Salby M.L. Fundamentals of Atmospheric Physics
Подождите немного. Документ загружается.

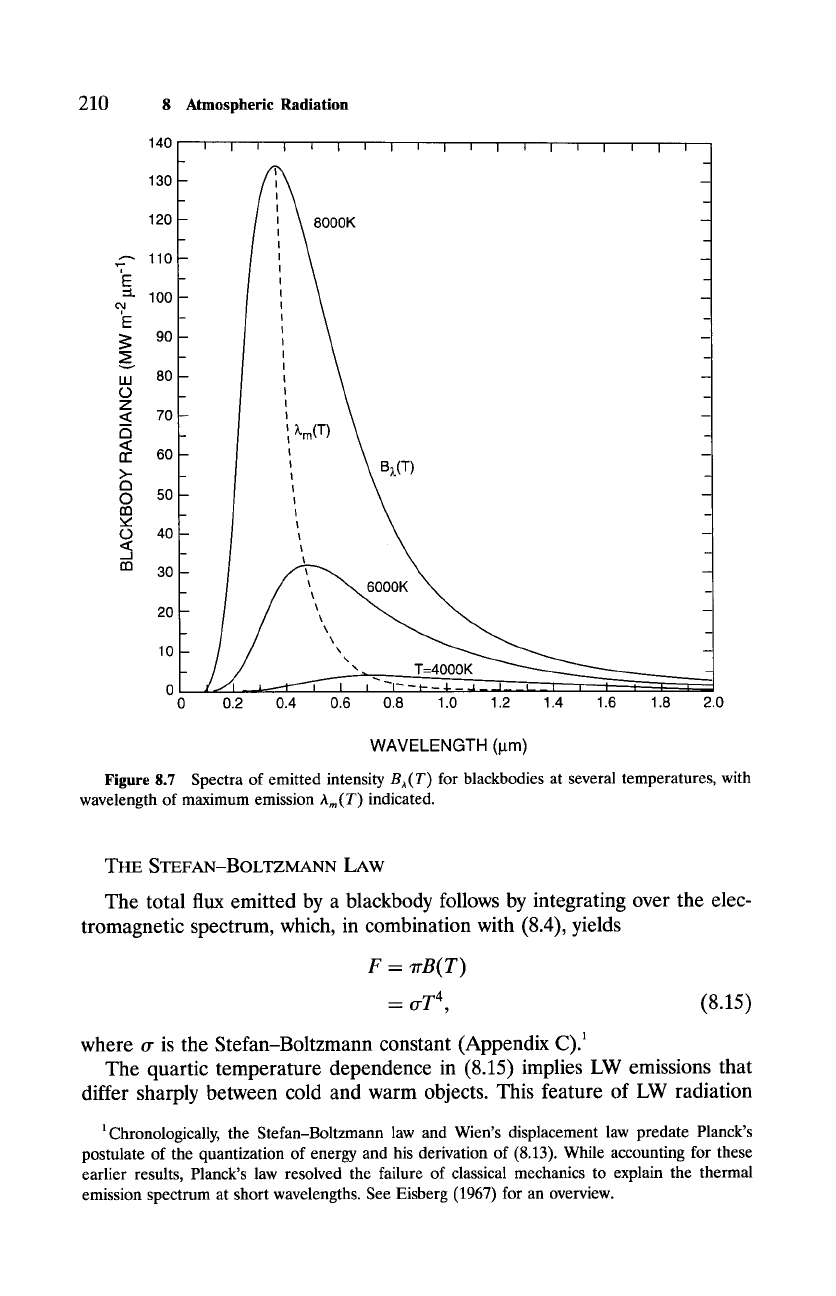
210
8
Atmospheric
Radiation
,,0'40f' '
120 I- 8000K
I I I i I I I I I I I i I m I
'_1
11o
"-7
E
lOO
04
i
E
90
v
I.U
80
0
z
< 70
D
<
rr 60
>..
a
O 5o
133
,y,
0 40
<
J
rn 30
, \
I
I
I
I
i
I
i
', km(T)
I
I
I
I
I
I
I
I
I
I
Bk.(T)
'~ f// ,,,
10 \
\
T=4OOOK
0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0
WAVELENGTH (pro)
Figure 8.7 Spectra of emitted intensity
B;,(T)
for blackbodies at several temperatures, with
wavelength of maximum emission Am(T) indicated.
THE STEFAN-BOLTZMANN LAW
The total flux emitted by a blackbody follows by integrating over the elec-
tromagnetic spectrum, which, in combination with (8.4), yields
F- IrB(T)
-- o'T 4,
(8.15)
where ~r is the Stefan-Boltzmann constant (Appendix C). 1
The quartic temperature dependence in (8.15) implies LW emissions that
differ sharply between cold and warm objects. This feature of LW radiation
1Chronologically, the Stefan-Boltzmann law and Wien's displacement law predate Planck's
postulate of the quantization of energy and his derivation of (8.13). While accounting for these
earlier results, Planck's law resolved the failure of classical mechanics to explain the thermal
emission spectrum at short wavelengths. See Eisberg (1967) for an overview.

8.2 Description of Radiative Transfer
211
is inherent to IR imagery like that shown in Figs. 1.23 and 1.25. The Sahara
desert in North Africa at 1200 GMT (Fig. 1.25a), which is very dark and has
temperatures in excess of 315 K, corresponds to emitted fluxes exceeding 550
W m -2. (This strong surface emission is mediated by the atmosphere, which
traps LW radiation and limits outgoing fluxes at the top of the atmosphere
to 350 W m -2 or less.) At the same time, LW emission from deep convective
towers over Indonesia, which have temperatures as cold as 180 K, is only of
order 60 W m -2. These large differences of LW emission symbolize geograph-
ical variations in the earth's energy budget. They are also symbolic of diurnal
variations, which follow from changes during the day of surface temperature
and cloud cover.
KIRCHHOFF'S LAW
The preceding laws determine the emission into a pencil of radiation during
its passage through a medium that behaves as a blackbody. Real substances
absorb and emit radiation at rates smaller than those of a perfect absorber and
emitter. A
graybody
absorbs and emits with efficiencies that are independent
of wavelength. The
absorptivity a x
of a substance is defined as the ratio of
the intensity it absorbs to
Bx(T).
According to (8.11), the absorptivity of a
layer approaches unity with increasing optical path length, at which point it
behaves as a blackbody. Similarly, the
emissivity E A
is defined as the ratio of
intensity emitted by a substance to
Bx(T),
which, according to the properties
of blackbody radiation, must likewise approach unity with increasing optical
path length.
Consider two infinite plates that communicate with one another only radia-
tively: one a blackbody and the other a graybody with constant absorptivity a
and emissivity e. Suppose the plates are in thermal equilibrium, so they ab-
sorb and emit energy in equal proportion. Suppose further that this state is
characterized by the plates having different temperatures. Introducing a con-
ducting medium between the plates will then drive the system out of thermal
equilibrium by permitting heat to flow from the warmer to the colder plate.
To restore thermal equilibrium, radiation must then transfer energy from the
colder plate back to the warmer plate~which violates the second law of ther-
modynamics. It follows that the
radiative equilibrium temperature
of the gray
plate, that temperature at which it emits energy at the same rate as it absorbs
energy from its surroundings, must be identical to that of the black plate.
For the gray plate to be in equilibrium, the flux of energy it emits,
E o'T 4,
must equal the flux of energy it absorbs from the black plate:
ao'T 4.
Apply-
ing
this reasoning to substances of arbitrary monochromatic absorptivity and
emissivity leads to the conclusion
E~ =aA. (8.16)
Known as
Kirchhoff's law,
(8.16) asserts that a substance emits radiation at
each wavelength as efficiently as it absorbs it.

212 8 Atmospheric
Radiation
In general, the radiative efficiency of a substance varies with wavelength. As
illustrated in Problem 8.12, an illuminated surface with an absorptivity of asw
in the SW and aLw in the LW has a radiative-equilibrium temperature that
varies according to the ratio asw/aLw. Thus, a surface like snow, which absorbs
weakly in the visible but strongly in the IR, has a lower radiative-equilibrium
temperature than a gray surface, for which the absorptivity is independent of
wavelength. Likewise, the selective transmission characteristics of the atmo-
sphere elevate the radiative-equilibrium temperature of the surface over what
it would be in the absence of an atmosphere. SW radiation that passes freely
through the atmosphere is absorbed at the surface. But LW radiation reemit-
ted by the surface to preserve thermal equilibrium is trapped by the overlying
atmosphere, which absorbs at those wavelengths (Problem 8.16). To offset re-
duced transmission, the surface must emit more LW radiation, which requires
a higher temperature (8.15), until the LW radiation rejected at the top of the
atmosphere just balances the SW radiation absorbed. The increased surface
temperature (e.g., the greenhouse effect) is related directly to the difference
in opacity between the visible and IR, the demonstration of which is left as an
exercise.
Kirchhoff's law is predicated on a state of
local thermodynamic equilibrium
(LTE), wherein temperature is uniform and radiation is isotropic within some
small volume. Those conditions are satisfied when energy transitions are dom-
inated by molecular collisions, which is true for the most important radiatively
active gases at pressures greater than 0.01 mb (e.g., at altitudes below 60 km).
At higher altitudes, LTE breaks down because the interval between collisions
is no longer short compared to the lifetime of excited states associated with
absorption and emission. Kirchhoff's law is then invalid.
With (8.16), the fractional energy emitted into a pencil of radiation along
an incremental distance
ds
can be expressed
d/,~
B,~
= de.~ - da~
= p~raAds
by Lambert's law. Then
dlx - p~raflxds,
(8.17)
where
JA -- B~ (T) (8.18)
denotes the monochromatic
source function
in the absence of scattering. Equa-
tion (8.18) holds under the conditions of Kirchhoff's law, namely, under LTE.
Otherwise, the contribution to the source function from emission is more
complex.

8.2 Description of Radiative Transfer 213
8.2.4 Scattering
Beyond absorption and emission, energy passing through a pencil of radiation
is also modified by scattering, which refers to the extraction and subsequent
reemission of energy by matter. A population of molecules possesses, in addi-
tion to the translational energy associated with temperature, electronic, vibra-
tional, and rotational energies. Those forms of internal energy can be excited
by absorbing a photon, which can then be released in several ways. The sim-
plest is when the excited internal energy is converted into translational energy
or "thermalized" through molecular collision, which corresponds to thermal
absorption of radiation. Thermal emission occurs through the reverse process.
The excited energy can also be reemitted--in wavelengths and directions dif-
ferent from those of the incident radiation (Fig. 8.8), which then constitutes
scattering. Analogous interactions occur between radiation and atmospheric
aerosol.
The foregoing process is described in terms of the monochromatic
scat-
tering cross section trsx,
which symbolizes the fractional area removed from a
pencil of radiation through scattering. Effects of absorption and scattering are
additive since both are linear. Then the monochromatic
extinction cross section
is defined by
k a = o-~;~ + o-,;~, (8.19.1)
(a)
~~1.6 ....
(b)
- - - J- ~~,) 30
.....
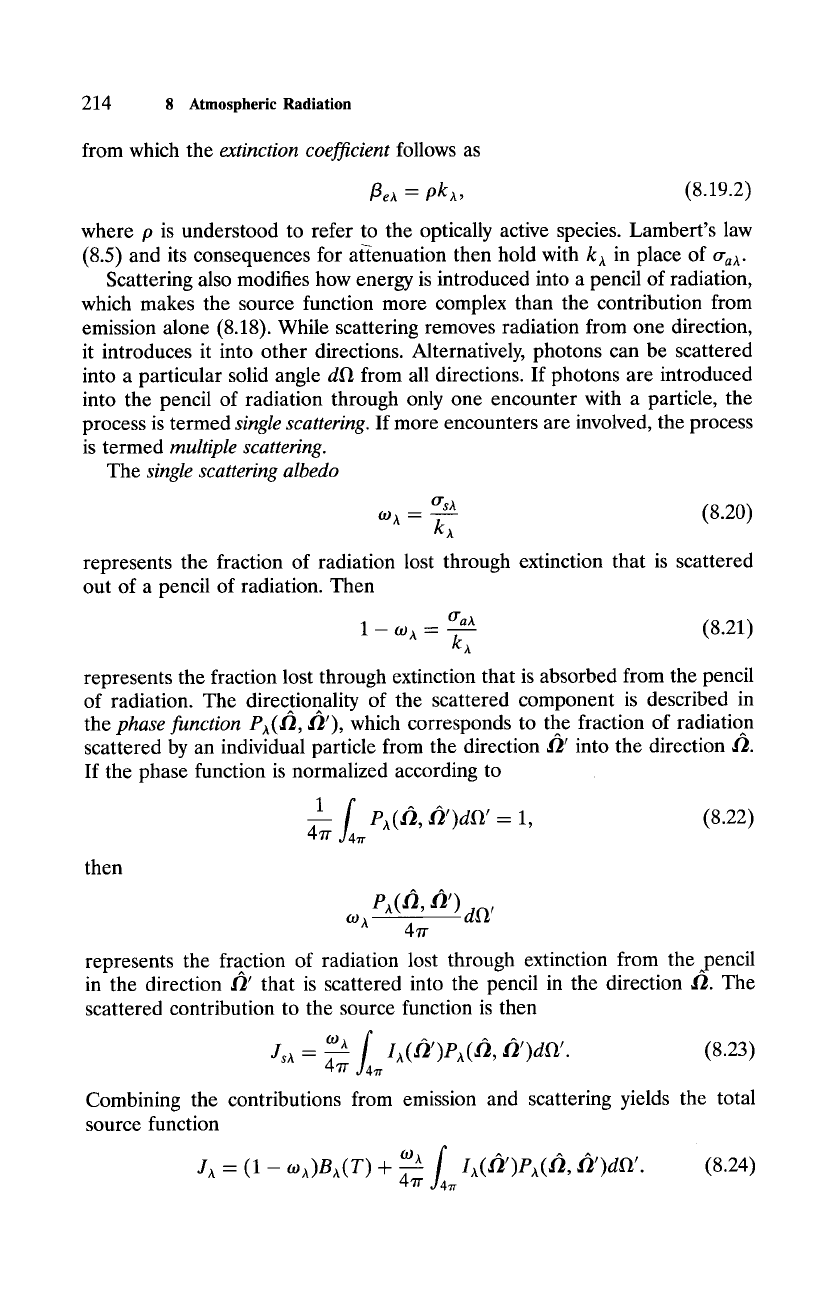
Figure
8.8 Angular distribution of radiation scattered from (a) small particles (of radius
a < < h), which is representative of
Rayleigh scattering
of SW radiation by air molecules (Sec.
9.4.1), and (b) large particles (a > > Z), which is representative of
Mie scattering
of SW radiation
by cloud droplets (Sec. 9.4.1). Phase function P is plotted in terms of the scattering angle O
and in (b) for a scattering population with the refractive index of water and an effective size
parameter
x e - 27r(ae/Z ) = 5. Note: The
compressed scale in (b) implies that energy redirected
by large particles is dominated by forward scattering. Larger particles produce even stronger
forward scattering (compare Fig. 9.27). Data in (b) courtesy of E Evans (U. Colorado).
214 8 Atmospheric
Radiation
from which the
extinction coefficient
follows as
[~eA =
pkA,
(8.19.2)
where p is understood to refer to the optically active species. Lambert's law
(8.5) and its consequences for attenuation then hold with k a in place of traa.
Scattering also modifies how energy is introduced into a pencil of radiation,
which makes the source function more complex than the contribution from
emission alone (8.18). While scattering removes radiation from one direction,
it introduces it into other directions. Alternatively, photons can be scattered
into a particular solid angle d12 from all directions. If photons are introduced
into the pencil of radiation through only one encounter with a particle, the
process is termed
single scattering.
If more encounters are involved, the process
is termed
multiple scattering.
The
single scattering albedo
Crsa (8.20)
r~ = kA
represents the fraction of radiation lost through extinction that is scattered
out of a pencil of radiation. Then
O'aA
(8.21)
1 - ro a =
ka
represents the fraction lost through extinction that is absorbed from the pencil
of radiation. The dire~ionality of the scattered component is described in
the
phase function
Pa(g~, g~'), which corresponds to the fraction of radiation
scattered by an individual particle from the direction g}' into the direction g}.
If the phase function is normalized according to
1 f g},
4rr an Pa(g]' )dO'- 1, (8.22)
7r
then
da'
~ 47r
represents the fraction of radiation lost through extinction from the ^pencil
in the direction g~' that is scattered into the pencil in the direction g~. The
scattered contribution to the source function is then
"f4
Js,~ = ~ Ia(~')Pa(g~, g~')dlT.
(8.23)
7I"
Combining the contributions from emission and scattering yields the total
source function
w,~ f ia(g],)pa(g}, g}')dfY
(8.24)
Jx - (a - ~~ + -~
,J4rr
8.3
Absorption Characteristics of Gases
215
8.2.5 The Equation of Radiative Transfer
Collecting the extinction of energy (8.19) and the introduction of energy (8.17)
yields the net rate at which the intensity inside a pencil of radiation changes
with distance s,
- -Ix + JA, (8.25)
pkAds
which is the
radiative transfer equation
in general form. In the absence of
scattering, (8.25) reduces to
dI~
pkads
= -I x + BA(T), (8.26)
where kA then equals the absorption cross section O-ax. Introducing the
optical
thickness
i
O
XA(s) = pkxds'
= -u(s),
which increases in the negative s direction (i.e., opposite to g]):
(8.27.1)
dXA =-pkAds,
(8.27.2)
casts the energy budget for a pencil of radiation into the canonical form
dlA
dxa
-- I x - BA(T ),
(8.28)
which is known as
Schwartzchild's equation.
The solution of Schwartzchild's equation can be expressed formally with
the aid of an integrating factor. Multiplying by
e-X~
allows (8.28) to be written
dxx
~(e-Xalx) -- _e-X~BA(T),
which upon integrating from 0 to
Xa(S)
yields
I,~(s) = I,~(O)e x~(s) - fo x~'(s)
B A [ T (X'A ) ] ex~ (s)-x'~ dx'x .
(8.29)
The first term in (8.29) describes an exponential decrease with optical path
length of the incident intensity IA(0), as embodied in Lambert's law (8.7).
The second term describes the cumulative emission and absorption between 0
and
XA(S).
If the properties T, p, and k A are known as functions of s, (8.29)
uniquely determines the intensity along the path of radiation.

216 8
Atmospheric Radiation
8.3 Absorption Characteristics of Gases
8.3.1 Interactions between Radiation and Molecules
The absorptivity of a medium approaches unity with increasing optical path
length (Sec. 8.2.1), irrespective of wavelength. Along finite path lengths (e.g.,
through an atmosphere of bounded mass), absorption may be large at some
wavelengths and small at others, according to the optical properties of the
medium. For a gas, the absorption spectrum a A is concentrated in a complex
array of lines that correspond to transitions between the discrete electronic,
vibrational, and rotational energy levels of molecules. Electronic transitions are
stimulated by radiation at UV and visible wavelengths, whereas vibrational and
rotational transitions occur at IR wavelengths. At pressures greater than about
0.1 mb (e.g., below 60 km), the internal energy acquired by absorbing a photon
is quickly thermalized. In addition to discrete absorption characteristics, a
continuum of absorption occurs at shorter wavelengths in connection with
the photodissociation and photoionization of molecules. The latter occur at
wavelengths of X-ray, UV, and, to a lesser degree, visible radiation and are
possible for all A shorter than the threshold to break molecular and electronic
bonds.
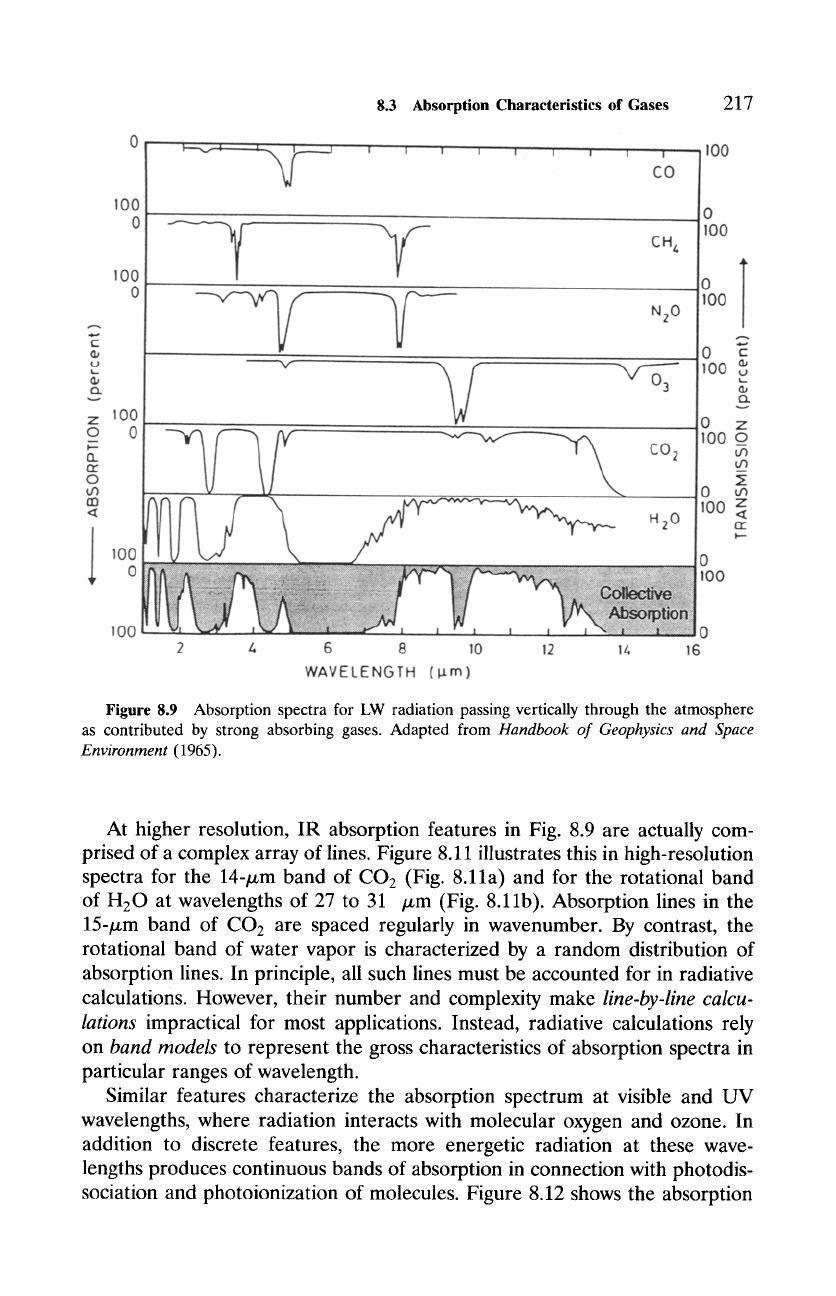
Figure 8.9 shows absorption spectra in the IR for optically active gases
corresponding to a vertical path through the atmosphere. All of the species
are minor constituents of air, the most important being water vapor, carbon
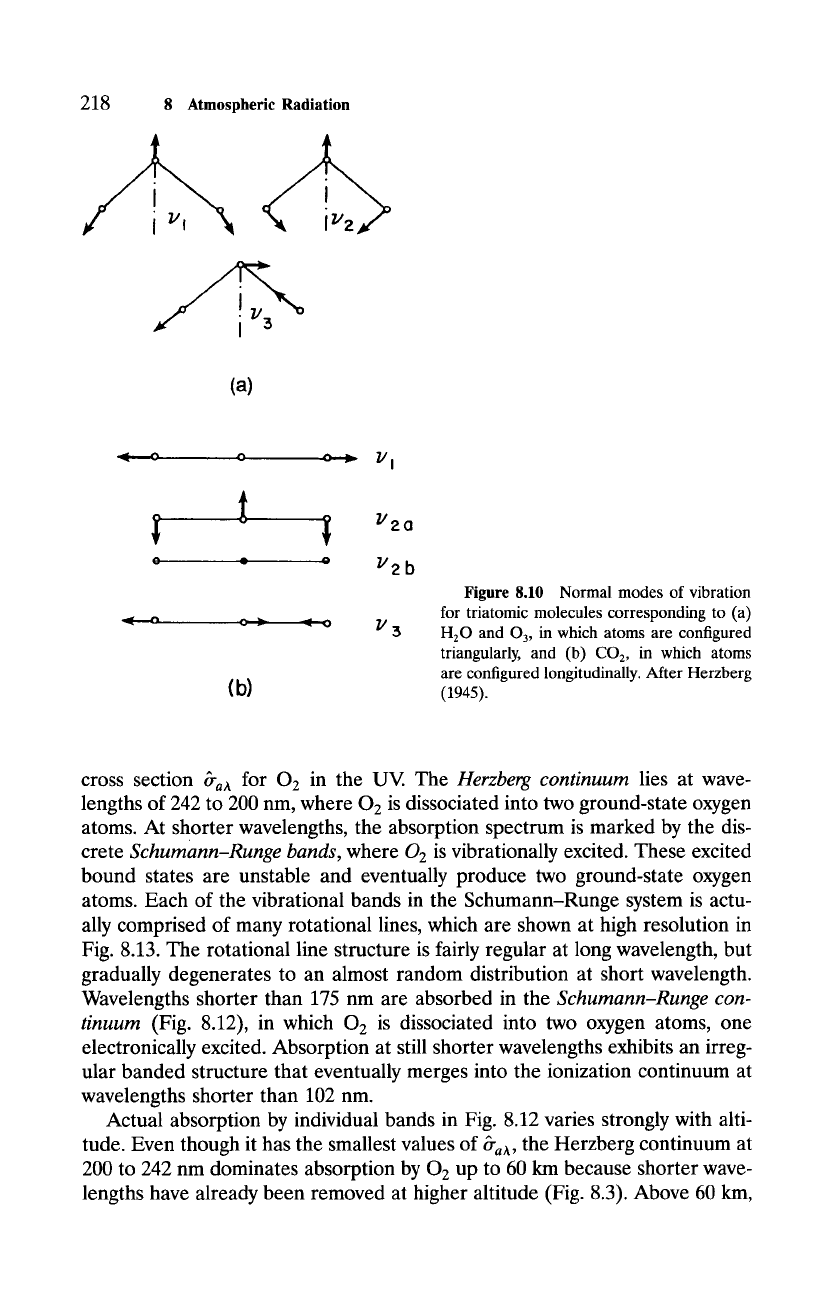
dioxide, and ozone. Each of these triatomic molecules is capable of under-
going simultaneous vibration-rotation transitions, which produce a clustering
of absorption lines. At coarse resolution, like spectra in Figs. 8.1 and 8.9,
these clusters appear as continuous bands of absorption. Water vapor absorbs
strongly in a broad band centered near 6.3/zm and in another at 2.7/zm. Both
bands correspond to transitions from vibrationally excited states: 6.3-pm ab-
sorption to the v2 vibrational mode (Fig. 8.10a), whereas the band at 2.7/xm
involves both va and v 3 modes of vibration. Rotational transitions of H20,
which are less energetic, lead to absorption at wavelengths longer than 12/zm
(compare Fig. 8.1). Carbon dioxide is excited vibrationally by wavelengths near
15 pm and also near 4.3/zm. The former corresponds to the transverse mode
v2 (Fig. 8.10b), whereas the latter corresponds to the longitudinal vibration
v 3. Ozone absorbs strongly at wavelengths near 9.6/xm in connection with vi-
brational transitions. Because it coincides with the atmospheric window at 8
to 12/xm (Fig. 8.1), this absorption band allows stratospheric ozone to inter-
act radiatively with the troposphere and the earth's surface. Except for ozone,
most of the absorption by these species takes place in the troposphere, where
absolute concentrations are large (compare Figs. 8.1b and c). Nitrous oxide,
methane, carbon monoxide, and chlorofluorocarbon (CFC) -11 and -12 also
have absorption lines within the range of wavelength shown in Fig. 8.9, but
are of secondary importance.
8.3 Absorption Characteristics of Gases
217
I00
0
u
L.
Q.
v
z 100
o
0
i--
n
Or
o
rn
,00
I00
2 4 6 8 I0 12 14
WAVELENGTH (lam)
CO
Y
CHa
N20
V
100
0
IO0
0
!00
o0 l
0 c
100 *
cl
0 z
100 o
0 m
100 z
0
100
0
6
Figure 8.9 Absorption spectra for LW radiation passing vertically through the atmosphere
as contributed by strong absorbing gases. Adapted from
Handbook
of Geophysics
and Space
Environment
(1965).
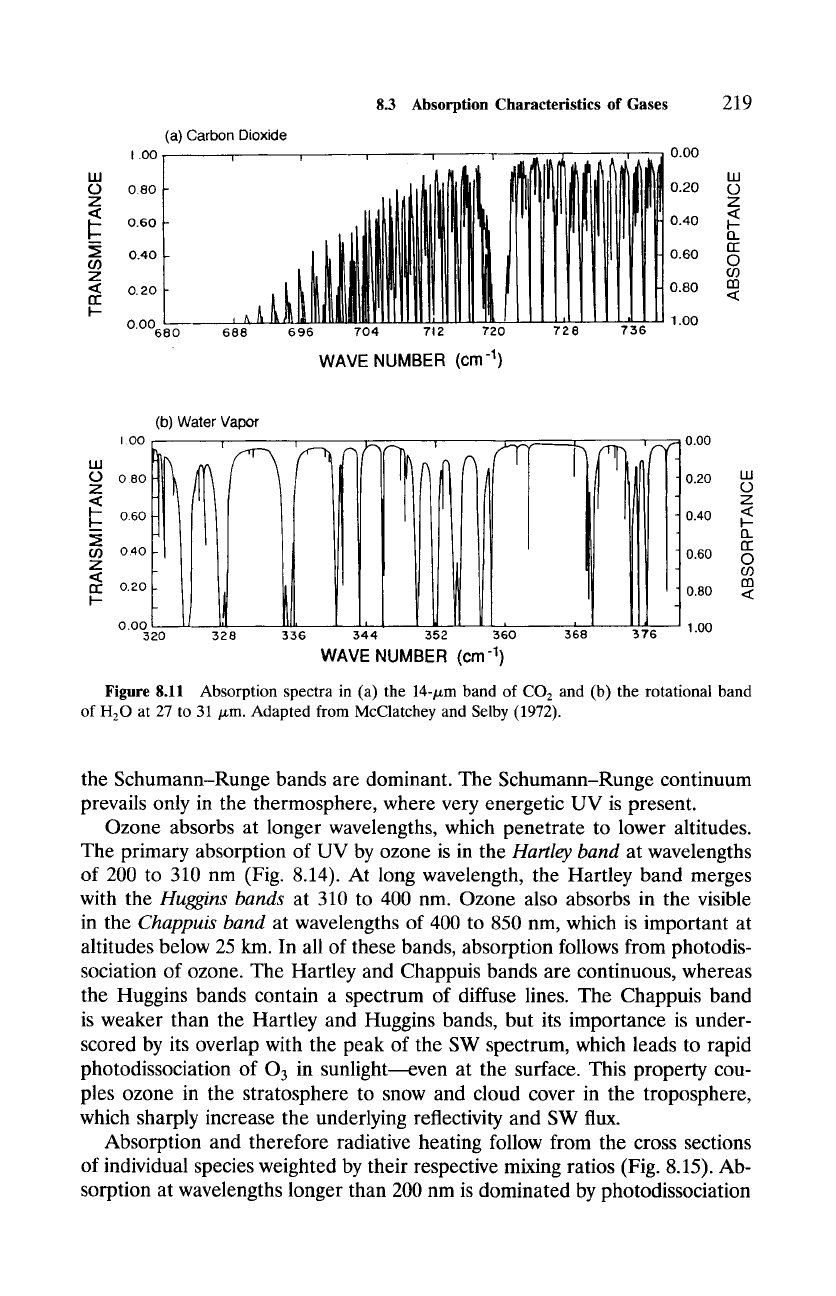
At higher resolution, IR absorption features in Fig. 8.9 are actually com-
prised of a complex array of lines. Figure 8.11 illustrates this in high-resolution
spectra for the 14-/xm band of CO2 (Fig. 8.11a) and for the rotational band
of H20 at wavelengths of 27 to 31 /xm (Fig. 8.11b). Absorption lines in the
15-/xm band of CO2 are spaced regularly in wavenumber. By contrast, the
rotational band of water vapor is characterized by a random distribution of
absorption lines. In principle, all such lines must be accounted for in radiative
calculations. However, their number and complexity make
line-by-line calcu-
lations
impractical for most applications. Instead, radiative calculations rely
on
band models
to represent the gross characteristics of absorption spectra in
particular ranges of wavelength.
Similar features characterize the absorption spectrum at visible and UV
wavelengths, where radiation interacts with molecular oxygen and ozone. In
addition to discrete features, the more energetic radiation at these wave-
lengths produces continuous bands of absorption in connection with photodis-
sociation and photoionization of molecules. Figure 8.12 shows the absorption
218 8 Atmospheric Radiation
/
(a)
o o ~ Z/I
Z/2Q
e = --" I/2b
(b)
Figure 8.10 Normal modes of vibration
for triatomic molecules corresponding to (a)
H20 and 03, in which atoms are configured
triangularly, and (b) CO2, in which atoms
are configured longitudinally. After Herzberg
(1945).
cross section &aA for
0 2
in the UV. The Herzberg continuum lies at wave-
lengths of 242 to 200 nm, where O2 is dissociated into two ground-state oxygen
atoms. At shorter wavelengths, the absorption spectrum is marked by the dis-
crete Schumann-Runge bands, where O2 is vibrationally excited. These excited
bound states are unstable and eventually produce two ground-state oxygen
atoms. Each of the vibrational bands in the Schumann-Runge system is actu-
ally comprised of many rotational lines, which are shown at high resolution in
Fig. 8.13. The rotational line structure is fairly regular at long wavelength, but
gradually degenerates to an almost random distribution at short wavelength.
Wavelengths shorter than 175 nm are absorbed in the Schumann-Runge con-
tinuum (Fig. 8.12), in which O2 is dissociated into two oxygen atoms, one
electronically excited. Absorption at still shorter wavelengths exhibits an irreg-
ular banded structure that eventually merges into the ionization continuum at
wavelengths shorter than 102 nm.
Actual absorption by individual bands in Fig. 8.12 varies strongly with alti-
tude. Even though it has the smallest values of &~, the Herzberg continuum at
200 to 242 nm dominates absorption by O2 up to 60 km because shorter wave-
lengths have already been removed at higher altitude (Fig. 8.3). Above 60 km,
uJ
0
z
<
fat)
z
<
rr
t--
I .00
0.80
0.60
0.40
0.20
0"00680 688
8.3
(a) Carbon Dioxide
I I I
696 704 7
Absorption Characteristics of Gases
219
0.00
0.20 (~
0.40
0.60 (~)
0.80 toO'-)
<
1.00
2 720 728 736
WAVE NUMBER (cm -1)
(b) Water Vapor
I O0 i 0.00
o.oo 0.40
04O 0.60
0.20 0.80
I-- <
0.00 - " 360 3~,8 1.00
320 ~2~ 3~o 3.,, 3~2 ~T6
WAVE NUMBER
(om -1)
Figure
8.11 Absorption spectra in (a) the 14-/xm band of CO2 and (b) the rotational band
of H20 at 27 to 31/xm. Adapted from McClatchey and Selby (1972).
the Schumann-Runge bands are dominant. The Schumann-Runge continuum
prevails only in the thermosphere, where very energetic UV is present.
Ozone absorbs at longer wavelengths, which penetrate to lower altitudes.
The primary absorption of UV by ozone is in the
Hartley band
at wavelengths
of 200 to 310 nm (Fig. 8.14). At long wavelength, the Hartley band merges
with the
Huggins bands
at 310 to 400 nm. Ozone also absorbs in the visible
in the
Chappuis band
at wavelengths of 400 to 850 nm, which is important at
altitudes below 25 km. In all of these bands, absorption follows from photodis-
sociation of ozone. The Hartley and Chappuis bands are continuous, whereas
the Huggins bands contain a spectrum of diffuse lines. The Chappuis band
is weaker than the Hartley and Huggins bands, but its importance is under-
scored by its overlap with the peak of the SW spectrum, which leads to rapid
photodissociation of
0 3
in sunlight---even at the surface. This property cou-
ples ozone in the stratosphere to snow and cloud cover in the troposphere,
which sharply increase the underlying reflectivity and SW flux.
Absorption and therefore radiative heating follow from the cross sections
of individual species weighted by their respective mixing ratios (Fig. 8.15). Ab-
sorption at wavelengths longer than 200 nm is dominated by photodissociation
