Askeland D.R., Fulay P.P. Essentials of Materials Science & Engineering
Подождите немного. Документ загружается.


Basic Refractories A number of refractories are based on MgO (magnesia, or peri-
clase). Pure MgO has a high melting point, good refractoriness, and good resistance
to attack by the basic environments often f ound in steel-making processes. Olivine
refractories contain forsterite, or Mg
2
SiO
4
, and also have high melting points. Other
magnesia refractories may include CaO or carbon. Typically, the basic refractories are
more expensive than the acid refractories.
Neutral Refractories These refractories, which include chromite and chromite-
magnesite, might be used to separate acid and basic refractories, preventing them from
attacking one another.
Special Refractories Carbon, or graphite, is used in many refractory applications,
particularly when oxygen is not present. Other refractory materials include zirconia
(ZrO
2
), zircon (ZrO
2
SiO
2
), and a variety of nitride s, carbides, and borides. Most
of the carbides, such as TiC and ZrC, do not resist oxidation well, and their high-tem-
perature applications are best suited to reducing conditions. However, silicon carbide
(SiC) is an exception; when SiC is oxidized at high temperatures, a thin layer of SiO
2
forms at the surface, protecting the SiC from further oxidation up to about 1500
C.
Nitrides and borides also have high-melting temp eratures and are less susceptible to
oxidation. Some of the oxides and nitrides are candidates for use in jet engines.
15-9 Other Ceramic Materials
In addition to their use in producing construction materials, appliances, structural
materials, and refractories, ceramics find a host of other applications, including the
following.
Cements Ceramic raw materials are joined using a binder that does not require firing
or sintering in a process called cementation. A chemical reaction converts a liquid resin
to a solid that joins the particles. In the case of sodium silicate, the introduction of CO
2
gas acts as a catalyst to dehydrate the sodium silicate solution into a glassy material:
xNa
2
O ySiO
2
H
2
O þ CO
2
! glass (not balanced)
Figure 15-16 shows silica sand grains used to produce molds for metal casting. The
liquid sodium silicate coats the sand grains and provides bridges between the sand
grains. Introduction of the CO
2
converts the bridges to a solid, joining the sand grains.
Figure 15-16
A photograph of silica sand grains bonded with
sodium silicate through the cementation
mechanism (60).
CHAPTER 15 Ceramic Materials490

Fine alumina-powder solutions catalyzed with phosph oric acid produce an alumi-
num phosphate cement:
Al
2
O
3
þ 2H
3
PO
4
! 2AlPO
4
þ 3H
2
O
When alumina particles are bonded with the aluminum phosphate cement, refractories
capable of operating at temperatures as high as 1650
C are produced.
Plaster of paris, or gypsum, is another material that is hardened by a cementation
reaction:
CaSO
4
1
2
H
2
O þ
3
2
H
2
O ! CaSO
4
2H
2
O
When the liquid slurry reacts, interlocking solid crystals of gypsum (CaSO
4
2H
2
O)
grow, with very small pores between the crystals. Larger amounts of water in the original
slurry provide more porosity, but they also decrease the strength of the final plaster. One
of the important uses of this material is for construction of walls in buildings.
The most common and important of the cementation reactions occurs in Portland
cement, which is used to produce concrete.
Coatings Ceramics are often used to provide protective coatings to other materials.
Common commercial coatings include glazes and enamels. Glazes are applied to the
surface of a ceramic material to seal a permeable clay body, to provide protection and
decoration, or for special purposes. Enamels are applied to metal surfaces. The enamels
and glazes are clay products that vitrify easily during firing. A common composition is
CaO Al
2
O
3
2SiO
2
.
Special colors can be produced in glazes and enamels by the addition of other
minerals. Zirconium silicate gives a white glaze, cobalt oxide makes the glaze blue,
chromium oxide produces green, lead oxide gives a yellow color, and a red glaze may
be produced by adding a mixture of selenium and cadmium sulfides.
One of the problems encountered with a glaze or enamel is surface cracking, or
crazing, which occurs when the glaze has a coe‰cient of thermal expansion di¤erent
than that of the underlying material. This is frequently the most important factor in
determining the composition of the coating.
Special coatings are used for advanced ceramics and high-service temperature
metals. SiC coatings are applied to carbon-carbon composite materials to improve their
oxidation resistance. Zirconia coatings are appli ed to nickel -based superalloys to pro-
vide thermal barriers that protect the metal from melting or adverse reactions.
Thin Films and Single Crystals Thin films of many complex and multi-component
ceramics are produced using di¤erent techniques such as sputtering, sol-gel, and chem-
ical-vapor deposition (CVD). Usually, the thickness of such films is less than 0.05 to 10
mm, and more likely greater than 2 mm. Many functional electronic ceramic thin films
are prepared and integrated onto silicon wafers, glasses, and other substrates. For ex-
ample, the magneti c strips on credit cards use iron oxide (g-Fe
2
O
3
or Fe
3
O
4
) thin films
for storing data. Indium tin oxide (ITO), a conductive and transparent material, is
coated on glass and used in applications such as touch-screen displays (Table 15-1).
Many other coatings are used on glass to make the glass energy e‰cient. Recently, a
self-cleaning glass using a TiO
2
coating has been developed. Similarly, thin films of ce-
ramics, such as PZT, PLZT, and BaTiO
3
-SrTiO
3
solid solutions, can be prepared and
used. Often, the films develop an orientation, or texture, which may be advantageous
for a given application. Single crystals of ceramics (e.g., SiO
2
or quartz, lithium niobate
(LiNbO
3
), sapphire, or yttrium aluminum garnate) are used in many electrical and
electro-optical applications. These crystals are grown from melts using techniques sim-
ilar to those described in Chapter 9.
15-9 Other Ceramic Materials 491

Fibers Fibers are produced from ceramic materials for several uses: as a reinforcement
in com posite materials, for weaving into fabrics, or for use in fiber-optic systems. Boro-
silicate glass fibers, the most commonly produced fibers, provide strength and sti¤ness
in fiberglass. Fibers can be produced from a variety of other ceramics, including alu-
mina, silicon carbide, silica, and boron carbide. The sol-gel process is also used to pro-
duce commercial fibers for many applications.
A special type of fibrous material is the silica tile used to provide the thermal pro-
tection system for NASA’s space shuttle. Silica fibers are bonded with colloidal silica to
produce an exceptionally lightweight tile with densities as low as 0.144 g/cm
3
; the tile is
coated with special high-emissivity glazes to permit protection up to 1300
C.
Joining and Assembly of Ceramic Components Ceramics are often made as mono -
lithic components rather than assemblies of numerous components. When two ceramic
parts are placed in contact under a load, stress concentrations at the brittle surface lead
to an increased probability of failure. In addition, methods for joining ceramic parts
into a larger assembly are limited. The brittle ceramics cannot be joined by fusion
welding or deformatio n bonding processes. At low temperatures, adhesive bonding
using polymer materials may be accomplished; ceramic cements may be used at higher
temperatures. Di¤usion bonding and brazing can be used to join ceramics and to join
ceramics to metals.
SUMMARY
V Ceramics are inorganic materials that have high hardness and high melting
points. These include single crystal and polycrystalline ceramics, glasses, and glass-
ceramics. Typical ceramics are electrical and thermal insulators with good chemical
stability and good strength in compression.
V Polycrystalline ceramics typically exhibit a brittle behavior, partly because of
porosity. Because most polycrystalline ceramics cannot plastically deform (unless
special conditions with respect to temperature and strain rates are created), the
porosity limits the ability of a ceramic material to withstand a tensile load.
V Ceramics play a critical role in a wide array of technologies related to electronic,
magnetic, optical, and energy. Many advanced ceramics play a very important role
in providing thermal insulation and high-temperature properties. Applications of
advanced ceramics range from credit cards, tiles for the space shuttle, medical
imaging, optical fibers that enable communication, and safe, energy-e‰cient
glasses. Traditional ceramics play a very important role as refractories for metals
processing and consumer applications.
V Ceramic-powder synthesis involves the treatment of ores using processes such as
crushing and grinding, followed by ball milling, and calcination. These processes
are inexpensive and widely used for making many ceramic powders.
V Ceramic processing is commonly conducted using compaction and sintering. For
specialized applications, isostatic compaction, hot pressing, and hot isostatic press-
ing (HIP) are used, especially to achieve higher-densification levels.
V Tape casting, slip casting, extrusion, and injection molding are some of the other
techniques used to form green ceramics into di¤erent shapes. These processes are
then followed by a burnout step in which binders and plasticizers are burnt o¤, and
the resultant ceramic is sintered.
CHAPTER 15 Ceramic Materials492

V Many silicates and other ceramics form glasses rather easily, since the kinetics of
crystallization are sluggish. Glasses can be formed in the form of sheets using float-
glass or fibers and other shapes. Silicate glasses are used in a significant number of
applications that include window glass, windshields, fiber optics, and fiberglass.
V Glass-ceramics are formed using controlled crystallization of inorganic glasses.
These materials are used widely for kitchenware and many other applications.
V Ceramics, in the form of fibers, thin films, coatings, and single crystals, have many
di¤erent applications.
GLOSSARY
Apparent porosity The percentage of a ceramic body that is composed of interconnected
porosity.
Bulk density The mass of a ceramic body per unit volume, including closed and interconnected
porosity.
Cementation Bonding ceramic raw materials into a useful product, using binders that form a
glass or gel without firing at high temperatures.
Ceramic An inorganic material with high melting temperature. Usually hard and brittle.
Ceramic bond Bonding ceramic materials by permitting a glassy product to form at high firing
temperatures.
Cermet A ceramic-metal composite (e.g., WC-Co) providing a good combination of hardness
with other properties such as toughness.
Cold isostatic pressing (CIP) A powder-shaping technique in which hydrostatic pressure is
applied during compaction. This is used for achieving a higher green ceramic density or compac-
tion of more complex shapes.
Devitrification The crystallization of glass.
Enamel A ceramic coating on metal.
Firing Heating a ceramic body at a high temperature to cause a ceramic bond to form.
Flux Additions to ceramic raw materials that reduce the melting temperature.
Glass An amorphous material derived by cooling of a melt.
Glass-ceramics Polycrystalline ceramics formed initially in the glassy state and later crystallized
during heat treatment to achieve improved strength and toughness.
Glass formers Oxides with a high bond strength that easily produce a glass during processing.
Glass temperature (T
g
) The temperature below which an undercooled liquid becomes a glass.
This is not a fixed temperature.
Glaze A ceramic coating applied to glass. The glaze contains glassy and crystalline ceramic
phases.
Green ceramic A ceramic that has been shaped into a desired form but has not yet been
sintered.
Glossary 493
Hot isostatic pressing (HIP) A powder-processing technique in which large pieces of metals,
alloys and ceramics can be produced using sintering under a hydrostatic pressure generated by a
gas.
Hot pressing A processing technique in which sintering is conducted under uniaxial pressure.
Hydroplastic forming A number of processes by which a moist ceramic clay body is formed into
a useful shape.
Injection molding A processing technique in which a thermoplastic mass (loaded with ceramic
powder) is mixed in an extruder-like setup and then injected into a die to form complex parts.
In the case of ceramics, the thermoplastic is burnt o¤.
Intermediates Oxides that, when added to a glass, help to extend the glassy network, although
the oxides normally do not form a glass themselves.
Laminated glass Annealed glass with a polymer (e.g., polyvinyl butyral, PVB) sandwiched in
between, used for car windshields.
Parison A crude glassy shape that serves as an intermediate step in the production of glassware.
The parison is later formed into a finished product.
Powder metallurgy Powder processing routes used for converting metal and alloy powders into
useful shapes.
Powder processing Unit operations conducted to convert powders into useful shapes (e.g.,
pressing, tape casting, etc.).
Reaction bonding A ceramic processing technique by which a shape is made using one material
that is later converted into a ceramic material by reaction with a gas.
Refractories A group of ceramic materials capable of withstanding high temperatures for pro-
longed periods of time.
Slip A liquid slurry that is poured into a mold. When the slurry begins to harden at the mold
surface, the remaining liquid slurry is decanted, leaving behind a hollow ceramic casting.
Slip casting Forming a hollow ceramic part by introducing a pourable slurry into a mold. The
water in the slurry is extracted into the porous mold, leaving behind a drier surface. Excess slurry
can then be decanted.
Spray drying A slurry of a ceramic powder is sprayed into a large chamber in the presence of
hot air. This leads to the formation of soft agglomerates that can flow well into the dies used
during powder compaction.
Synthesis Steps conducted to make a ceramic powder.
Tape casting A process for making thin sheets of ceramics using a ceramic slurry consisting of
binders, plasticizers, etc. The slurry is cast with the help of a blade onto a plastic substrate. The
resultant green tape is then dried, cut, and machined and used to make electronic ceramic and
other devices.
Tempered glass A high-strength glass that has a surface layer where the stress is compressive,
induced thermally during cooling or by the chemical di¤usion of ions.
True porosity The percentage of a ceramic body that is composed of both closed and inter-
connected porosity.
Vitrification Melting, or formation of a glass.
CHAPTER 15 Ceramic Materials494

PROBLEMS
3
Section 15-1 Applications of Ceramics
15-1 What are the primary types of atomic bonds in
ceramics?
15-2 Explain the meaning of following terms:
ceramics, inorganic glasses, and glass-ceramics.
15-3 Explain why ceramics typically are processed as
powders. How is this similar to or di¤erent from
the processing of metals?
15-4 What do the terms ‘‘glaze’’ and ‘‘enamel’’ mean?
15-5 What material is used to make the tiles that pro-
vide thermal protection in NASA’s space shuttle?
15-6 Which ceramic materials are most widely used?
15-7 Explain how ceramic materials can be classified
in di¤erent ways.
15-8 State any one application of the following ce-
ramics: (a) alumina, (b) silica, (c) barium titanate,
(d) zirconia, (e) boron carbide, and (f) diamond.
Section 15-2 Properties of Ceramics
15-9 What are some of the typical characteristics of
ceramic materials?
15-10 Why is the tensile strength of ceramics much
lower than their compressive strength?
15-11 Plastic deformation due to dislocation motion
is important in metals; however, this is not a
very important consideration for the properties
of ceramics and glasses. Explain.
15-12 Can ceramic materials show superplastic be-
havior or are they always brittle? Explain.
15-13 Explain why the strength of ceramics tends to
show a wide scatter in their mechanical prop-
erties.
Section 15-3 Synthesis and Processing
of Ceramic Powders
15-14 What is the driving force to sintering?
15-15 What is the driving force to grain growth?
15-16 What mechanisms of di¤usion play the most
important role in the solid-state sintering of
ceramics?
15-17 Explain the use of the following processes (use a
sketch as needed): (a) uniaxial compaction and
sintering, (b) hot pressing, (c) HIP, and (d) tape
casting.
Section 15-4 Characteristics of Sintered
Ceramics
15-18 What are some of the important characteristics
of sintered ceramics?
15-19 What typical density levels are obtained in sin-
tered ceramics?
15-20 What do the terms ‘‘apparent porosity’’ and
‘‘true porosity’’ of ceramics mean?
15-21 The specific gravity of Al
2
O
3
is 3.96 g/cm
3
.A
ceramic part is produced by sintering alumina
powder. It weighs 80 g when dry, 92 g after it
has soaked in water, and 58 g when suspended
in water. Calculate the apparent porosity, the
true porosity, and the closed pores.
15-22 Silicon carbide (SiC) has a specific gravity of
3.1 g/cm
3
. A sintered SiC part is produced,
occupying a volume of 500 cm
3
and weighing
1200 g. After soaking in water, the part weighs
1250 g. Calculate the bulk density, the true
porosity, and the volume fraction of the total
porosity that consists of closed pores.
Section 15-5 Inorganic Glasses
15-23 What is the main reason why glass formation is
easy in silicate systems?
15-24 Can glasses be formed using metallic materials?
15-25 Define the terms ‘‘glass formers’’, ‘‘intermedi-
ates’’, and ‘‘modifiers’’.
15-26 What does the term ‘‘glass temperature’’ mean?
Is this a fixed temperature for a given composi-
tion of glass?
15-27 How many grams of BaO can be added to 1 kg
of SiO
2
before the O:Si ratio exceeds 2.5 and
glass-forming tendencies are poor? Compare
this with the case when Li
2
O is added to SiO
2
.
15-28 Calculate the O:Si ratio when 30 wt% Y
2
O
3
is
added to SiO
2
. Will this material provide good
glass-forming tendencies?
Section 15-6 Glass-Ceramics
15-29 How is a glass-ceramic di¤erent from a glass
and a ceramic?
15-30 What are the advantages of using glass-ceramics
as compared to either glasses or ceramics?
15-31 Draw a typical heat-treatment profile encoun-
tered in processing of glass-ceramics.
15-32 What are some of the important applications of
glass-ceramics?
Problems 495

16
Polymers
Have You Ever Wondered?
9 What are compact disks (CDs) made from?
9 What is Silly Putty
<
made from?
9 What polymer is used in chewing gum?
9 Which was the first synthetic fiber ever made?
9 Why are some plastics ‘‘dishwasher safe’’ and some not?
9 What are bulletproof vests made from?
9 What polymer is used for non-stick cookware?
The word mer means a ‘‘unit.’’ In this context,
the term mer refers to a unit group of atoms or
molecules that defines a characteristic arrange-
ment for a polymer. A polymer can be thought of
as a material made by combining several mers or
units. Polymers are materials consisting of giant
or macromolecules, chain-like molecules having
average molecular weights from 10,000 to
more than 1,000,000 g/mol. They are built by
joining many mers or units through chemical
bonding. Molecular weight is defined as the sum
of atomic masses in each molecule. Most poly-
mers, solids or liquids, are carbon-based; how-
ever, they can be inorganic (e.g., silicones based
on a Si-O network). Plastics are materials that
are composed principally of polymers containing
496

additives such as glass fibers, fillers, pigments,
and the like that further enhance their proper-
ties. Plastics include thermoplastics (commodity
and engineering), thermoset materials, and elas-
tomers (natural or synthetic). In this book, we use
the terms plastics and polymers interchangably.
Polymerization is the process by which small
molecules consisting of one unit (known as a
monomer) or a few units (known as oligomers) are
chemically joined to create these giant mole-
cules. Polymerization normally begins with the
production of long chains in which the atoms
are strongly joined by covalent bonding. Plastics
are used in an amazing number of applications
including clothing, toys, home appliances, struc-
tural and decorative items, coatings, paints, ad-
hesives, automobile tires, biomedical devices, car
bumpers and interiors, foams, andpackaging. Poly-
mers are often used in composites, both as fibers
and as a matrix. Liquid crystal displays (LCDs)
are based on polymers. We also use polymers
to make eyeglasses with photochromic lenses.
Plastics are often used to make electronic com-
ponents because of their insulating ability and
low dielectric constant. More recently, signifi-
cant developments have occurred in the area of
flexible electronic devices based on the useful
piezoelectricity, semiconductivity, optical and
electro-optical properties seen in some polymers.
Polymers such as polyvinyl acetate (PVA) are
water-soluble. Many such polymers can be dis-
solved in water or organic solvents to be used as
binders, surfactants, or plasticizers in processing
ceramics and semiconductors, and as additives to
many consumer products. Polyvinyl butyral
(PVB), a polymer, makes up part of the laminated
glass used for car windshields (Chapter 15).
Polymers are probably used in more technologies
than any other class of materials.
Commercial—or standard commodity—poly-
mers are lightweight, corrosion- resistant mate-
rials with low strength and stiffness, and they are
not suitable for use at high temperatures. These
polymers are, however, relatively inexpensive and
are readily formed into a variety of shapes, rang-
ing from plastic bags to mechanical gears to
bathtubs. Engineering polymers are designed to
give improved strength or better performance at
elevated temperatures. These materials are pro-
duced in relatively small quantities and often are
expensive. Some of the engineering polymers
can perform at temperatures as high as 350
C;
others—usually in a fiber form—have strengths
that are greater than that of steel.
Polymers also have many useful physical
properties. Some polymers such as acrylics like
Plexiglas
TM
and Lucite
TM
are transparent and can
substitute for glasses. Although most polymers
are electrical insulators, special polymers (such
as the acetals) and polymer-based composites
possess useful electrical conductivity. Teflon
TM
has a low coefficient of friction and is the coating
for nonstick cookware. Polymers also resist cor-
rosion and chemical attack.
16-1 Classification of Polymers
Polymers are classified in several ways: by how the molecules are synthesized, by their
molecular structure, or by their chemical family. One way to classify polymers is to
state if the polymer is a linear polymer or a branched polymer (Figure 16-1). A linear
polymer consists of spaghetti-like molecular chains. In a branched polymer, there are
primary polymer chains and secondary o¤shoots of smaller chains that stem from these
main chains. Note that even though we say ‘‘linear’’, the chains are actually not in the
form of straight lines. A better method to describe polymers is in terms of their me-
chanical and thermal behavior. Table 16-1 compares the three major polymer categories.
16-1 Classification of Polymers 497
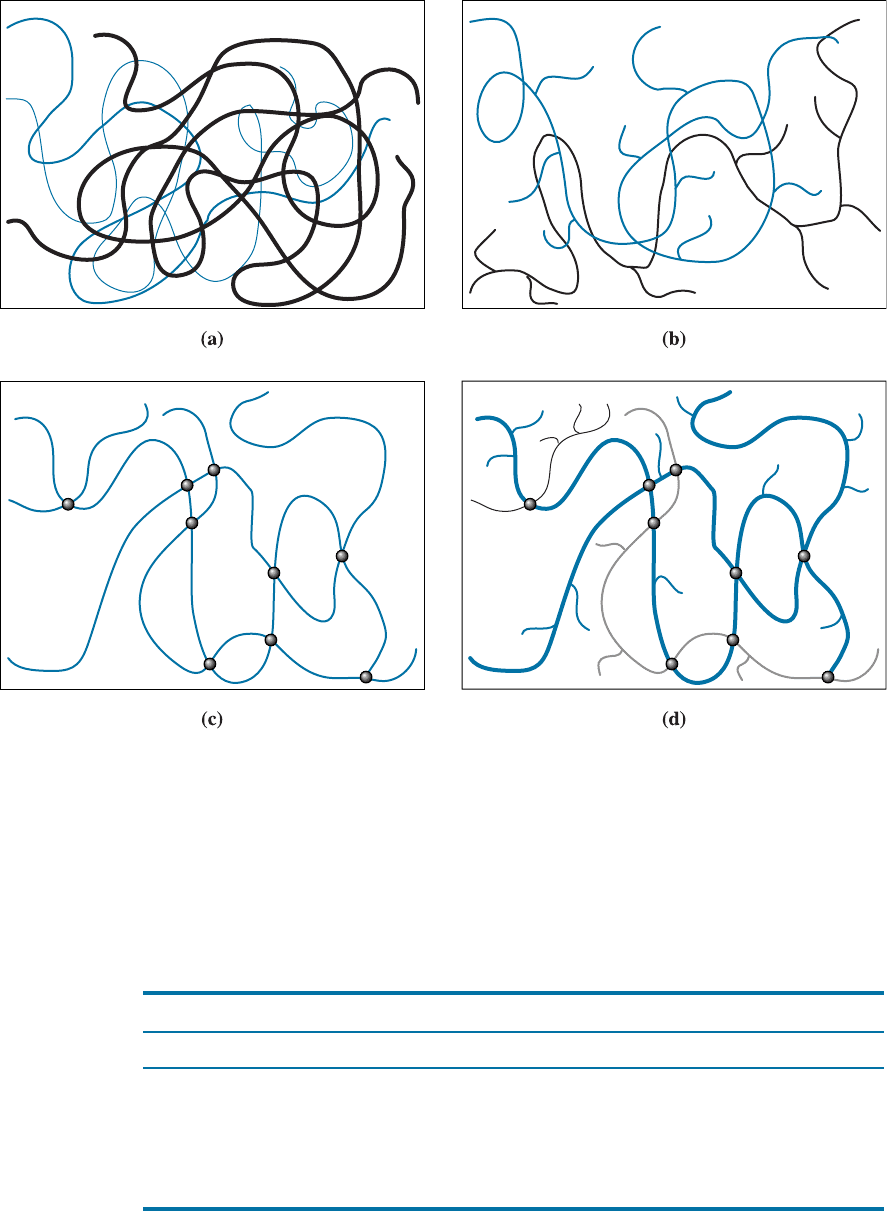
Figure 16-1 Schematic showing linear and branched polymers. Note that branching can
occur in any type of polymer (e.g., thermoplastics, thermosets, and elastomers). (a) Linear
unbranched polymer: notice chains are not straight lines and not connected. Different polymer
chains are shown using different shades and design to show clearly that each chain is not
connected to another. (b) Linear branched polymer: chains are not connected, however they
have branches. (c) Thermoset polymer without branching: chains are connected to one another
by covalent bonds but they do not have branches. Joining points are highlighted with solid
circles. (d) Thermoset polymer that has branches and chains that are interconnected via
covalent bonds. Different chains and branches are shown in different shades for better
contrast. Places where chains are actually chemically bonded are shown with filled circles.
TABLE 16-1 9 Comparison of the three polymer categories
Behavior General Structure Example
Thermoplastic Flexible linear chains (straight or branched) Polyethylene
Thermosetting Rigid three-dimensional network (chains may be
linear or branched)
Polyurethanes
Elastomers Thermoplastics or lightly cross-linked thermosets,
consist of spring-like molecules
Natural rubber
CHAPTER 16 Polymers498
Thermoplastics are composed of long chains produced by joining together mono-
mers; they typically behave in a ductile manner. The chains may or may not have
branches. Individual chains are intertwined. There are relatively weak van der Waals
bonds between atoms of di¤erent cha ins. This is somewhat similar to a few trees that
are tangled up together. The trees may or may not have branches, each tree is on its
own and not connected to another. The chains in thermoplastics can be untangled by
application of a tensile stress. Thermoplastics can be amorphous or crystalline. Upon
heating, thermoplastics soften and melt. They are processed into shapes by heating to
elevated temperatures. Thermoplastics are easily recycled.
Thermosetting polymers are composed of long chains (linear or branched) of mole-
cules that are strongly cross-linked to one another to form three-dimensional network
structures. Network or thermosetting polymers are like a bunch of strings that are
knotted to one another in several places and not just tangled up. Each string may have
other side strings attached to it. Thermosets are generally stronger, but mor e brittle,
than thermoplastics. Thermosets do not melt upon heating but begin to decompose.
They cannot easily be reprocessed after the cross-linking reaction has occurred and
hence recycling is di‰cult.
Elastomers These are known as rubbers. They have an elastic deformation >200%.
These may be thermoplastics or lightly cross-linked thermosets. The polymer chains
consist of coil-like molecules that can reversibly stretch by applying a force.
Thermoplastic elastomers are a special group of polymers. They have the processing
ease of thermoplastics and the elastic behavior of elastomers.
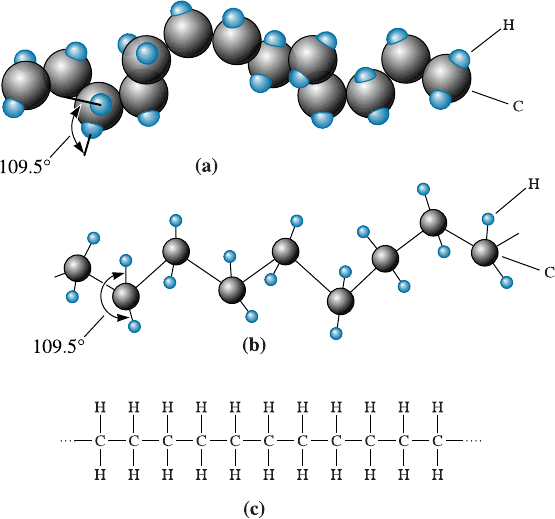
Representative Structures Figure 16-2 shows three ways we could represent a segment
of polyethylene, the simplest of the thermoplastics. The polymer chain consists of a
Figure 16-2 Three ways to represent the structure of polyethylene: (a) a solid three-dimensional
model, (b) a three-dimensional ‘‘space’’ model, and (c) a simple two-dimensional model.
16-1 Classification of Polymers 499
