Salby M.L. Fundamentals of Atmospheric Physics
Подождите немного. Документ загружается.


250 8
Atmospheric
Radiation
oxidation. These and other processes controlling optically active species are
temperature dependent, which introduces the possibility of feedback between
the two sides of (8.81).
Noteworthy is the mechanism controlling water vapor, which is the pri-
mary absorber in the IR. Temperature dependence in the Clausius-Clapeyron
relation (4.38) makes the water vapor abundance vary sharply with Ts. The
exponential dependence of saturation vapor pressure implies that an increase
of Ts can sharply increase the water vapor content of the atmosphere. En-
hanced cloud cover, which is likewise implied, further increases z~. By (8.81),
the increased optical depth results in a higher equilibrium surface tempera-
ture, which, in turn, increases the saturation vapor pressure and H20 content
of the atmosphere, and so forth.
Positive feedbacks like the one between temperature and water vapor sup-
port potentially large changes of
Ts
and other properties that characterize
climate. A paradigm of such changes is the so-called "runaway greenhouse ef-
fect," which is used to explain the present state of the Venusian atmosphere.
The evolution of a planet's atmosphere is thought to occur through slow dis-
charge of gases from the planet's surface. Atmospheric uptake of those gases
is limited by their saturation vapor pressures, which depend only on tempera-
ture (e.g., on T~). Because those gases are responsible for atmospheric opacity,
their saturation vapor pressures translate into the optical depth
"r s.
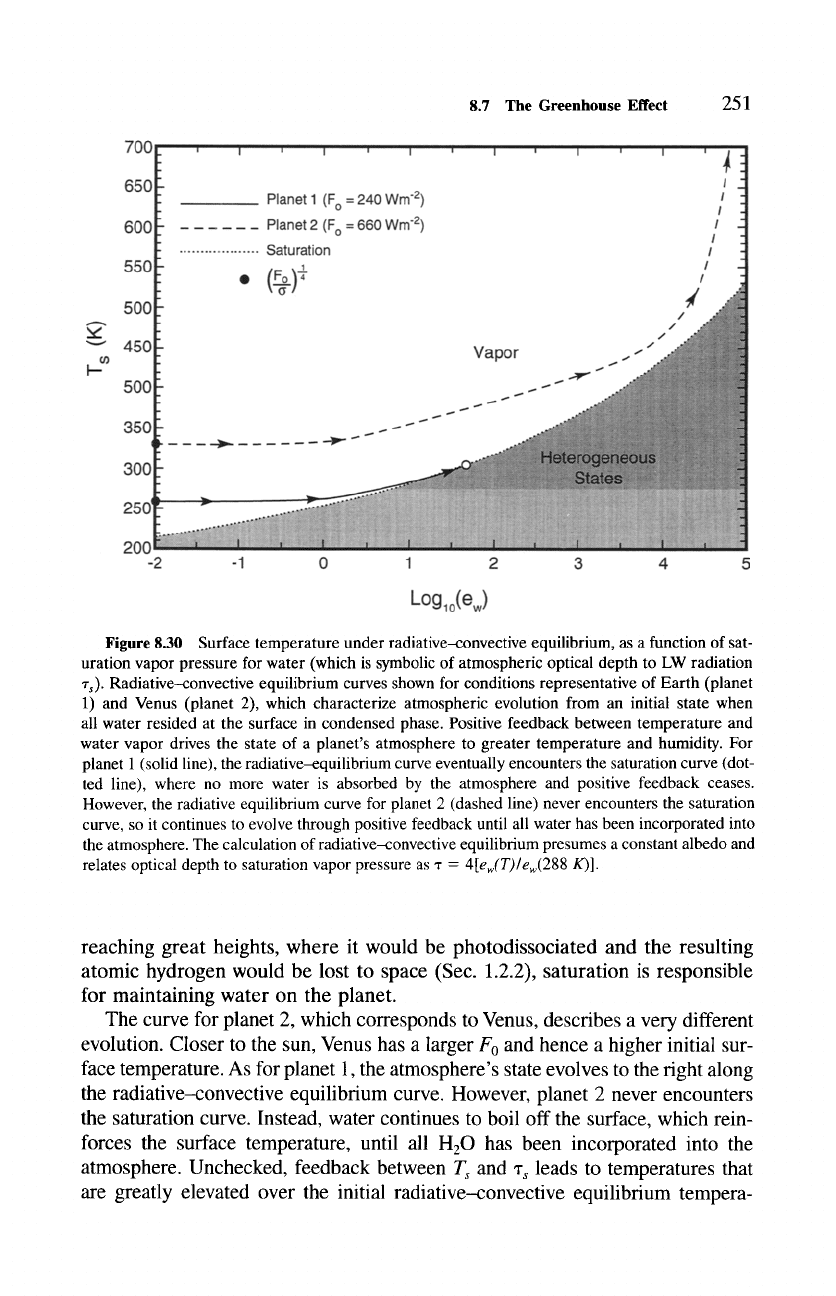
Plotting
T~ against log(ew), which is symbolic of
log('rs),
produces the saturation curve
(dotted line) in Fig. 8.30 separating heterogeneous states of water from vapor
phase alone.
The optical depth z~ and incident solar flux F 0 determine the surface tem-
perature of the planet, for example, via (8.81) under radiative equilibrium
or its counterpart (8.70) under radiative--convective equilibrium. Then the
surface temperature T~ defines a family of radiative-convective equilibrium
curves, two of which are superposed in Fig. 8.30. The curve for a particular
F 0 can be thought to represent the evolution of a given planet. For small
'r s,
T~ _~ (Fo/o')~,
which reflects the initial state of the planet when all of its water
resided at the surface in condensed phase. The situation for Earth is repre-
sented in the curve for planet 1 (solid line). Initially unsaturated, the atmo-
sphere incorporates water vapor from the surface, with concomitant increases
of its opacity ~'~ and surface temperature T~. Positive feedback between tem-
perature and water vapor then drives the state of the atmosphere to the right
along its radiative--convective equilibrium curve. Eventually, the atmosphere's
state encounters the saturation curve, beyond which further increases of
"r s
and
Ts
are prevented. For the conditions of planet 1, saturation is achieved at
a surface temperature of about 287 K. Subsequent increases of water vapor
are offset by condensation, which returns H20 to the planet's surface. Those
conditions describe a saturated system that contains multiple phases of water,
reflecting the present state of Earth's atmosphere. By preventing H20 from
8.7 The Greenhouse Effect
251
7001:
,
I
'
,
'
i
L
650~-- _ _ Planet 1 (F o =
Wm -2)
240
600~1" Planet 2 (F o = 660 Wm "2)
Saturation
5001-
,,r
450
U)
I---
500
3501z-
....-- ---"
I ' I ~ I
Vapor ~ ~ "
I
i
I
I
I
I
/
00 :~.~,e..-:#~:rr.-.~ ~,, ..... ...,..~.~r .,.....e..~:.~ ~:~:~:~ ~. ~:~ , :,.~- ~:~ {~..-... ~:,,:..'.%..:..t~s~.-.~..~ :~.,~&,..~..#~..,:gv .~,---.;~:~.~- ~ ~.~:;r ~. - 9 %,~.~ ri,~i.;..T:~-:: :{. * ::~.~-~t~,..~:.~$,.,:#~:z~':~,~ ~ :. . ~ .: ~,,. ~r .
-2 -1 0 1 2 3 4 5
Log,o(e~)
Figure 8.30 Surface temperature under radiative-convective equilibrium, as a function of sat-
uration vapor pressure for water (which is symbolic of atmospheric optical depth to LW radiation
~'s)- Radiative-convective equilibrium curves shown for conditions representative of Earth (planet
1) and Venus (planet 2), which characterize atmospheric evolution from an initial state when
all water resided at the surface in condensed phase. Positive feedback between temperature and
water vapor drives the state of a planet's atmosphere to greater temperature and humidity. For
planet 1 (solid line), the radiative-equilibrium curve eventually encounters the saturation curve (dot-
ted line), where no more water is absorbed by the atmosphere and positive feedback ceases.
However, the radiative equilibrium curve for planet 2 (dashed line) never encounters the saturation
curve, so it continues to evolve through positive feedback until all water has been incorporated into
the atmosphere. The calculation of radiative-convective equilibrium presumes a constant albedo and
relates optical depth to saturation vapor pressure as -r =
4[ew(T)/ew(288 K)].
reaching great heights, where it would be photodissociated and the resulting
atomic hydrogen would be lost to space (Sec. 1.2.2), saturation is responsible
for maintaining water on the planet.
The curve for planet 2, which corresponds to Venus, describes a very different
evolution. Closer to the sun, Venus has a larger F0 and hence a higher initial sur-
face temperature. As for planet 1, the atmosphere's state evolves to the right along
the radiative-convective equilibrium curve. However, planet 2 never encounters
the saturation curve. Instead, water continues to boil off the surface, which rein-
forces the surface temperature, until all H20 has been incorporated into the
atmosphere. Unchecked, feedback between
Ts
and % leads to temperatures that
are greatly elevated over the initial radiative-convective equilibrium tempera-

252 s Atmospheric Radiation
ture. This is symbolic of the 750 K mean surface temperature of Venusmwhich
is some 400 K warmer than would result in the absence of its atmosphere.
Atmospheric composition suggested by the evolution of planet 2 also differs
fundamentally from that of planet 1. For initial water abundance comparable
to that of Earth, the Venusian atmosphere would reach a state in which H20
is its primary constituent. Water vapor would then be vulnerable to photodis-
sociation by UV, allowing atomic hydrogen to escape to space and free oxygen
to recombine at the surface through oxidation processes. In this fashion, atmo-
spheric water vapor would be systematically depleted and replaced by carbon
dioxide, which is consistent with the predominance of CO2 in the present-day
Venusian atmosphere.
For Earth, gases other than water vapor also contribute to the greenhouse
effect. Absorption by carbon dioxide is situated near the maximum of the LW
emission spectrum (Fig. 8.5). In tandem with the steadily increasing concen-
tration of CO2 (Fig. 1.11), this feature suggests that increased surface tem-
peratures are inevitable if the present trend continues. CFCs, methane, and
nitrous oxide follow carbon dioxide in importance. All have anthropogenic
sources that have been associated with increasing concentrations.
Were the preceding feedback to operate in isolation, small changes of tem-
perature (e.g., stimulated by occasional volcanic eruptions or anthropogenic
activities) could result in large shifts of the Earth's climate. In fact, the
temperaturemwater vapor feedback is but one of several believed to shape
climate. The involvement of properties like surface reflectivity (e.g., snow and
ice cover), clouds, and the distributions of optically active species is strongly
suggested by models of radiative-convective equilibrium, which exhibit marked
sensitivity to how such ingredients are prescribed. Clouds and aerosol, which
are treated in the next chapter, are especially important because they sharply
alter the absorption and scattering characteristics of the atmosphere.
Suggested Reading
An Introduction to Atmospheric Radiation (1980) by Liou is a very readable
treatment of radiative transfer. It includes discussions of solar variability and
band models.
Fundamentals of Modern Physics (1967) by Eisberg provides an excellent
overview of Planck's theory and the emergence of modem physics. It includes
a basic treatment of line broadening.
Aeronomy of the Middle Atmosphere (1986) by Brasseur and Solomon and Mid-
dle Atmosphere Dynamics (1987) by Andrews et al. contain excellent treatments
of radiative transfer in the stratosphere and mesosphere. They also include de-
scriptions of solar variability and its implications to UV radiation.
An advanced treatment of radiative transfer can be found in Atmospheric Ra-
diation (1989) by Goody and Yung, which contains an enlightening comparison
of two-stream approximations.

Problems
253
Atmospheric Ozone: Assessment of Our Understanding of Processes Controlling
Its Present Distribution and Change
(WMO, 1986) includes a detailed overview
of solar variability based on satellite measurements.
Special Functions and Their Applications
(1972) by N. Lebedev contains a
proper development of the exponential integral.
Hartmann (1994) provides an excellent overview of the earth's energy budget,
including a treatment of horizontal energy transport by the general circulation.
Ramanathan
et al.
(1989) provides an overview of recent satellite measure-
ments of the earth's radiation budget and observations of the solar constant.
Manabe and Strickler (1964) is a classic calculation of radiative-convective
equilibrium, which contains an estimate of thermal damping time inside the
troposphere.
Climate Change: The IPCC Scientific Assessment
(Houghton
et al.,
1990) con-
tains an overview of climate feedback mechanisms and assessments of how
well they are understood.
Problems
8.1. Calculate the brightness temperature of the earth based on (a) an ob-
served broadband OLR of 235 W m -2, (b) the outgoing narrowband flux
(Fig. 8.5) at A = 11 pm, (c) the outgoing narrowband flux at A = 9.6/zm,
and (d) the outgoing narrowband flux at A = 15 pm.
8.2. Derive the Stefan-Boltzmann law (8.15).
8.3. As the sun cools, its spectrum will shift toward longer wavelengths. Esti-
mate the change in the earth's equivalent blackbody temperature if the
peak in the SW spectrum is displaced from its current position of 0.48
/xm to a yellower wavelength of 0.55/zm.
8.4. Orbital ellipticity brings the earth some 3.5% nearer the sun during Jan-
uary than during July, which is responsible for the slight asymmetry of
insolation apparent in Fig. 1.28. (a) Calculate the corresponding change
in the earth's equivalent blackbody temperature. (b) Discuss this change
in relation to other changes that are introduced by hemispheric asym-
metries of the earth's surface and atmospheric circulation.
8.5. Consider a 250 K isothermal atmosphere that contains a single absorbing
gas of uniform mixing ratio 0.05. The specific absorption cross section
of that gas is given as a function of wavelength by
trax--a'Oexp[-(A-A~ la [m2kg-1]'
where A 0 = 0.5/zm and a = 0.01/zm. (a) Calculate, as a function of A,
the optical path length for downward-traveling radiation from the top of

254 8 Atmospheric
Radiation
the atmosphere to the height z. (b) Plot the absorptivity of this layer, as
a function of h, for z = 5, 3, and 1 scale heights.
8.6. Show that a layer having optical thickness Au << 1 has absorptivity
Aa - Au.
8.7. Prove Kirchhoff's law (8.16) in generality.
8.8. The top of the photosphere has a radius of 7.0 x 108 m. If the mean
earth-sun distance is 1.5 x 1011 m, calculate the equivalent blackbody
temperature of the photosphere.
8.9. Venus has an albedo of 0.77 and a mean distance from the sun of 1.1 x
1011 m. (a) Calculate the equivalent blackbody temperature of Venus.
(b) Discuss this value in relation to the observed surface temperature of
750 K.
8.10. Consider an isothermal atmosphere that is optically thick to LW radia-
tion. (a) How much of the LW radiance emitted to space at zero zenith
angle originates in its uppermost three optical depths? (b) Determine
the altitude range, in scale heights, occupied by the layer between optical
depths of 0.1 and 3.0 under isothermal conditions.
8.11. A fiat plate sensor on board a satellite behaves as a graybody with
constant absorptivity a. Calculate its radiative-equilibrium temperature
when the sensor faces the sun if (a) a - 0.8 and (b) a - 0.2.
8.12. As for Problem 8.11, but if the sensor has different absorptivities to SW
and LW radiation of (a)
asw
and
aLW,
respectively, and (b)
asw
= 0.2
and
aLW
= 0.8.
8.13. Under the conditions of Problem 8.12, calculate the sensor's temperature
as a function of time after the satellite has been rotated to face the sensor
away from the sun, if it has an area of 0.1 m 2 and a specific heat of 2
jK -1.
8.14. The hood of an automobile can be modeled as a gray flat plate with LW
absorptivity 0.9 and the atmosphere as a gray layer with LW absorptivity
0.8. In this framework, estimate the hood's temperature for a solar zenith
angle of 25 ~ if (a) the automobile's color is light with a SW absorptivity
of 0.2 and (b) the automobile's color is dark with a SW absorptivity of
0.6. (c) What physical process would, in practice, mediate the values in
parts (a) and (b)?
8.15. A simple model approximates the earth's surface as a graybody and the
atmosphere as a gray layer with LW absorptivities of 1.0 and 0.8, respec-
tively. Use this model to estimate the radiative-equilibrium temperature
of the ground in the presence of 50% cloud cover (fully reflective) and
under (a) perpetual summertime conditions over vegetated terrain: a so-
lar zenith angle of 25 ~ and the surface having a SW absorptivity of 0.6,
and (b) perpetual wintertime conditions over snow-covered terrain: a

Problems
255
8.16.
8.17.
8.18.
8.19.
8.20.
8.21.
8.22.
8.23.
solar zenith angle of 60 ~ and the surface having a SW absorptivity of
0.2.
Consider a simple model of the earth comprised of an isothermal gray
layer with SW and LW absorptivities
asw
and
aLw,
respectively, and a
black underlying surface, both of which are illuminated by a SW flux F 0
and are in thermal equilibrium. The energy budget can be formulated
by tracing energy through repeated absorptions and reemissions by the
surface and atmosphere. (a) Develop an arithmetic progression for the
fractional energy absorbed by the atmosphere during successive trans-
missions from the surface to construct a series representation for the net
energy absorbed by the atmosphere. (b) Use the series representation
to calculate the radiative-equilibrium temperature of the atmosphere
for F 0 - (1-
d)(Fs/4 )
= 240 W m -2 (Sec. 1.3),
asw -
0.20, and
aLw -0.94,
which are representative of values in Fig. 1.27.
Use the results of Problem 8.16 to determine the radiative-equilibrium
temperature of the surface (a) for the conditions given, (b) in the absence
of an atmosphere, and (c) as in part (a), but with the atmosphere's LW
absorptivity increased to
aLw -0.98.
Estimate the level where the collisional half-width equals the Doppler
half-width for (a) the water vapor absorption line (Fig. 8.11) at A -1 -- 352
cm -1 and (b) the CO2 absorption line at A -1 - 712
cm -1.
Consider a discretely stratified atmosphere comprised of N isothermal
layers, each of which is transparent to SW radiation but has a LW ab-
sorptivity a and which, collectively, are in radiative equilibrium with an
incident SW flux F 0 and a black underlying surface. (a) Derive expres-
sions for the flux
Fs
emitted by the surface for N - 1, N - 2, and
then generalize those expressions to arbitrary N. (b) Let
a - 'rs/N
and
take the limit N --+ ~ to recover expression (8.81) for a continuously
stratified atmosphere. (c) Verify that, even though they have different
optical characteristics, the atmospheres for different N all have the same
equivalent blackbody temperature. Explain why.
Derive expressions (8.38) for the upwelling and downwelling intensities
in a plane parallel atmosphere as functions of optical depth.
Show that the upwelling and downwelling fluxes for horizontally isotropic
radiation are described in terms of the exponential integral E 2 by (8.45).
An 80% solar eclipse occurs during morning, when the tempera-
ture would normally be 20 ~ C. Use a gray atmosphere in radiative-
equilibrium with a black surface to estimate the change of surface
temperature that would result were the eclipse to persist indefinitely
(compare Problem 8.33).
Show that the two-stream transformation (8.54) casts the budgets of
upwelling and downwelling radiation into the form of (8.55).

256 8
Atmospheric
Radiation
8.24.
8.25.
8.26.
8.27.
8.28.
8.29.
Consider a gray atmosphere that is moisture laden, in hydrostatic equilib-
rium, and has a LW specific absorption coefficient k. The atmosphere is
in radiative equilibrium with a black surface, which, in turn, is in thermal
equilibrium with a downwelling SW flux F 0. (a) Provide an expression
relating the radiative-equilibrium temperature TeE to height z. Discuss
the form of this relationship and how it can be solved for TRe(Z). (b)
Determine TRe(Z) in a neighborhood of the surface if the atmosphere
is optically thick (e.g., in the limit k --+ c~) and if the relative change of
temperature there can be ignored. (c) Under the conditions of part (b)
but in light of hydrostatic stability and if air near the surface is almost
saturated, derive an expression involving the height of the tropopause.
Discuss how the tropopause height depends on surface temperature and
the physical processes controlling it.
Consider a gray atmosphere with a LW specific absorption coefficient k
and an underlying surface with SW and LW absorptivities asw and aLW,
respectively. (a) Determine the distribution of radiative-equilibrium
temperature as a function of optical depth, asw, and aLW. (b) For
aLW = 1, discuss the limiting behavior: asw ~ O. (c) For asw = 1,
discuss the limiting behavior: aLw ~ O.
Discuss how the competing influences of radiative transfer and con-
vective mixing control stratification, in light of the diurnal variation of
insolation.
Show that the upwelling flux inside a gray atmosphere that is in radiative
equilibrium with a black underlying surface is given by (8.71).
Revisit the radiative-convective equilibrium in Figs. 8.21 and 8.22. (a)
Determine the temperature distribution under the same conditions, but
now using the CAPE (Sec. 7.4.1) of the radiative equilibrium atmosphere
to infer the tropopause height zT and presuming the air to be virtually
saturated. (b) Plot the profiles of upwelling and downwelling fluxes under
this radiative-convective equilibrium. (c) Plot the profile of radiative
heating.
The Venusian atmosphere is composed chiefly of carbon dioxide, with
g = 8.8 m s -2, Cp = 8.44 • 102 J kg -1
K -1
for CO2, a mean distance
from the sun of 0.70 that of Earth, and an albedo of d = 0.77. (a) Use
radiative equilibrium and the observed surface temperature Ts = 750 K
to estimate the optical depth of the Venusian atmosphere. (b) Calcu-
late the temperature distribution under radiative-convective equilibrium
based on (1) the profile of optical depth (8.69), with h = RTs/g, (2)
the presumption that temperatures inside the convective layer are hot
enough to ignore condensation, and (3) in place of the constraint used
in Sec. 8.5.2 to determine the tropopause height, requiring the temper-
ature at the midlevel of the hydrostatically unstable layer to equal that

Problems 257
8.30.
8.31.
8.32.
8.33.
8.34.
under radiative equilibrium. (c) Plot the profiles of upwelling radiation,
downwelling radiation, and radiative heating.
The CO2 absorption band is already saturated in the earth's atmosphere
(Fig. 8.1). In light of this feature, explain how increased levels of CO2
could result in global warming.
Derive the expression for Newtonian cooling (8.79) from the full repre-
sentation of radiative heating.
(a) Calculate the characteristic timescale of thermal damping in the
troposphere based on P0 = 1.2 kg
m -3,
the equivalent blackbody tem-
perature
T e
= 255 K (which is symbolic of where most OLR is emitted),
and a characteristic depth of 5 km (which corresponds to the preced-
ing temperature under radiative-convective equilibrium: Fig. 8.21). (b)
What is the heat capacity of the surface under this approximation?
Use Newtonian cooling in Problem 8.32 to estimate the amplitude
ATs
of the diurnal cycle of surface temperature, where AT~ is approximated
by half the nocturnal depression of temperature from its daytime maxi-
mum. Estimate
ATs
under equinoctial conditions, for a surface temper-
ature maximum of 300 K, and for an optical depth (a) ~'s = 4, which is
representative of the global-mean conditions, and (b) ~s = 1, which is
representative of arid conditions.
Construct a counterpart to Fig. 8.30 for the runaway greenhouse effect,
using the same initial values but based on radiative equilibrium. At what
temperature does the earth's atmosphere become saturated? Compare
this value with the one obtained under radiative-convective equilibrium.

Chapter 9
Aerosol and Clouds
Radiative transfer is modified importantly by clouds. Owing to their high
reflectivity in the visible, clouds shield the earth-atmosphere system from so-
lar radiation and thus represent cooling in the shortwave (SW) energy budget.
Conversely, the strong infrared (IR) absorptivity of water and ice particles
sharply increases the optical depth of the atmosphere, which magnifies the
greenhouse effect and represents warming in the longwave (LW) energy bud-
get. Atmospheric aerosol has optical properties similar to clouds.
We develop cloud processes from a morphological description of atmo-
spheric aerosol, without which clouds would not form in the earth's atmo-
sphere. We then examine the microphysics controlling cloud formation. Macro-
physical characteristics of clouds and accompanying microphysical properties
are developed in terms of environmental conditions controlling the formation
of particular cloud types. These elemental considerations culminate in descrip-
tions of radiative and chemical processes that operate inside clouds and figure
in climate.
9.1 Morphology of Atmospheric Aerosol
Small particulates are produced and removed through a variety of processes,
which make the composition, size, and distribution of atmospheric aerosol
widely variable (Table 9.1). Aerosol concentrations are smallest over the
oceans (Fig. 1.22), where a particle number density of n
= 10 3 cm -3
is repre-
sentative, and greatest over industrial areas, where n > 105 cm -3 is observed.
These and other distinctions lead to two broad classes of tropospheric aerosol:
continental
and
marine.
9.1.1 Continental Aerosol
Continental aerosol includes (1) crustal species that are produced by ero-
sion of the earth's surface, (2) combustion and secondary components related
to anthropogenic activities, and (3) carbonaceous components consisting of
hydrocarbons and elemental carbon. Crustal aerosol is produced in subtrop-
ical deserts like the Sahara, the southwestern United States, and southern
258
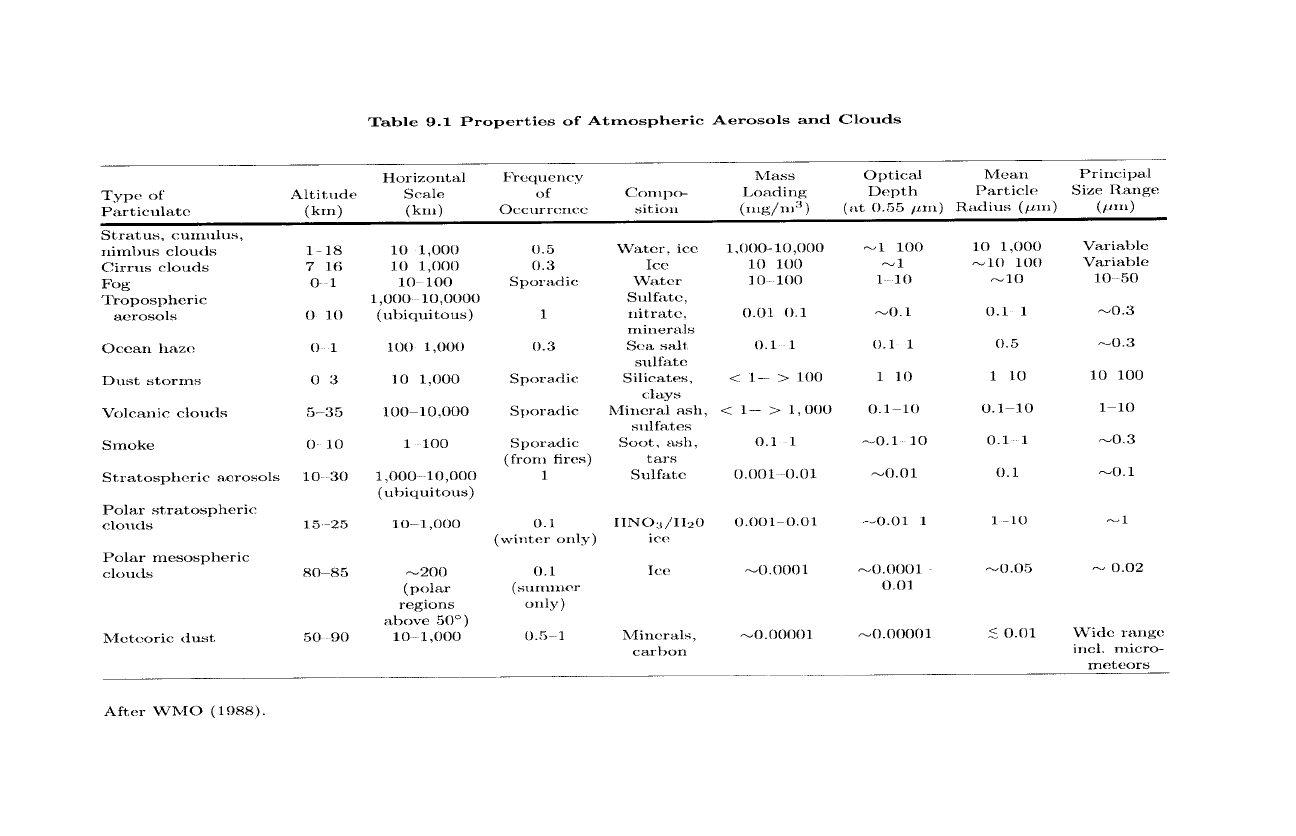
Table
9.1
Properties
of
Atmospheric
Aerosols and
Clouds
Horizontal
Frequcricy
&lass
Optical
Mean
Principal
Typc.
of
Altitude
Scale
of
C"n1p"-
Loading
Depth
Particlr Size Range
Particulatc
(krri)
(kiii)
Occurronw
sitioii
(riig/~ii")
(at
0.55
/LII~)
Radius
(,uiri)
(/mi)
Stratus,
ciirnrilus.
Iiimbiis
clouds
Cirnis
cloiids
Fog
Tropospheric
aerosols
Occari
hazc
Dust
storms
Volcanic
cloricls
Smoke
Stratosphcric
a.crosols
Polar stratosphcric
clo11ds
Polar
mesospheric
Cl<>ll<lS
blct voric dust,
1
18
7
16
01
0
10
0
1
03
5-35
0
10
10
30
15-25
80-85
50
90
10
1,000
10 1,000
10
100
1,000
10,0000
(ubiquitous)
100
1,000
10
1.000
100-10,000
1
100
1.000
10,000
(ubiquitous)
10-1,000
0.5
0.3
Sporadic
1
0.3
Sporadic
Sporadic
Sporadic
(fronl
fircs)
1
0.1
Water.
ice
Icc.
Wittcr
Sulfate,
nitrate.
minerals
Sca
salt
s11lfate
Silicates,
clays
Mincral ash,
SlllfiLtCS
Soot,
ash.
tars
Sulfatc
1.000-10,000
10
100
10 100
0
01
0
1
0
1
1
<
I-
>
100
<
1
-
>
1,
000
01
1
0.001
0.01
0.00
1-0.0
1
(winter
orlly)
ic?
-200
0.1
ICY.
-0.000
1
(polar
(siirni
iicr
regions
o11ly)
above
SOo)
10
1,000
0.5-
1
Miricrals,
-0.00001
carbon
-1
100
-1
110
-0
1
0.1 1
110
0
1-10
-01
10
-0.01
-0 .0
1
-
1
-0
0001
0
01
-0
.oooo
1
10
1,000
-10
100
-10
0.1
1
0.5
1
10
0.1-10
01
1
0.1
1-10
-
0.05
5
0.01
Variable
Variable
10 50
-0.3
-0.3
10 100
1-10
-0.3
-0.1
--I
-
0.02
Widc
raiipc
irlcl. micro-
meteors
After
WMC>
(
1988).
