Askeland D.R., Fulay P.P. Essentials of Materials Science & Engineering
Подождите немного. Документ загружается.

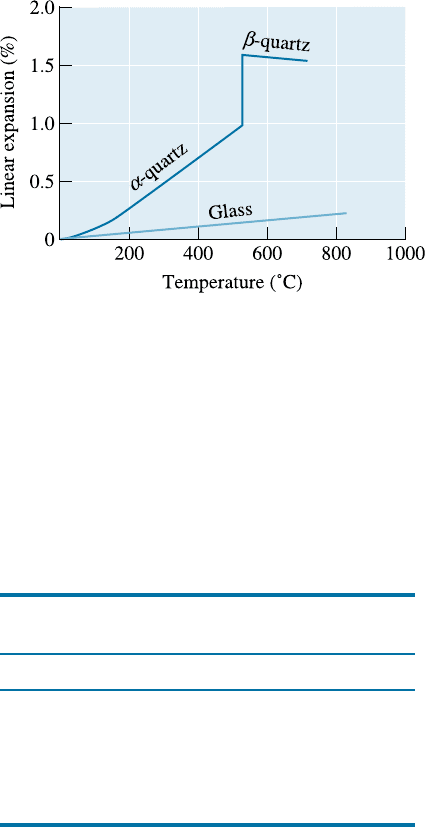
Silicate Glasses The silicate glasses are the most widely used. Fused silica, formed
from pure SiO
2
, has a high-melting point, and the dimensional changes during heating
and cooling are small (Figure 15-6). Generally, however, the silicate glasses contain
additional oxides (Table 15-5). While oxides such as silica behave as glass formers,an
intermediate oxide (such as lead oxide or aluminum oxide) does not form a glass by
itself but is incorporated into the network structure of the glass formers. A third group
of oxides, the modifiers, break up the network structure and eventually cause the glass
to devitrify, or crystallize.
Modified Silicate Glasses Modifiers break up the silica network if the oxygen-to-
silicon ratio (O:Si) increases significantly. When Na
2
O is added to silica glass, for ex-
ample, the sodium ions enter holes within the network rather than becoming part of the
network. However, the oxygen ion that enters with the Na
2
O does become part of the
network (Figure 15-7). When this happens, there are not enough silicon ions to combine
with the extra oxygen ions and keep the network intact. Eventually, a high O:Si ratio
causes the remaining silica tetrahedra to form chains, rings, or compounds, and the
silica no longer transforms to a glass. When the O:Si ratio is above about 2.5, silica
glasses are di‰cult to form; above a ratio of three, a glass forms only when special
precautions are taken, such as the use of rapid cooling rates.
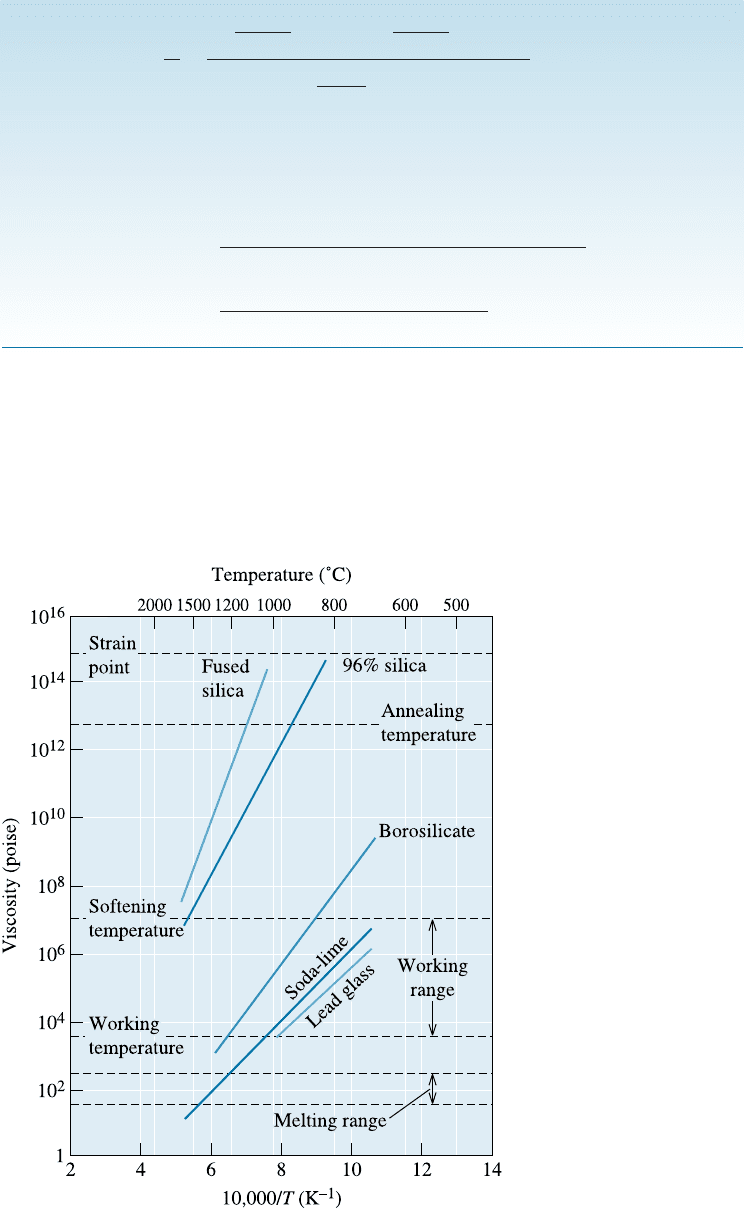
Modification also lowers the melting point and viscosity of silica, making it possible
to produce glass at lower temperatures. The e¤ect of Na
2
O additions to silica is shown
in Figure 15-8. As you can see, the addition of Na
2
O produces eutectics with very low
melting temperatures. Adding CaO, which reduces the solubility of the glass in water,
further modifies these glasses. The example that follows shows how to design a glass.
Figure 15-6
The expansion of quartz. In addition
to the regular—almost linear—
expansion, a large, abrupt expansion
accompanies the a-tob-quartz
transformation. However, glasses
expand uniformly.
TABLE 15-5 9 Division of the oxides into glass
formers, intermediates, and modifiers
Glass Formers Intermediates Modifiers
B
2
O
3
TiO
2
Y
2
O
3
SiO
2
ZnO MgO
GeO
2
PbO
2
CaO
P
2
O
5
Al
2
O
3
PbO
V
2
O
3
BeO Na
2
O
CHAPTER 15 Ceramic Materials480
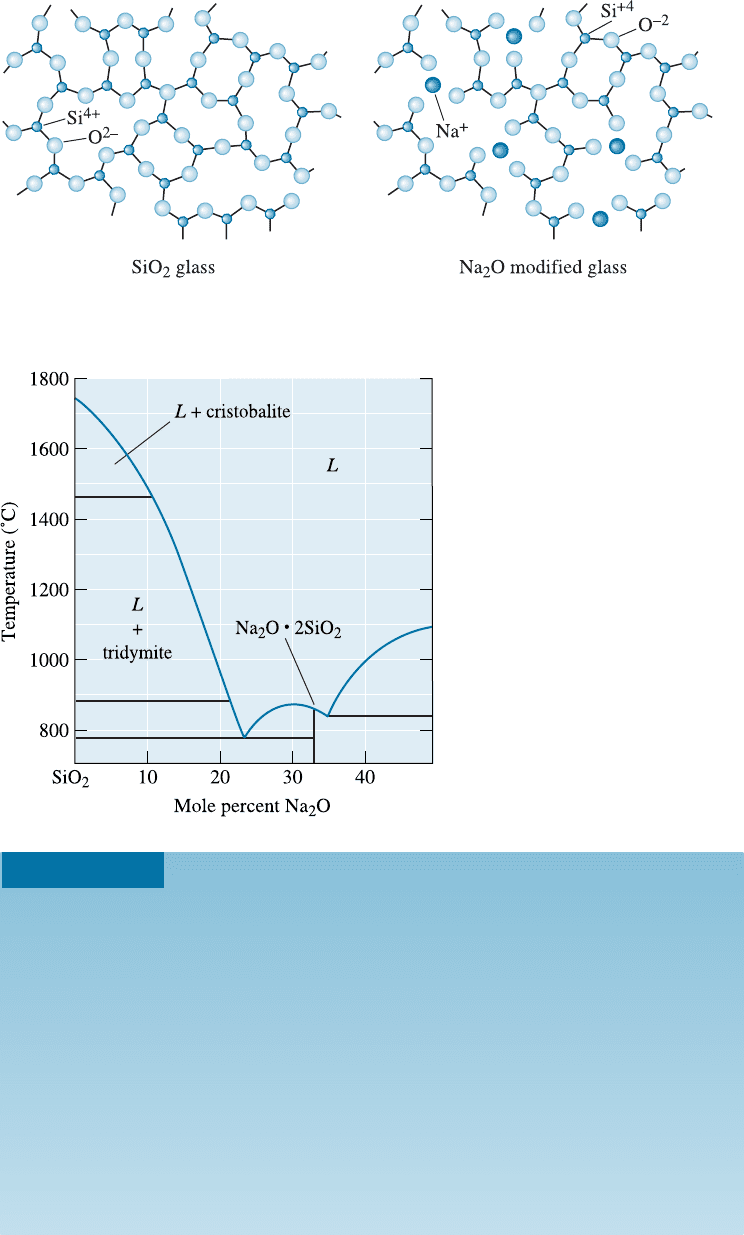
Figure 15-7 The effect of Na
2
O on the silica glass network. Sodium oxide is a modifier,
disrupting the glassy network and reducing the ability to form a glass.
Figure 15-8
The SiO
2
-Na
2
O phase diagram.
Additions of soda (Na
2
O) to silica
dramatically reduce the melting
temperature of silica by forming
eutectics.
EXAMPLE 15-2
Design of a Glass
We produce good chemical resistance in a glass when we introduce B
2
O
3
into
silica. To assure that we have good glass-forming tendencies, we wish the O:Si
ratio to be no more than 2.5, but we also want the glassware to have a low-
melting temperature to make the glass-forming process easier and more eco-
nomical. Design such a glass.
SOLUTION
Because B
2
O
3
reduces the melting temperature of silica, we would like to add
as much as possible. We also, however, want to assure that the O:Si ratio is no
more than 2.5, so the amount of B
2
O
3
is limited. As an example, let us deter-
mine the amount of B
2
O
3
we must add to obtain exactly an O:Si ratio of 2.5.
Let f
B
be the mole fraction of B
2
O
3
added to the glass, and ð1 f
B
Þ be the
mole fraction of SiO
2
:
15-5 In organic Glasses 481
O
Si
¼
3
O ions
B
2
O
3
ðf
B
Þþ 2
O ions
SiO
2
ð1 f
B
Þ
1
Si ion
SiO
2
ð1 f
B
Þ
¼ 2:5
3f
B
þ 2 2f
B
¼ 2:5 2:5f
B
or f
B
¼ 0:143
Therefore, we must produce a glass containing no more than 14.3 mol% B
2
O
3
.
In weight percent:
wt% B
2
O
3
¼
ðf
B
Þð69:62 g=molÞ
ðf
B
Þð69:62 g=molÞþð1 f
B
Þð60:08 g=molÞ
100
wt% B
2
O
3
¼
ð0:143Þð69:62Þ
ð0:143Þð69:62Þþð0:857Þð60:08Þ
100 ¼ 16:2
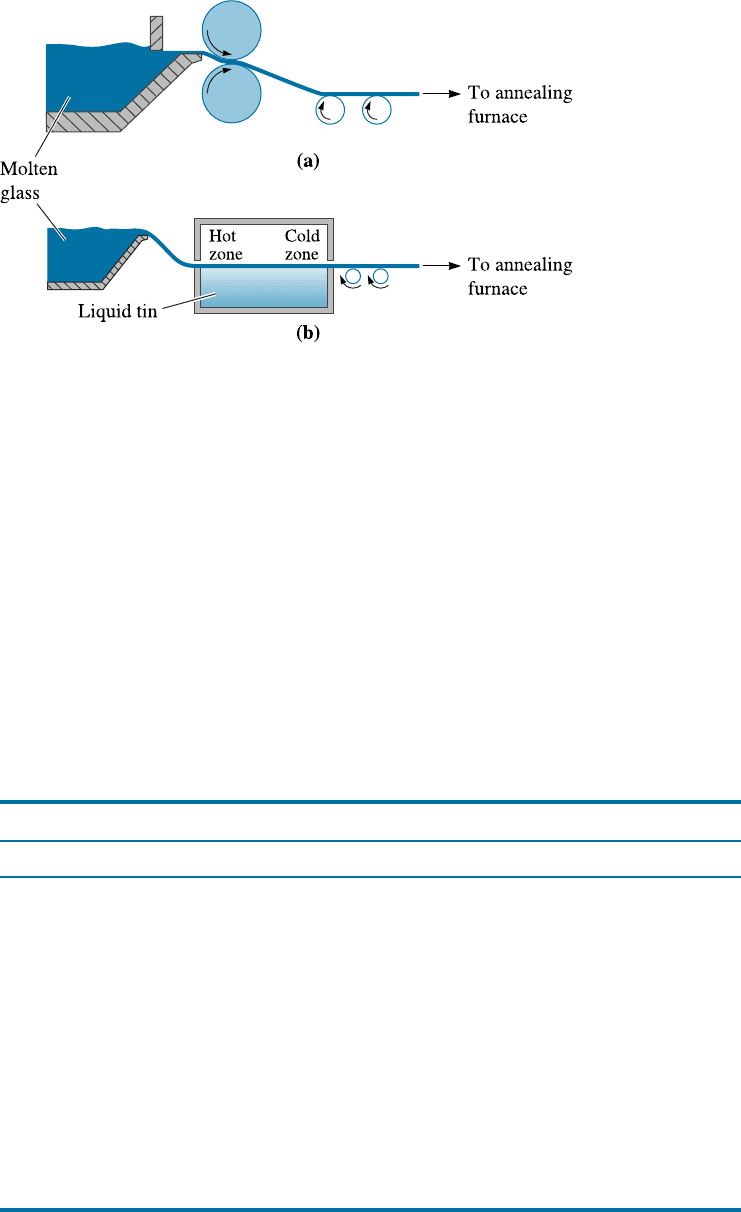
Glasses are manufactured into useful articles at a high temperature with viscosity con-
trolled so that the glass can be shaped without breaking. Figure 15-9 helps us under-
stand the processing in terms of the viscosity ranges.
1. Liquid range. Sheet and plate glass are produced when the glass is in the molten
state. Techniques include rolling the molten glass through water-cooled rolls or floating
Figure 15-9
The effect of temperature
and composition on the
viscosity of glass.
CHAPTER 15 Ceramic Materials482
the molten glass over a pool of liquid tin (Figure 15-10). This float-glass process pro-
duces an exceptionally smo oth surface on the glass. The development of the float-glass
process was a genuine breakthrough in the area of glass processing. Basic float-glass
composition has been essentially unchanged for many years (Table 15-6). Glasses for
automotive applications contain small amounts of iron oxide to control absorption of
light and infrared radiation.
Some glass shapes, including large optical mirror s, are produced by casting the
molten glass into a mold, then assuring that cooling is as slow as possible to minimize
residual stresses and avoid cracking of the glass part. Glass fibers may be produced by
drawing the liquid glass through small openings in a platinum die [Figure 15-11(c)].
Typically, many fibers are produced simultaneously for a single die.
2. Working range. Shapes such as those of containers or light bulbs can be formed
by pressing, drawing, or blowing glass into molds (Figure 15-11). A hot gob of liquid
Figure 15-10 Techniques for manufacturing sheet and plate glass: (a) rolling and (b) floating
the glass on molten tin.
TABLE 15-6 9 Compositions of typical glasses (in weight percent)
Glass SiO
2
Al
2
O
3
CaO Na
2
OB
2
O
3
MgO PbO Others
Fused silica 99
Vycor
TM
96 4
Pyrex
TM
81 2 4 12
Glass jars 74 1 5 15 4
Window glass 72 1 10 14 2
Plate glass/Float glass 73 1 13 13
Light bulbs 74 1 5 16 4
Fibers 54 14 16 10 4
Thermometer 73 6 10 10
Lead glass or crystal 67 6 17 10% K
2
O
Optical flint 50 1 19 13% BaO, 8% K
2
O, ZnO
Optical crown 70 8 10 2% BaO, 8% K
2
O
E-glass fibers 55 15 20 10
S-glass fibers 65 25 10
15-5 In organic Glasses 483
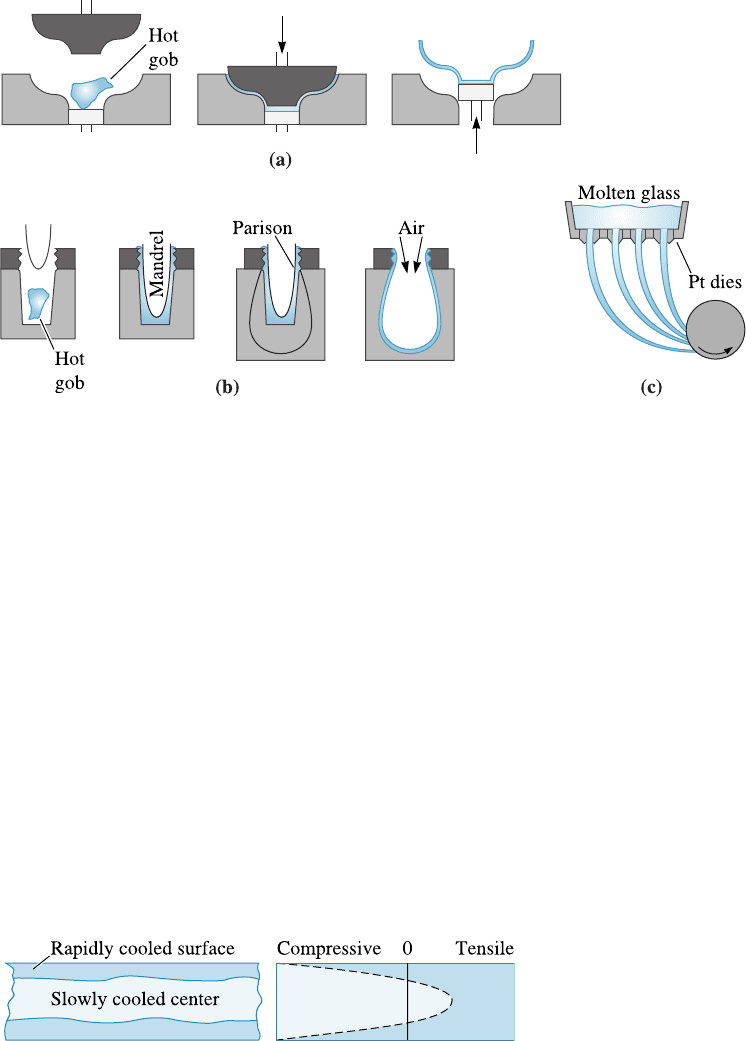
glass may be preformed into a crude shape (parison), then pressed or blown into a
heated die to produce the final shape. The glass is heated to the working range so that
the glass is formable, but not ‘‘runny.’’
3. Annealing range. Some glass parts may be annealed to reduce residual stresses
introduced during forming. Large glass castings, for example, are often annealed and
slowly cooled to prevent cracking. Some glasses may be heat treated to cause devitrifi-
cation, or the precipitation of a crystalline phase from the glass.
Tempered glass is produced by quenching the surface of plate glass with air, causing
the surface layers to cool and contract. When the center cools, its contraction is re-
strained by the already rigid surface, which is placed in compression (Figure 15-12). Tem-
pered glass is capable of withstanding much higher tensile stresses and impact blows
than untempered glass. Tempered glass is used in car and home windows, shelving for
refrigerators, ovens, furniture, and many other applications where safety is important.
Laminated glass, consisting of two annealed glass pieces with a polymer (such as poly-
vinyl butyral or PVB) in between, is shatterproof and used to make car windshields.
Glass Compositions Pure SiO
2
must be heated to very high temperatures to obtain
viscosities that permit economical forming. Most commercial glasses are based on silica
(Table 15-6); modifiers such as soda (Na
2
O) are added to break down the network
structure and form eutectics with low melting temperatures, whereas lime (CaO) is
added to reduce the solubili ty of the glass in water. The most common commercial glass
Figure 15-11 Techniques for forming glass products: (a) pressing, (b) press and blow process,
and (c) drawing of fibers.
Figure 15-12
Tempered glass is cooled
rapidly to produce
compressive residual
stresses at the surface.
CHAPTER 15 Ceramic Materials484

contains approximately 75% SiO
2
, 15% Na
2
O, and 10% CaO and is known as soda-
lime glass.
Borosilicate glasses, which contain about 15% B
2
O
3
, have excellent chemical and
dimensional stability. Their uses include laboratory glassware (Pyrex
TM
) and containers
for the disposal of high-level radioactive nuclear waste. Calcium aluminoborosilicate
glass—or E-glass—is used as a general-purpose fiber for composite materials, such as
fiberglass. Aluminosil icate glass, with 20% Al
2
O
3
and 12% MgO, and high-silica
glasses, with 3% B
2
O
3
, are excellent for high-temperature resistance and for protection
against heat or thermal shock. The S-glass, a magnesium aluminosilicate, is used to
produce high-strength fibers for composite materials. Fused silica, or virtually pure
SiO
2
, has the best resistance to high temperature, thermal shock, and chemical attack,
although it is also expensive.
Special optical qualities can also be obtained, including sensitivity to light. Photo-
chromic glass, which is darkened by the ultraviolet portion of sunlight, is used for sun-
glasses. Photosensitive glass darkens permanently when exposed to ultraviolet light;
if only selected portions of the glass are exposed and then immersed in hydrofluoric
acid, etchings can be produced. Polychromatic glasses are sensitive to all light, not just
ultraviolet radiation. Similarly, nanosized crystals of semiconductors such as cadmium
sulfide (CdS) are nucleated in silicate glasses in a process known as striking. These
glasses exhibit lively colors and also have useful optical properties.
15-6 Glass-Ceramics
Glass-ceramics are crystalline materials that are derived from amorphous glasses. Usu-
ally, glass-ceramics have a substantial level of crystallinity (@>70–99%). The formation
of glass-ceramics was discovered serendipitously by Don Stookey. With glass-ceramics,
we can take advantage of the formability of glass. Also a product that contains very
low porosity can be obtained by producing a shape with conventional glass-forming
techniques, such as pressing or blowing. Glass, however, has poor creep resistance. We
then crystallize the glass using heterogeneous nucleation by such oxides as TiO
2
and/or
ZrO
2
. These oxides react with the glass and with each other and provide the nuclei that
ultimately lead to glass crystallization. Phase separation of glasses plays an important
role in formation of the nuclei. In some commercial glass-ceramics (e.g., Visionware
TM
)
nano-sized crystallites are formed and the resultant material remains optically trans-
parent.
The first step in producing a glass-ceramic is to assure that crystallization does not
occur during cooling from the forming temperature. A continuous and isothermal
cooling transformation diagram, much like the CCT and TTT diagrams for steels, can
be seen for silicate-based glasses. Figure 15-13(a) shows a TTT diagram for a glass. If
glass cools too slowly, a transformation line is crossed ; nucleation and growth of the
crystals begin, but in an uncontrolled manner. Addition of modif ying oxides to glass,
much like addition of alloying elements to steel, shifts the transformation curve to
longer times and prevents devitrification even at slow cooling rates. As noted in pre-
vious chapters, strictly speaking we should make use of CCT (and not TTT) diagrams
for this discussion.
Nucleation of the crystalline phase is controlled in two ways. First, the glass con-
tains agents, such as TiO
2
, that react with other oxides and form phases that provide
the nucleation sites. Second, a heat treatment is designed to provide the appropriate
number of nuclei; the temperature should be relatively low in order to maximize the rate
15-6 Glass-Ceramics 485
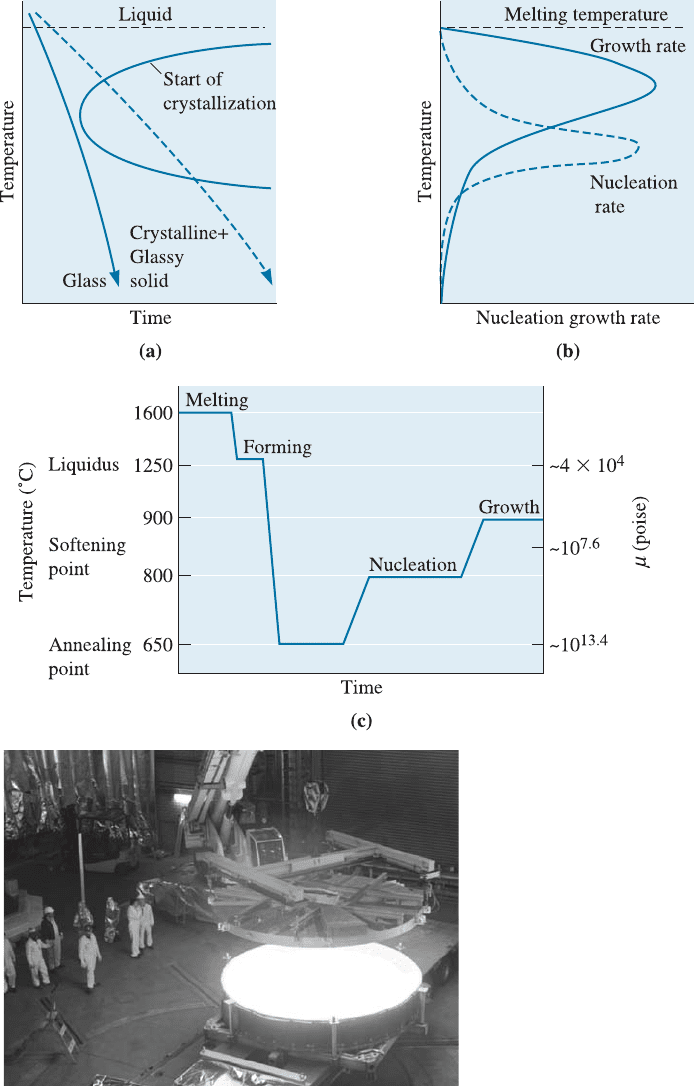
Figure 15-13 Producing a glass-ceramic: (a) Cooling must be rapid to avoid the start of
crystallization. Isothermal and continuous cooling curves for lunar glass. (b) The rate of
nucleation of precipitates is high at low temperatures, whereas the rate of growth of the
precipitates is high at higher temperatures. (c) A typical heat-treatment profile for glass-
ceramics fabrication, illustrated here for Li
2
O-Al
2
O
3
-SiO
2
glasses. (d) A large mirror blank of
the Zerodur
TM
made by Schott Glass. (Source: Photo Courtesy of Schott Glass.)
CHAPTER 15 Ceramic Materials486

of nucleation [Figure 15-13(b)]. However, the overall rate of crystallization depends on
the growth rate of the crystals once nucleation occurs; higher temperatures are required
to maximize the growth rate. Consequently, a heat-treatment schedule similar to that
shown in Figure 15-13(c) for the Li
2
O-Al
2
O
3
-SiO
2
glass-ceramics (Pyroceram
TM
) can be
used. The low-temperature step provides nucleation sites, and the high-temperature step
speeds the rate of growth of the crystals; as much as 99% of the part may crystallize.
This special structure of glass-ceramics can provide good mechanical strength and
toughness, often with a low coe‰cient of thermal expansion and high-t emperature
corrosion resistance. Perhaps the most important glass-ceramic is based on the Li
2
O-
Al
2
O
3
-SiO
2
system. These materials are used for cooking utensils (Corning Ware
TM
)
and ceramic tops for stoves. Other glass-ceramics are used in communication, com-
puter, and optical applications. Figure 15-13(d) shows the processing of a large
telescope mirror being prepared using the Zerodur
TM
glass-ceramic materia l. This
glass-ceramic is especially suited for telescope mirror blanks because it has a very small
coe‰cient of thermal expansion (Chapter 2).
15-7 Processing and Applications of Clay Products
Crystalline ceramics are often manufactured into useful articles by preparing a shape, or
compact, composed of the raw materials in a fine powder form. The powders are then
bonded by chemical reaction, partial or complete vitrification (melting), or sintering.
Clay products form a group of traditional ceramics used for producing pipe, brick,
cooking ware, and other common products. Clay, such as kaolinite, and water serve as
the initial binder for the ceramic powders, which are typically silica. Other materials,
such as feldspar [(K, Na)
2
O Al
2
O
3
6SiO
2
] serve as fluxing (glass-forming) agents dur-
ing later heat treatment.
Forming Techniques for Clay Products The powders, clay, flux, and water are mixed
and formed into a shape (Figures 15-3 and 15-4). Dry or semi-dry mixtures are me-
chanically pressed into ‘‘green’’ (unbaked) shapes of su‰cient strength to be handled.
For more uniform compaction of complex shapes, isostatic pressing may be done; the
powders are placed into a rubber mold and subjected to high pressures through a gas or
liquid medium. Higher moisture contents permit the powders to be more plastic or
formable. Hydroplastic forming processes, including extrusion, jiggering, and hand
working, can be applied to these plastic mixes. Ceramic slurries containing large
amounts of organic plasticizers, rather than water, can be injected into molds.
Still higher moisture contents permit the formation of a slip, or pourable slurry,
containing fine ceramic powder. The slip is poured into a porous mold. The water in the
slip nearest to the mold wall is drawn into the mold, leaving behind a soft solid which
has a low-moisture content. When enough water has been drawn from the slip to pro-
duce a desired thickness of solid, the remain ing liquid slip is poured from the mold,
leaving behind a hollow shell (Figure 15-4). Slip casting is used in manufacturing
washbasins and other commercial products. After forming, the ceramic bodies—or
greenware—are still weak, contain water or other lubricants, and are porous, and sub-
sequent drying and firing are required.
Drying and Firing of Clay Products During drying, excess moisture is removed and
large dimensional changes occur. Initially, the water between the clay platelets—the
interparticle water—evaporates and provides most of the shrinkage. Relatively little
15-7 Proce ssing and Applications of Clay Products 487
dimensional change occurs as the remaining wa ter between the pores evaporates. The
temperature and humidity are carefully controlled to provide uniform drying through-
out the part, thus minimizing stresses, distortion, and cracking.
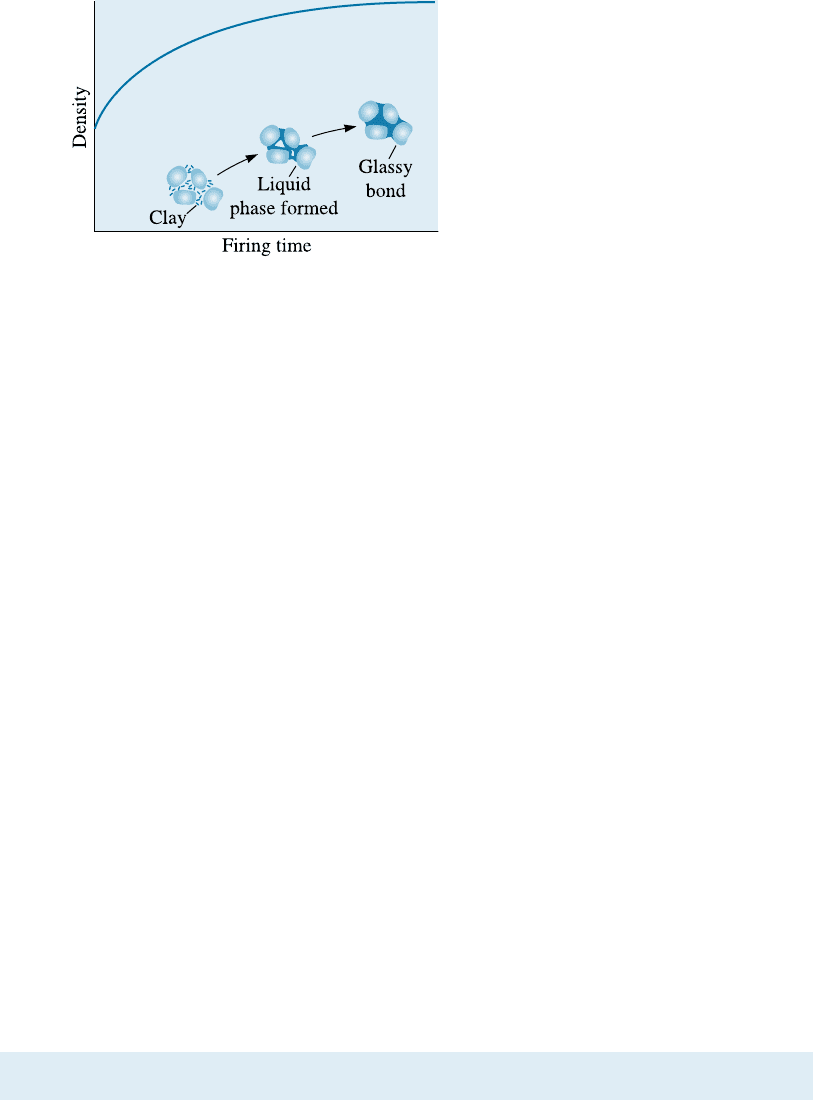
The rigidity and strength of a ceramic part are obtained during firing. During
heating, the clay dehydrates, eliminating the hydrated water that is part of the kaolinite
crystal structure, and vitrification begins (Figure 15-14). Impurities and the fluxing
agent react with the ceramic particles (SiO
2
) and clay, producing a low-melting-point
liquid phase at the grain surfaces. The liquid helps eliminate porosity and, after cooling,
changes to a rigid glass that binds the ceramic particles. This glassy phase provides a
ceramic bond, but it also causes additional shrinkage of the entire ceramic body.
The grain size of the final part is determined primarily by the size of the original
powder particles. Furthermore, as the amount of flux increases, the melting temperature
decreases; more glass forms, and the pores become rounder and smaller. A smaller ini-
tial grain size accelerates this process by providing more surface area at which vitri-
fication can occur.
Applications of Clay Products Many structural clay products and whitewares are
produced using these processes. Brick and tile used for construction are pressed or ex-
truded into shape, dried, and fired to produce the ceramic bond. Higher firing temper-
atures or finer original particle sizes produce more vitrification, less porosity, and higher
density. The higher density improves mechanical properties but reduces the insulating
qualities of the brick or tile.
Earthenware are porous clay bodies fired at relatively low temperatures. Little
vitrification occurs; the porosity is very high and interconnected, and earthenware
ceramics may leak. Consequently, these products must be covered with an impermeable
glaze.
Higher firing temperatures, which provide more vitrification and less porosity,
produce stoneware. The stoneware, which is used for drainage and sewer pipe, contains
only 2% to 4% porosity. Ceramics known as china and porcelain require even higher
firing temperatures to cause complete vitrification and virtually no porosity.
15-8 Refractories
Refractory materials are important components of the equipment used in the produc-
tion, refining, and handling of metals and glasses, for constructing heat-treating furna-
ces, and for other high-temperature processing equipment. The refractories must surviv e
at high temperatu res without being corroded or we akened by the surrounding environ-
Figure 15-14
During firing, clay and other fluxing
materials react with coarser particles to
produce a glassy bond and reduce porosity.
CHAPTER 15 Ceramic Materials488
ment. Typical refractories are composed of coarse oxide particles bonded by a finer
refractory material. The finer material melts during firing, providing bonding. In some
cases, refractory bricks contain about 20 to 25% apparent porosity to provide improved
thermal insulation.
Refractories are often divided into three groups—acid, basic, and neutral—based
on their chemical behavior (Table 15-7).
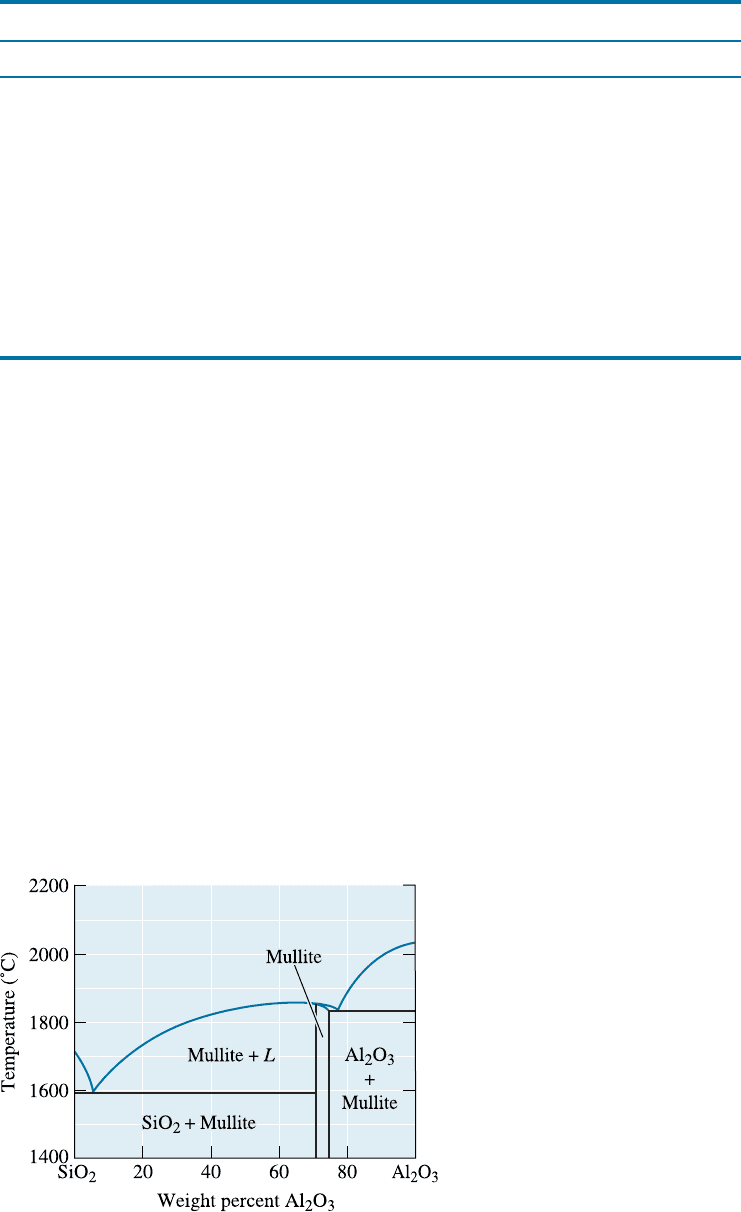
Acid Refractories Common acidic refractories include silica, alumina, and fireclay
(an impure kaolinite). Pure silica is sometimes used to contain molten metal. In some
applications, the silica may be bonded with small amounts of boron oxide, which melts
and produces the ceramic bond. When a small amount of alumina is added to silica, the
refractory contains a very low-melting-point eutectic microconstituent (Figure 15-15)
and is not suited for refractory applications at temperatures above about 1600
C, a
temperature often required for steel making. However, when larger amounts of alumina
are added, the microstructure contains increasing amounts of mullite, 3Al
2
O
3
2SiO
2
,
which has a high melting temperature. These fireclay refractories are generally relatively
weak, but they are inexpensive. Alumina concentrations above about 50% constitute
the high-alumina refractories.
TABLE 15-7 9 Compositions of typical refractories (weight percents)
Refractory SiO
2
Al
2
O
3
MgO Fe
2
O
3
Cr
2
O
3
Acidic
Silica 95–97
Superduty firebrick 51–53 43–44
High-alumina firebrick 10–45 50–80
Basic
Magnesite 83–93 2–7
Olivine 43 57
Neutral
Chromite 3–13 12–30 10–20 12–25 30–50
Chromite-magnesite 2–8 20–24 30–39 9–12 30–50
(From Ceramic Data Book, Cahners Publishing Co., 1982.)
Figure 15-15
A simplified SiO
2
-Al
2
O
3
phase
diagram, the basis for alumina
silicate refractories.
15-8 Refractories 489
