Salby M.L. Fundamentals of Atmospheric Physics
Подождите немного. Документ загружается.

260
9
Aerosol and Clouds
Asia (see Fig. 9.40). Combustion and secondary aerosols originate chiefly in
the industrialized regions of Europe and North America. These continental
source regions give the Northern Hemisphere greater number densities than
the Southern Hemisphere. Carbonaceous aerosol has large sources in tropical
regions due to agricultural burning and, through reaction with other species,
to vegetative emissions.
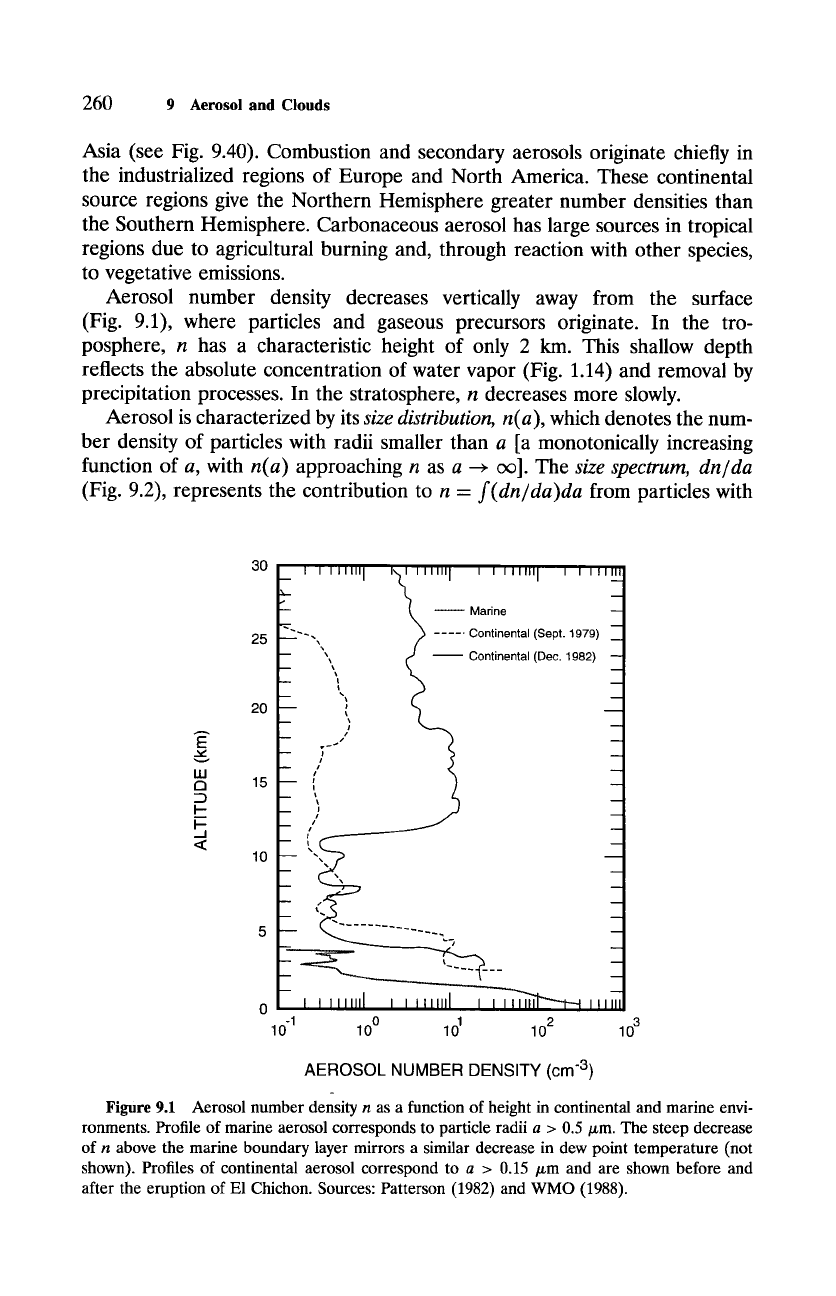
Aerosol number density decreases vertically away from the surface
(Fig. 9.1), where particles and gaseous precursors originate. In the tro-
posphere, n has a characteristic height of only 2 km. This shallow depth
reflects the absolute concentration of water vapor (Fig. 1.14) and removal by
precipitation processes. In the stratosphere, n decreases more slowly.
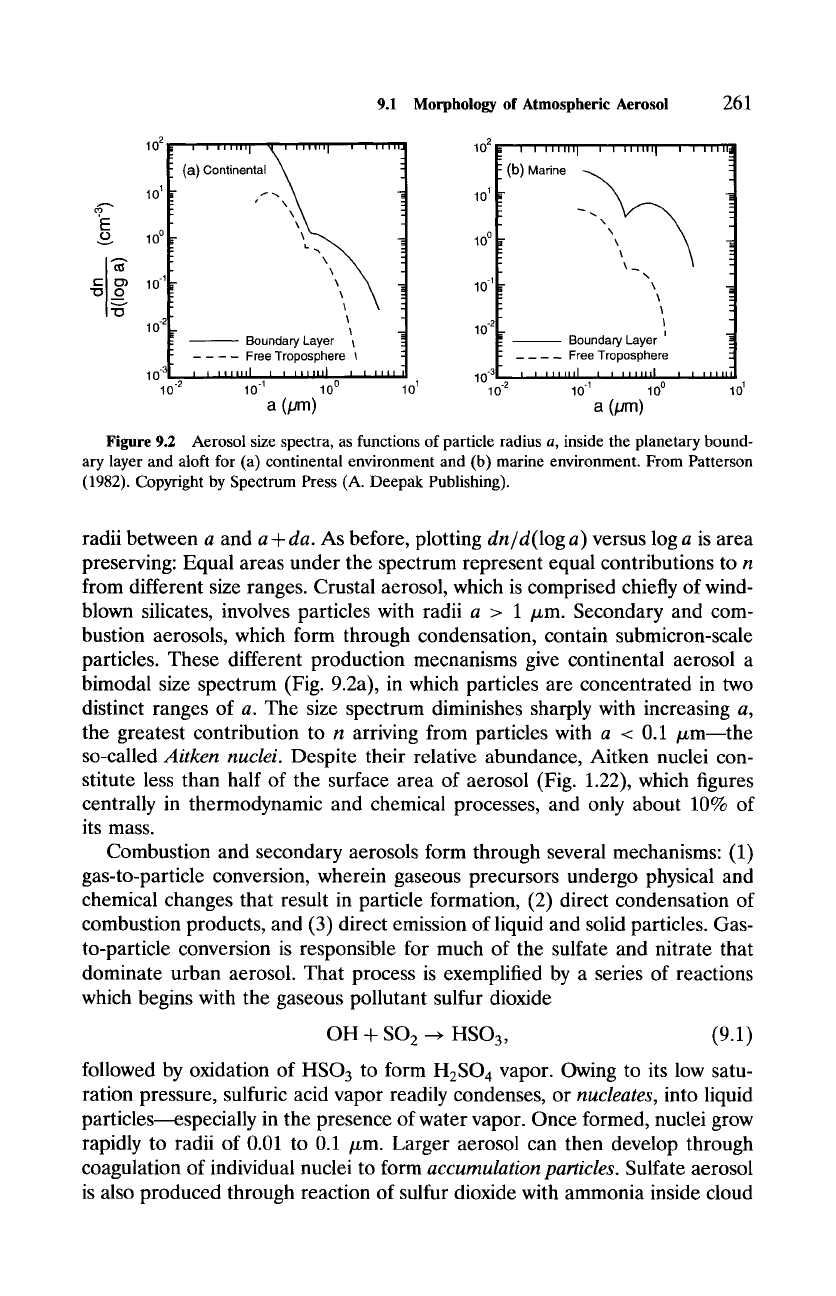
Aerosol is characterized by its size distribution, n(a), which denotes the num-
ber density of particles with radii smaller than a [a monotonically increasing
function of a, with n(a) approaching n as a ~ c~]. The size spectrum, dn/da
(Fig. 9.2), represents the contribution to n = f(dn/da)da from particles with
E
v
LH
a
F--
.J
<
30
25
20
15
10
_ I I I
I
IIII I
I
~ t
t
1
- ?
/
_ /
t
I
/
/
1
/ I Illlllli i llllllli
10 1 10 0 101
I I I~Jllll
I I ItllLI
..................... Marine
u
Continental (Sept.
1979) _
Continental (Dec. 1982) --
m
i i lilili~J"~ IIIII
10 2 103
AEROSOL NUMBER DENSITY (cm 3)
Figure 9.1 Aerosol number density n as a function of height in continental and marine envi-
ronments. Profile of marine aerosol corresponds to particle radii a > 0.5/zm. The steep decrease
of n above the marine boundary layer mirrors a similar decrease in dew point temperature (not
shown). Profiles of continental aerosol correspond to a > 0.15 /zm and are shown before and
after the eruption of El Chichon. Sources: Patterson (1982) and WMO (1988).
9.1 Morphology
of Atmospheric Aerosol
261
102 102
101 101
1 0 ~ 1 0 ~
] 0 .2 ] 0 .2
10
102 101 10 ~ 101
a (pro)
10 -3
10 -2
(b) Marine
.....
~N
N. "
\
\
I
Boundary lager
Free Troposphere
] I I I I II 11 I I I I I It II I I I I I II
10 1 100 101
a (pm)
Figure 9.2 Aerosol size spectra, as functions of particle radius a, inside the planetary bound-
ary layer and aloft for (a) continental environment and (b) marine environment. From Patterson
(1982). Copyright by Spectrum Press (A. Deepak Publishing).
radii between a and
a + da.
As before, plotting
dn/d(log a)
versus log a is area
preserving: Equal areas under the spectrum represent equal contributions to n
from different size ranges. Crustal aerosol, which is comprised chiefly of wind-
blown silicates, involves particles with radii a > 1 /zm. Secondary and com-
bustion aerosols, which form through condensation, contain submicron-scale
particles. These different production mechanisms give continental aerosol a
bimodal size spectrum (Fig. 9.2a), in which particles are concentrated in two
distinct ranges of a. The size spectrum diminishes sharply with increasing a,
the greatest contribution to n arriving from particles with a < 0.1 ~m--the
so-called
Aitken nuclei.
Despite their relative abundance, Aitken nuclei con-
stitute less than half of the surface area of aerosol (Fig. 1.22), which figures
centrally in thermodynamic and chemical processes, and only about 10% of
its mass.
Combustion and secondary aerosols form through several mechanisms: (1)
gas-to-particle conversion, wherein gaseous precursors undergo physical and
chemical changes that result in particle formation, (2) direct condensation of
combustion products, and (3) direct emission of liquid and solid particles. Gas-
to-particle conversion is responsible for much of the sulfate and nitrate that
dominate urban aerosol. That process is exemplified by a series of reactions
which begins with the gaseous pollutant sulfur dioxide
OH + SO 2 -+ HSO3,
(9.1)
followed by oxidation of HSO 3 to form H2SO 4 vapor. Owing to its low satu-
ration pressure, sulfuric acid vapor readily condenses, or
nucleates,
into liquid
particles---especially in the presence of water vapor. Once formed, nuclei grow
rapidly to radii of 0.01 to 0.1/xm. Larger aerosol can then develop through
coagulation of individual nuclei to form
accumulation panicles.
Sulfate aerosol
is also produced through reaction of sulfur dioxide with ammonia inside cloud
262
9
Aerosol and Clouds
droplets, which, upon evaporating, leave behind sulfate particles. In the tro-
posphere, (NH4)2SO4, NHnHSO4, and H2SO 4 are all present, whereas super-
cooled H2SO 4 droplets are the prevalent form of sulfate in the stratosphere.
Nitrogen emissions from combustion and natural sources lead to analogous
products. Sulfate and nitrate are both important in the troposphere, but nitrate
is especially prevalent in urban areas because it is an inevitable by-product of
combustion.
Carbonaceous aerosols like soot consist of submicron-size particles. Natural
sources include pollen and spores, as well as complex hydrocarbon vapors, like
isoprene. Emitted via plant transportation, those vapors react with oxides of
nitrogen and subsequently nucleate.
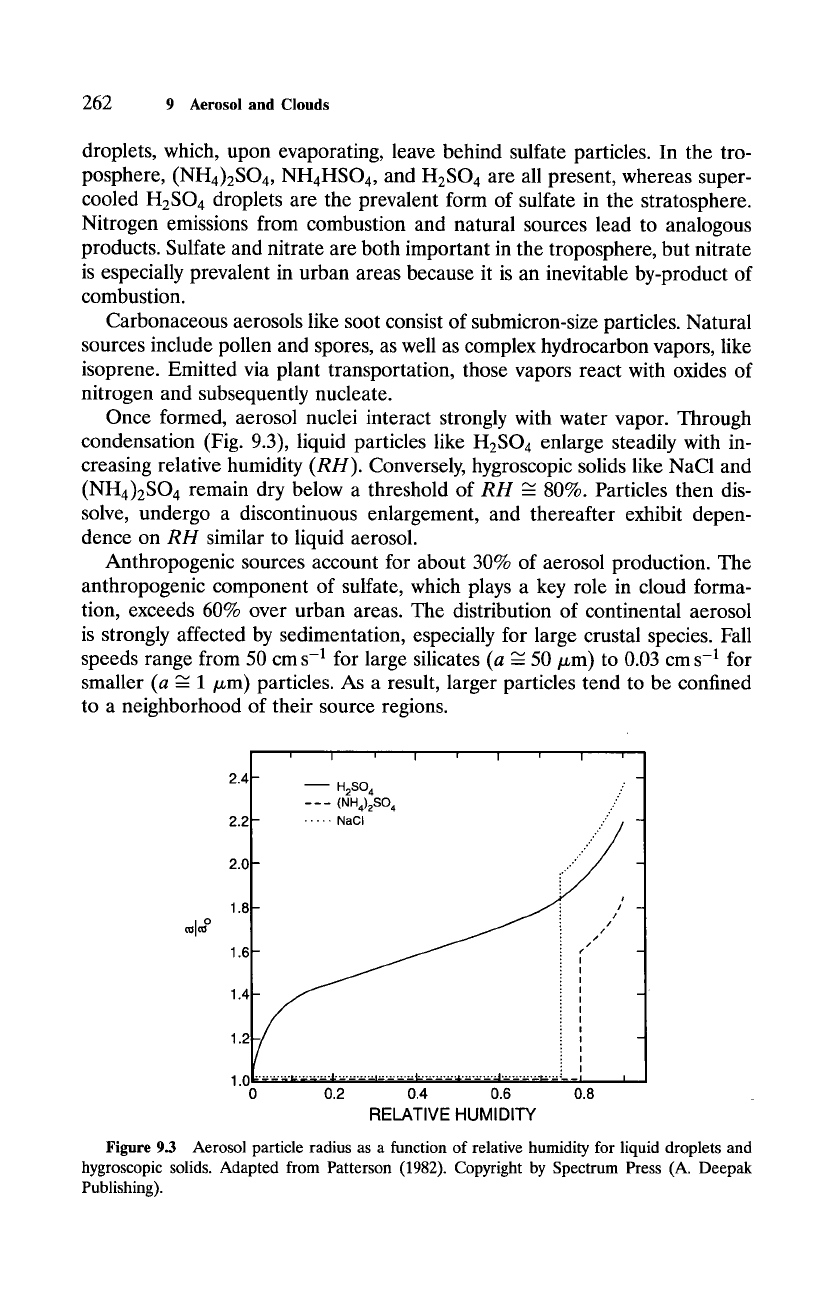
Once formed, aerosol nuclei interact strongly with water vapor. Through
condensation (Fig. 9.3), liquid particles like H2SO 4 enlarge steadily with in-
creasing relative humidity
(RH).
Conversely, hygroscopic solids like NaC1 and
(NH4)2SO 4 remain dry below a threshold of
RH ~-
80%. Particles then dis-
solve, undergo a discontinuous enlargement, and thereafter exhibit depen-
dence on
RH
similar to liquid aerosol.
Anthropogenic sources account for about 30% of aerosol production. The
anthropogenic component of sulfate, which plays a key role in cloud forma-
tion, exceeds 60% over urban areas. The distribution of continental aerosol
is strongly affected by sedimentation, especially for large crustal species. Fall
speeds range from 50 cm s -1 for large silicates (a ~ 50/zm) to 0.03 cm s -1 for
smaller (a -~ 1/xm) particles. As a result, larger particles tend to be confined
to a neighborhood of their source regions.
i
|
I
|
I | I
|
I
| /
1
2.4
__ H2SO 4
--- (NH4)2SO 4 .."
2.2,- ..... NaCI .. --
2.0 "'"'""
,,'1
i /'
i ,
1.0
0 0.2 0.4 0.6 0.8
RELATIVE HUMIDITY
Figure 9.3 Aerosol particle radius as a function of relative humidity for liquid droplets and
hygroscopic solids. Adapted from Patterson (1982). Copyright by Spectrum Press (A. Deepak
Publishing).
9.1 Morphology of Atmospheric Aerosol
263
Top Jet Drop
O t 5 cm Ejection Height
50 IJm Diameter
O
O
9 9 9 O 9 9 Film Drops
9 9 9 9
9 15-20 Produced
o~.... ~e
9 9
/ ~ \
9 9 Submicronto
Film Cap r.ea-~ i i / ! T 101JmDiameter
5x10 IJm A
1 to 3 IJm Thlck~ /..~//5 00 IJm Diameter
Rise
Velocity
3.5 cm/sec
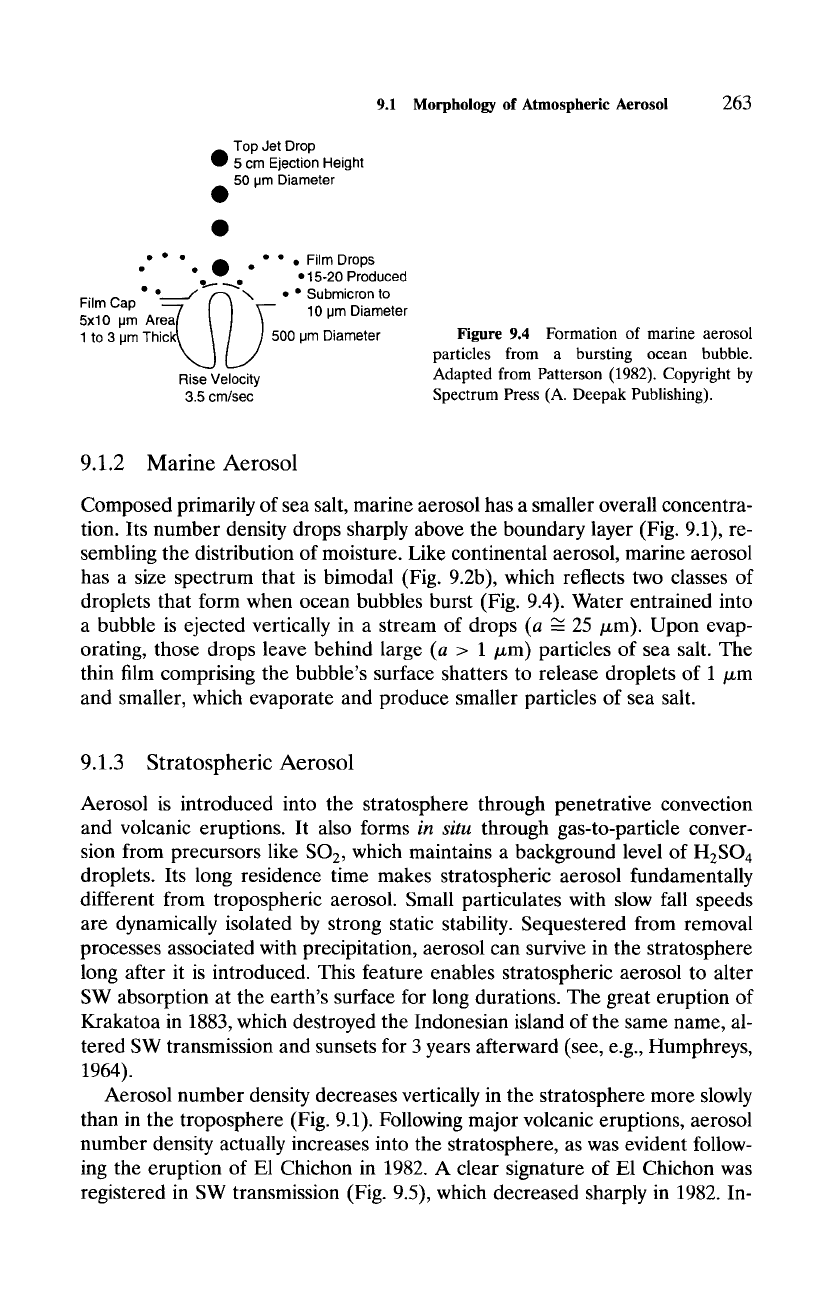
Figure 9.4 Formation of marine aerosol
particles from a bursting ocean bubble.
Adapted from Patterson (1982). Copyright by
Spectrum Press (A. Deepak Publishing).
9.1.2 Marine Aerosol
Composed primarily of sea salt, marine aerosol has a smaller overall concentra-
tion. Its number density drops sharply above the boundary layer (Fig. 9.1), re-
sembling the distribution of moisture. Like continental aerosol, marine aerosol
has a size spectrum that is bimodal (Fig. 9.2b), which reflects two classes of
droplets that form when ocean bubbles burst (Fig. 9.4). Water entrained into
a bubble is ejected vertically in a stream of drops (a --- 25/zm). Upon evap-
orating, those drops leave behind large (a > 1/xm) particles of sea salt. The
thin film comprising the bubble's surface shatters to release droplets of 1 ~m
and smaller, which evaporate and produce smaller particles of sea salt.
9.1.3 Stratospheric Aerosol
Aerosol is introduced into the stratosphere through penetrative convection
and volcanic eruptions. It also forms
in situ
through gas-to-particle conver-
sion from precursors like SO2, which maintains a background level of H2SO 4
droplets. Its long residence time makes stratospheric aerosol fundamentally
different from tropospheric aerosol. Small particulates with slow fall speeds
are dynamically isolated by strong static stability. Sequestered from removal
processes associated with precipitation, aerosol can survive in the stratosphere
long after it is introduced. This feature enables stratospheric aerosol to alter
SW absorption at the earth's surface for long durations. The great eruption of
Krakatoa in 1883, which destroyed the Indonesian island of the same name, al-
tered SW transmission and sunsets for 3 years afterward (see, e.g., Humphreys,
1964).
Aerosol number density decreases vertically in the stratosphere more slowly
than in the troposphere (Fig. 9.1). Following major volcanic eruptions, aerosol
number density actually increases into the stratosphere, as was evident follow-
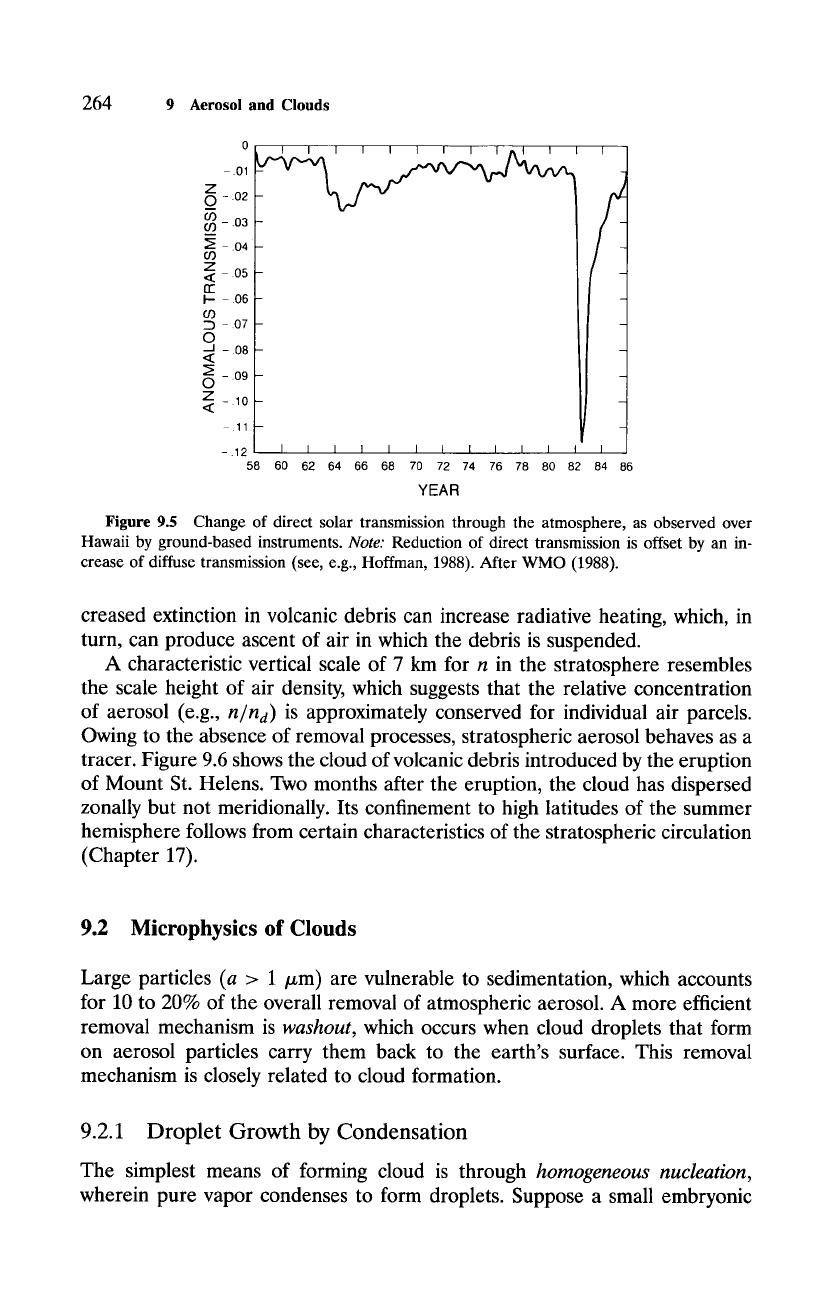
ing the eruption of E1 Chichon in 1982. A clear signature of E1 Chichon was
registered in SW transmission (Fig. 9.5), which decreased sharply in 1982. In-
264
9
Aerosol and
Clouds
0
-
.01
z
0 - .02
or)
0') - .03
~;
-
.04
CO
Z
< - .05
n-"
I--
-
.06
or)
:)
-
.07
0
-J - 08
<
- .09
0
Z
-
.10
<
-.11
-
.12
i
I I I I I I I I I I I I
58 60 62 64 66 68 70 72 74 76 78 80 82 84 86
YEAR
Figure 9.5 Change of direct solar transmission through the atmosphere, as observed over
Hawaii by ground-based instruments.
Note:
Reduction of direct transmission is offset by an in-
crease of diffuse transmission (see, e.g., Hoffman, 1988). After WMO (1988).
creased extinction in volcanic debris can increase radiative heating, which, in
turn, can produce ascent of air in which the debris is suspended.
A characteristic vertical scale of 7 km for n in the stratosphere resembles
the scale height of air density, which suggests that the relative concentration
of aerosol (e.g.,
n/nd)
is approximately conserved for individual air parcels.
Owing to the absence of removal processes, stratospheric aerosol behaves as a
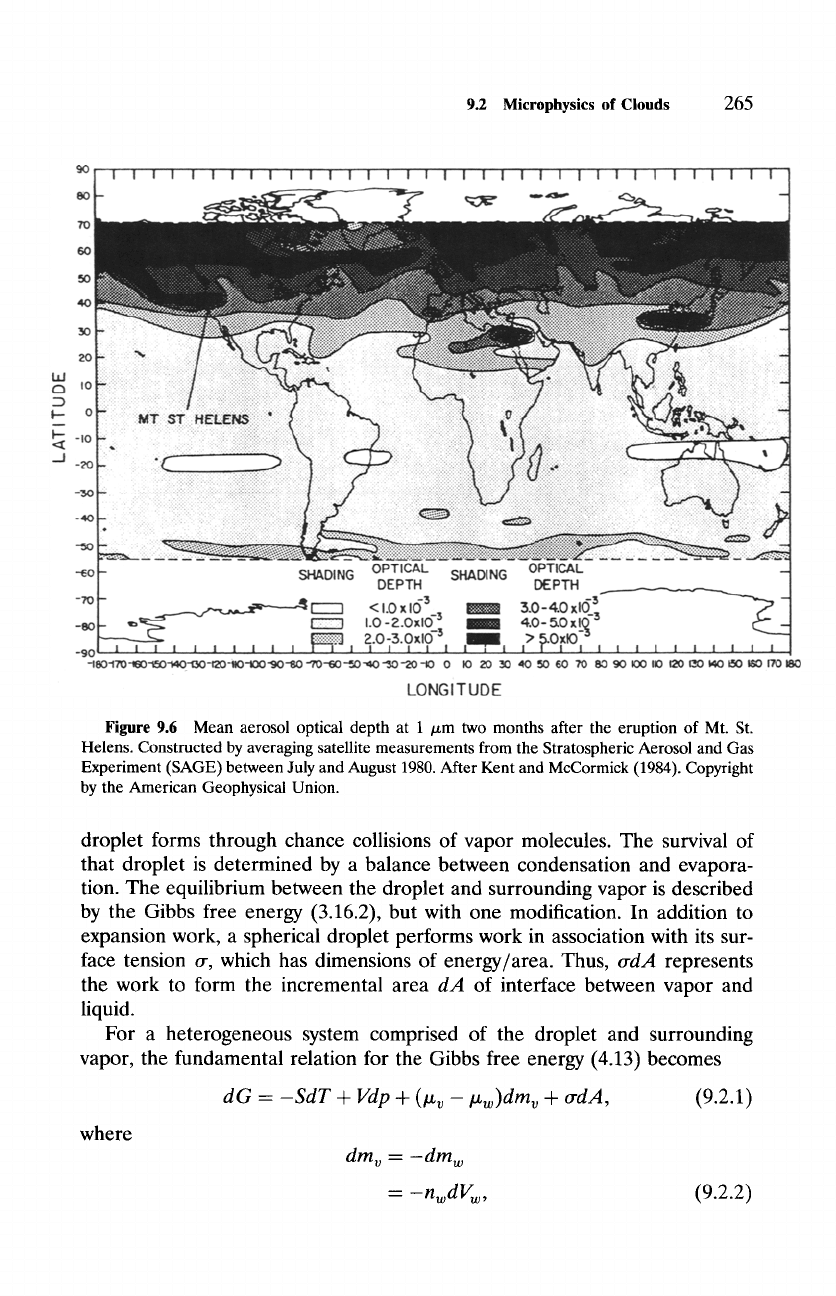
tracer. Figure 9.6 shows the cloud of volcanic debris introduced by the eruption
of Mount St. Helens. Two months after the eruption, the cloud has dispersed
zonally but not meridionally. Its confinement to high latitudes of the summer
hemisphere follows from certain characteristics of the stratospheric circulation
(Chapter 17).
9.2 Microphysics of Clouds
Large particles (a > 1/zm) are vulnerable to sedimentation, which accounts
for 10 to 20% of the overall removal of atmospheric aerosol. A more efficient
removal mechanism is
washout,
which occurs when cloud droplets that form
on aerosol particles carry them back to the earth's surface. This removal
mechanism is closely related to cloud formation.
9.2.1 Droplet Growth by Condensation
The simplest means of forming cloud is through
homogeneous nucleation,
wherein pure vapor condenses to form droplets. Suppose a small embryonic
9.2
Microphysics of Clouds
265
90
80
70
60
~)
40
~., . : . . -r .x~. :~-.-~..-.-.... :...:.:.:.:d+:.:.::
173 ~0 . i/::- " " - ..... ! ~'::'::~.
E3
" : :- ::t ::: ""i::: :::::::::::::::::::::::::: - ::
I-- -~o - " ........ -.i :iii-liiii~iiiiii:~:i~:~-:.::i:~: 9 :.:..i .........
.... .. -. :, ========================== :. :.:
-30 -- . 9 .. :-.":: ::-:::i.i::::::.?...: . .- "
. :-..--.. ::.....: ============================== :...:.:.:.:.:::~ :.
.......... ii
_40 _ . :i:.~::iiiiiii:i:~i~ii~!:ii~:~
" . ....... :.i+:-i.)i~i~i.ii!:i
.)i. :
-4rdO ~ " : ~~~.t~..~.,.f:'" "" """ " """" " '
...... : i:~i~ i:.i::
i iii
i! :::: i~i iii; ~i~iii::iiiii! i:~:i!i.~...:!:/:ii~ .. :::i ..... +i~:-i:ii:i:~i:~::i::!~.!~i.r
:... : ....:...:..::.:.:.: :::::...:.....::-: .::.:.::.:.:.:.:-::.:.:.:.:-::.. 9 :..:..:-:~:...... ......:::.:.:. ..
.... . : ..:.. :::::::::::::::::::::::::::::::
.............................. :.:! ......,
================================================================================================= : _-L.:~.-~.:~:i~
~o SHADING OPTICAL SI-IADING OPTICAL -'!
DEPTH DEPTH _
-70 ~E"---I <,.Oxl() 3 I~ 3.O-4.0xl(~3_~f--~~~'~ d
-,o ~ ,.o-z.o,,o -~ m, 4.o-~o,tq-' ~'--!
z,.o,3.o~,o -~ ,R", ,>~oi,,~,~,,,,,,,, , ,--I
-9q I I I I I I I I I I ~ I I _ I I ,
-180"lTO'll~-If~O-14O-130-l'20-IK)i:)O-90-1~O-'rO-60~~-~ 0 I0 20 ~ 40 ~1060 70 80 90 I00110 1201~0 IdlOlSO 160 I'/0180
LONGITUDE
Figure 9.6 Mean aerosol optical depth at 1 /xm two months after the eruption of Mt. St.
Helens. Constructed by averaging satellite measurements from the Stratospheric Aerosol and Gas
Experiment (SAGE) between July and August 1980. After Kent and McCormick (1984). Copyright
by the American Geophysical Union.
droplet forms through chance collisions of vapor molecules. The survival of
that droplet is determined by a balance between condensation and evapora-
tion. The equilibrium between the droplet and surrounding vapor is described
by the Gibbs free energy (3.16.2), but with one modification. In addition to
expansion work, a spherical droplet performs work in association with its sur-
face tension cr, which has dimensions of energy/area. Thus,
odA
represents
the work to form the incremental area
dA
of interface between vapor and
liquid.
For a heterogeneous system comprised of the droplet and surrounding
vapor, the fundamental relation for the Gibbs free energy (4.13) becomes
where
dG --SdT + Vdp + (tz v - p,w)dmv + ordA,
(9.2.1)
dmv - -dmw
= -nwdV w,
(9.2.2)

266 9 Aerosol and Clouds
and
n w
and Vw denote the number density and volume of the droplet,
respectively. ~
The difference of chemical potential between the vapor and liquid phases
can be expressed in terms of the vapor and saturation vapor pressures by
appealing to the fundamental relation (3.16) for the individual phases. 2 Under
an isothermal and reversible change of pressure
de,
for the vapor and
d~v - v,,de
(9.3.1)
dtzw - vwde
(9.3.2)
for the liquid. Subtracting gives
d(t~ - l~w) - (v~- vw)de
~- vvde.
(9.4)
Then applying the gas law for an individual molecule of vapor
ev o = KT
(9.5)
(e.g., Lee
et al.,
1973), where K is the Boltzmann constant, gives
d(i~o - tZw) - KTdln e,
which upon integrating from the saturation pressure (at which /z o - ~w)
obtains
/zv -/z w - KT ln(e-~). (9.6)
Incorporating (9.6) into (9.2) and integrating from a reference state of pure
vapor (with the vapor pressure maintained) yields the change of Gibbs free
energy
AG=
-VwnwKTln(-~w)+OrA.
Thus, forming a spherical droplet of radius a corresponds to the change of
Gibbs free energy
4 (~w)
AG =--47ra2o - -
-~Tra3nwKTln .
(9.7)
1
In phase transformations away from saturation, the system is out of chemical equilibrium, so
the chemical potentials in (9.2) need not be equal.
2 Surface tension introduces a pressure difference between the droplet and surrounding vapor,
which makes the pressure during a phase transformation variable. Strictly, that process should
be treated as isothermal and isochoric, for which F is the appropriate free energy. However,
discrepancies with G under isothermal and isobaric conditions are small enough to be ignored
(see, e.g., Pruppacher and Klett, 1978).
9.2 Microphysics of Clouds
267
1.6
1.4
1.2
1.0
v 0.8
,I.--
o
x
(.9 0.6
0.4
0.2
m
0
-0.2
0
/
I
/
J
/
/
e
e w
/
/
/
- 0.999
/
/
/
/
/
/
//
/ e
,,
~ = 1.u
/ / e w
/
/
/.
/
e
- 1.002
e w
/
/
i I i I i I t I t I t I i I I I t I I
.1 .2 .3 .4 .5 .6 .7 .8 .9 1.0
a (pm)
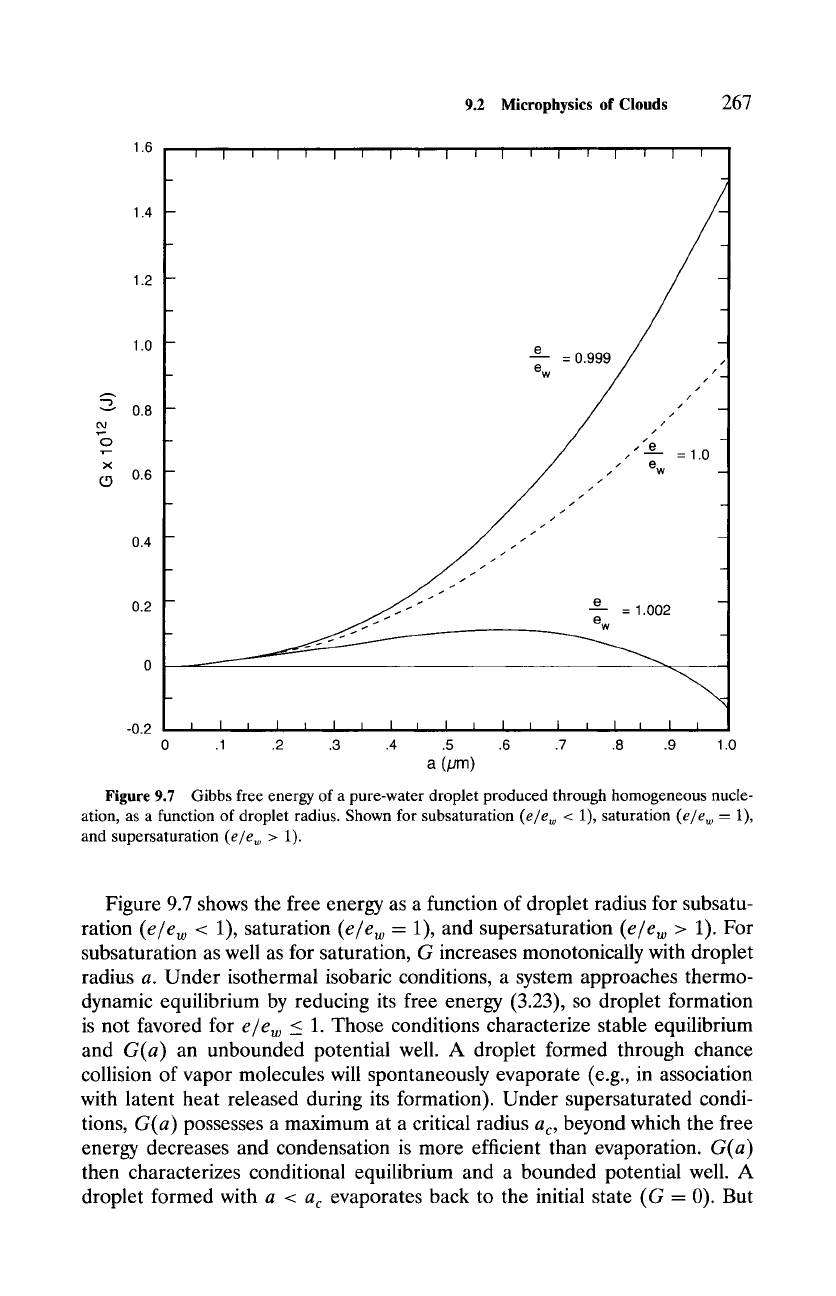
Figure 9.7 Gibbs free energy of a pure-water droplet produced through homogeneous nucle-
ation, as a function of droplet radius. Shown for subsaturation (e/ew < 1), saturation (e/e w = 1),
and supersaturation (e/ew > 1).
Figure 9.7 shows the free energy as a function of droplet radius for subsatu-
ration (e/ew < 1), saturation (e/ew - 1), and supersaturation
(e/e w
> 1). For
subsaturation as well as for saturation, G increases monotonically with droplet
radius a. Under isothermal isobaric conditions, a system approaches thermo-
dynamic equilibrium by reducing its free energy (3.23), so droplet formation
is not favored for e/ew _< 1. Those conditions characterize stable equilibrium
and
G(a)
an unbounded potential well. A droplet formed through chance
collision of vapor molecules will spontaneously evaporate (e.g., in association
with latent heat released during its formation). Under supersaturated condi-
tions,
G(a)
possesses a maximum at a critical radius
ac,
beyond which the free
energy decreases and condensation is more efficient than evaporation.
G(a)
then characterizes conditional equilibrium and a bounded potential well. A
droplet formed with a <
ac
evaporates back to the initial state (G - 0). But
268
9
Aerosol and Clouds
one formed with a > a c grows spontaneously through condensation of vapor
and is said to be activated.
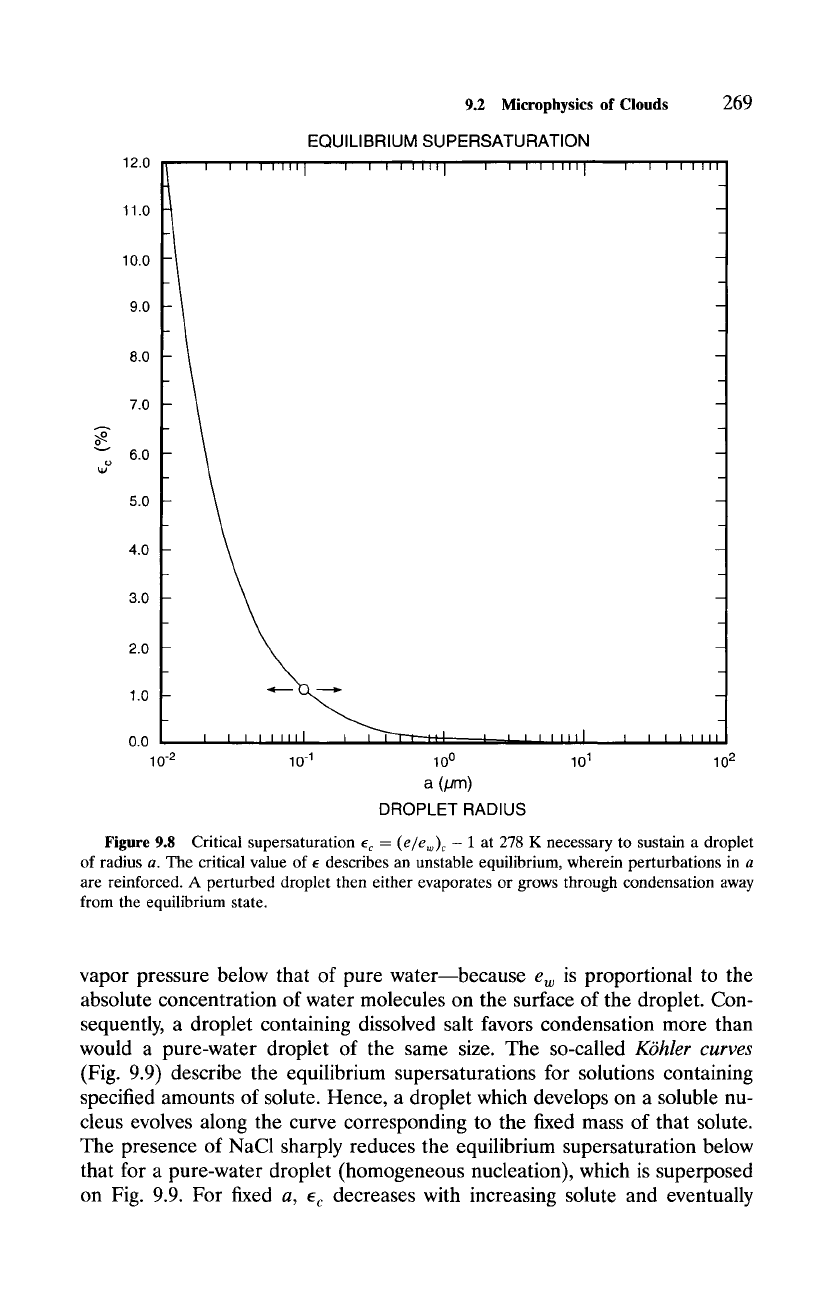
The critical radius for a given temperature and vapor pressure follows by
differentiating (9.7):
2tr
ac =
e "
(9.8)
nwKTln(~)
Known as Kelvin's formula, (9.8) can be rearranged for the critical or equilib-
rium supersaturation
(L)
e c = - 1, (9.9)
c
above which a droplet of radius a grows spontaneously through condensa-
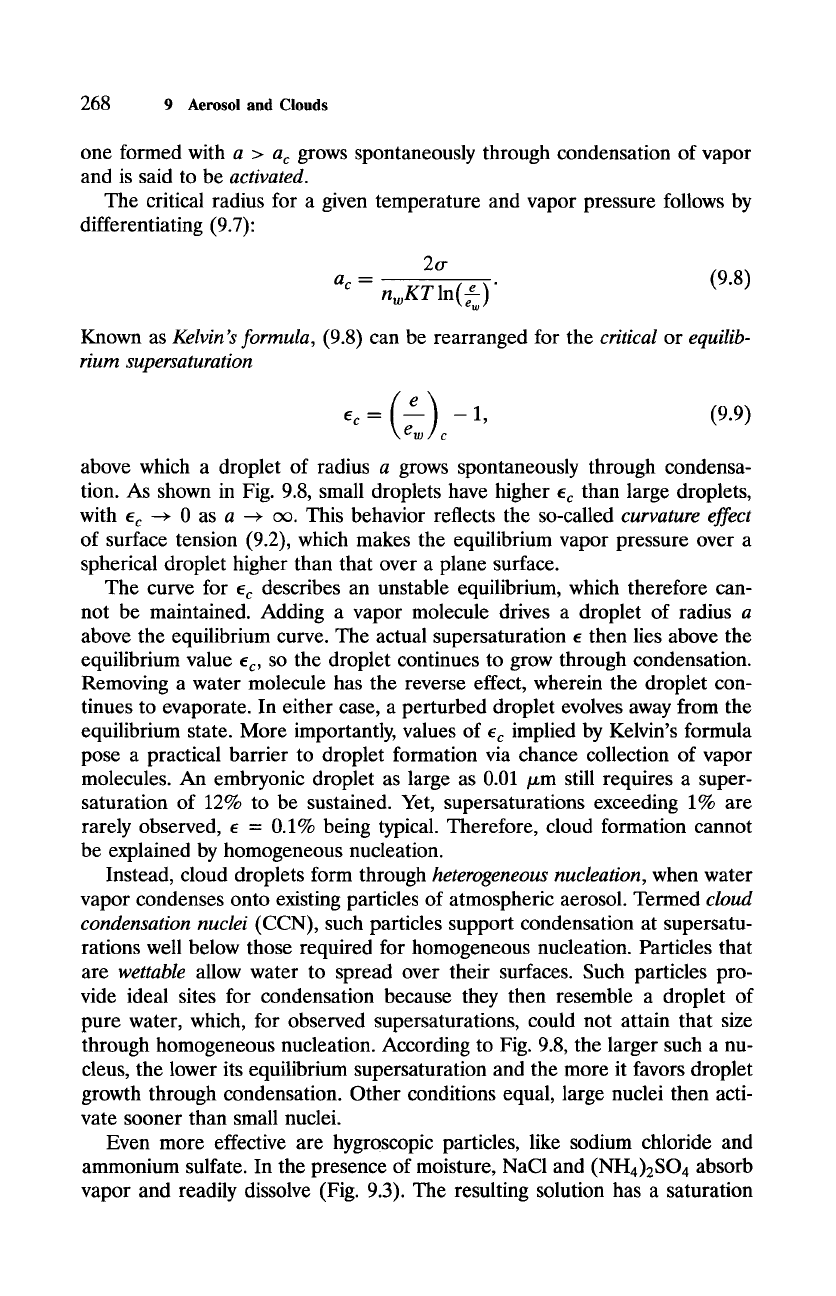
tion. As shown in Fig. 9.8, small droplets have higher ec than large droplets,
with ec ~ 0 as a ~ c~. This behavior reflects the so-called curvature effect
of surface tension (9.2), which makes the equilibrium vapor pressure over a
spherical droplet higher than that over a plane surface.
The curve for ec describes an unstable equilibrium, which therefore can-
not be maintained. Adding a vapor molecule drives a droplet of radius a
above the equilibrium curve. The actual supersaturation e then lies above the
equilibrium value e c, so the droplet continues to grow through condensation.
Removing a water molecule has the reverse effect, wherein the droplet con-
tinues to evaporate. In either case, a perturbed droplet evolves away from the
equilibrium state. More importantly, values of ec implied by Kelvin's formula
pose a practical barrier to droplet formation via chance collection of vapor
molecules. An embryonic droplet as large as 0.01 /zm still requires a super-
saturation of 12% to be sustained. Yet, supersaturations exceeding 1% are
rarely observed, e = 0.1% being typical. Therefore, cloud formation cannot
be explained by homogeneous nucleation.
Instead, cloud droplets form through heterogeneous nucleation, when water
vapor condenses onto existing particles of atmospheric aerosol. Termed cloud
condensation nuclei (CCN), such particles support condensation at supersatu-
rations well below those required for homogeneous nucleation. Particles that
are wettable allow water to spread over their surfaces. Such particles pro-
vide ideal sites for condensation because they then resemble a droplet of
pure water, which, for observed supersaturations, could not attain that size
through homogeneous nucleation. According to Fig. 9.8, the larger such a nu-
cleus, the lower its equilibrium supersaturation and the more it favors droplet
growth through condensation. Other conditions equal, large nuclei then acti-
vate sooner than small nuclei.
Even more effective are hygroscopic particles, like sodium chloride and
ammonium sulfate. In the presence of moisture, NaC1 and (NH4)2SO 4 absorb
vapor and readily dissolve (Fig. 9.3). The resulting solution has a saturation
v
9.2 Microphysics of Clouds
EQUILIBRIUM SUPERSATURATION
12.0
11.0
10.0
9.O
! k
8.0
7.0
6.0
5.0
4.0
3.0
2.0
1.0
0.0
10 -2 10 -1 10 ~ 101 10 2
a (gm)
DROPLET RADIUS
269
Figure 9.8 Critical supersaturation ec = (e/ew)c - 1 at 278 K necessary to sustain a droplet
of radius a. The critical value of e describes an unstable equilibrium, wherein perturbations in a
are reinforced. A perturbed droplet then either evaporates or grows through condensation away
from the equilibrium state.
vapor pressure below that of pure water--because
e w
is proportional to the
absolute concentration of water molecules on the surface of the droplet. Con-
sequently, a droplet containing dissolved salt favors condensation more than
would a pure-water droplet of the same size. The so-called
K6hler curves
(Fig. 9.9) describe the equilibrium supersaturations for solutions containing
specified amounts of solute. Hence, a droplet which develops on a soluble nu-
cleus evolves along the curve corresponding to the fixed mass of that solute.
The presence of NaC1 sharply reduces the equilibrium supersaturation below
that for a pure-water droplet (homogeneous nucleation), which is superposed
on Fig. 9.9. For fixed a, e~ decreases with increasing solute and eventually
