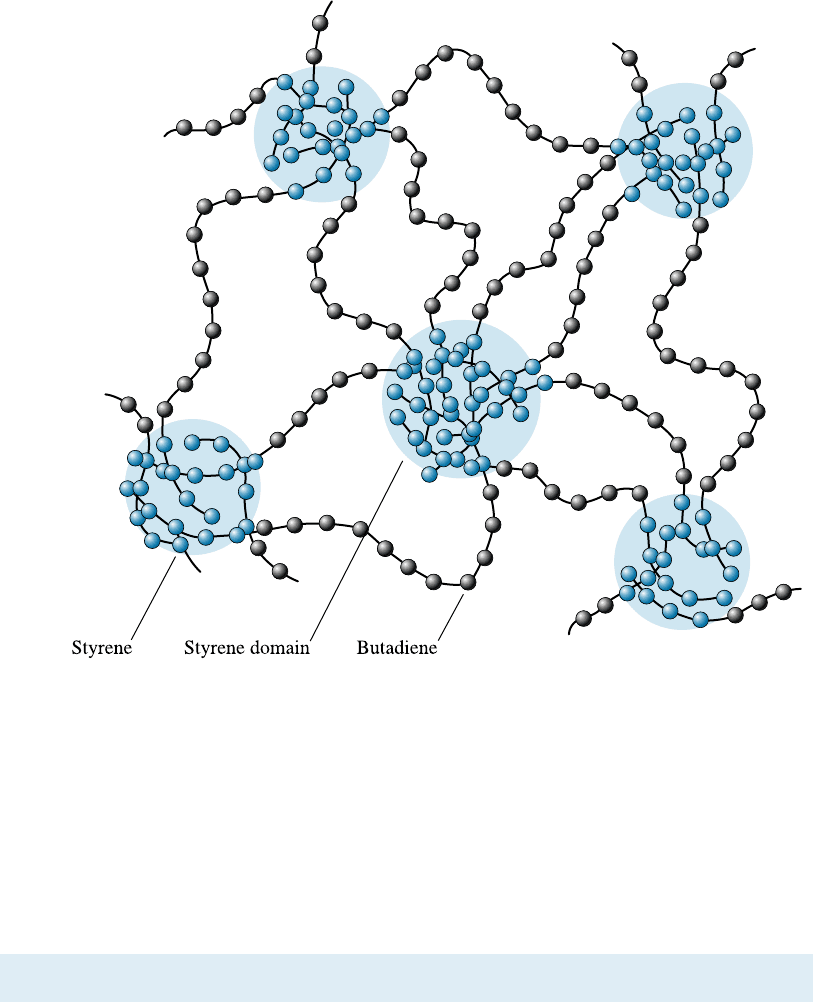
on cross-linking to produce a large amount of elastic deformation. Figure 16-23 shows
the structure of a styrene-butadiene block copolymer engineered so that the styrene
repeat units are located only at the ends of the chains. Approximately 25% of the chain
is composed of styrene. The styrene ends of several chains form spherical-shaped
domains. The styrene has a high glass temperature; consequently, the domains are
strong and rigid and tightly hold the chains together. Rubbery areas containing buta-
diene repeat units are located between the styrene domains; these portions of the poly-
mer have a glass temperature below room temperature and therefore behave in a soft,
rubbery manner. Elastic deformation occurs by recoverable movement of the chains;
however, sliding of the chains at normal temperatures is prevented by the styrene
domains.
The styrene-butadiene block copolymers di¤er from the BS rubber discussed earlier
in that cross-linking of the butadiene monomers is not necessary and, in fact, is un-
desirable. When the thermoplastic elastomer is heated, the styrene heats above the
glass temperature, the domains are destroyed, and the polymer deforms in a viscous
manner—that is, it behaves as any other thermoplastic, making fabrication very easy.
When the polymer cools, the domains reform and the polymer reverts to its elastomer
TABLE 16-9 9 Properties of selected elastomers
Tensile
Strength
(MPa)
%
Elongation
Density
(g/cm
3
)
Polyisoprene 21 800 0.93
Polybutadiene 24 0.94
Polyisobutylene 28 350 0.92
Polychloroprene (Neoprene) 24 800 1.24
Butadiene-styrene (BS or SBR rubber) 21 2000 1.0
Butadiene-acrylonitrile 5 400 1.0
Silicones 7 700 1.5
Thermoplastic elastomers 35 1300 1.06
16-8 Elastomers (Rubbers) 527
characteristics. The thermoplastic elastomers consequently behave as ordinary thermo-
plastics at elevated temperatures and as elastomers at low temperatures. This behavior
also permits the thermoplastic elastomers to be more easily recycled than conventional
elastomers. A useful fluoroelastome r for high temperature and corrosive environments
is Viton
TM
. It is used for seals, O-rings, etc.
16-9 Thermosetting Polymers
Thermosets are highly cross-linked polymer chains that form a three-dimensional
network stru cture. Because the chains cannot rotate or slide, these polymers possess
good strength, sti¤ness, and hardness. However, thermose ts also have poor ductility
and impact properties and a high gl ass temperature. In a tensile test, thermosetting
polymers display the same behavior as a brittle metal or a ceramic.
Thermosetting polymers often begin as linear chains. Depending on the type of
repeat units and the degree of polymerization, the initial polymer may be either a solid
or a liquid resin; in some cases, a two- or three-part liquid resin is used (as in the case of
Figure 16-23 The structure of the styrene-butadiene (SB) copolymer in a thermoplastic
elastomer. The glassy nature of the styrene domains provides elastic behavior without cross-
linking of the butadiene.
CHAPTER 16 Polymers528
the two tubes of epoxy glue that we often use). Heat, pressure, mixing of the various
resins, or other methods initiate the cross-linking process. Cross-linking is not rever-
sible; once formed, the thermosets cannot be reused or recycled conveniently.
The functional groups for a number of common thermosetting polymers are sum-
marized in Table 16-10, and representative properties are given in Table 16-11.
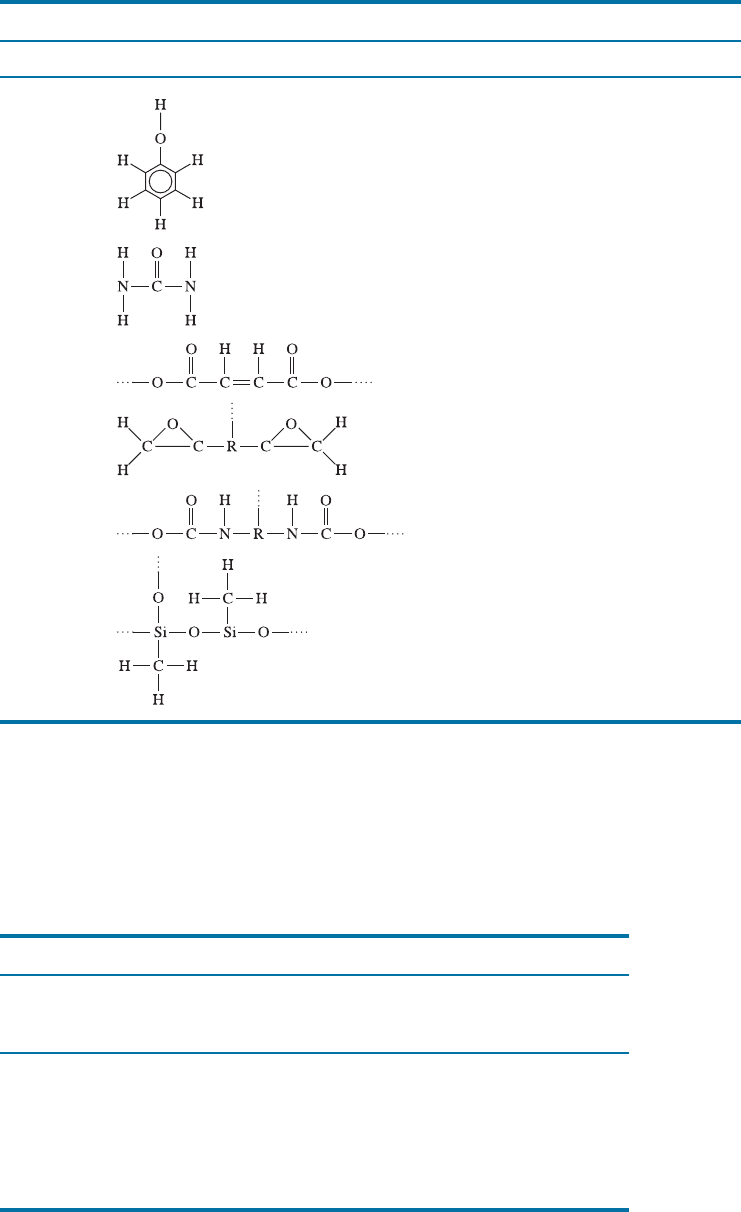
TABLE 16-10 9 Functional units and applications for selected thermosets
Polymer Functional Units Typical Applications
Phenolics Adhesives, coatings, laminates
Amines
Adhesives, cookware, electrical moldings
Polyesters
Electrical moldings, decorative laminates,
polymer matrix in fiberglass
Epoxies
Adhesives, electrical moldings, matrix for
composites
Urethanes
Fibers, coatings, foams, insulation
Silicone
Adhesives, gaskets, sealants
TABLE 16-11 9 Properties of typical thermosetting polymers
Tensile
Strength
(MPa)
%
Elongation
Elastic
Modulus
(MPa)
Density
(g/cm
3
)
Phenolics 62 2 9 1.27
Amines 69 1 11 1.50
Polyesters 90 3 5 1.28
Epoxies 103 6 4 1.25
Urethanes 69 6 1.30
Silicone 28 0 8 1.55
16-9 Thermosetting Polymers 529
