Salby M.L. Fundamentals of Atmospheric Physics
Подождите немного. Документ загружается.


130 5
Transformations of Moist
Air
heat from the gas phase, which cools the parcel, introduces negative buoyancy,
and thus promotes continued descent. Any condensate that previously precip-
itated out of the parcel is not available to reabsorb latent heat that it released
during ascent, which therefore remains in the gas phase.
The foregoing behavior is responsible for confining water vapor near the
earth's surface. Introduced over warm oceans, water vapor is extracted from air
that is displaced upward. Moisture is lost altogether when condensate precipi-
tates back to the earth's surface, after cloud particles have become sufficiently
large. In this fashion, thermodynamics, in combination with hydrostatic strat-
ification, maintains upper levels of the atmosphere in a very dry state. Even
inside a convective tower, mixing ratio decreases vertically because chemi-
cal equilibrium requires r to equal
r c,
which decreases steadily with altitude.
By limiting its vertical transport, thermodynamics prevents water vapor from
reaching great altitudes, where it would be photodissociated by energetic ra-
diation and ultimately destroyed when the free hydrogen produced is lost to
space (Sec. 1.2.2).
Due to exchange of latent heat with the condensed phase, the gas phase
of an air parcel is not adiabatic above the LCL---even though the entire
system may still be. Consequently, the potential temperature of the gas phase
is no longer conserved. Since the parcel's mass is dominated by dry air, the
transformation of mass has only a minor effect on the energetics of the parcel.
But the transfer of latent heat that attends the phase transformation has a
major effect by serving as an internal heat source for the system. Latent heat
released to the gas phase during condensation offsets cooling due to adiabatic
expansion work that is performed by the parcel during ascent. Conversely,
latent heat absorbed from the gas phase during vaporization offsets warming
due to adiabatic compression work that is performed on the parcel during
descent. Owing to the transfer of latent heat, the parcel's temperature no
longer changes with altitude at the dry adiabatic lapse rate, but rather varies
more slowly under saturated conditions.
5.4.2 The Pseudo-Adiabatic Process
If expansion work occurs fast enough for heat transfer with the environment
to remain negligible and if none of its moisture precipitates out, the parcel
is closed and its behavior above the LCL is described by a reversible satu-
rated adiabatic process. That process depends weakly on the abundance of
condensate present (e.g., on how much of the system's enthalpy is represented
by condensate) and therefore on the LCL of the parcel. However, because it
is present only in trace abundance, the variation of condensate unnecessarily
complicates the parcel's description under saturated conditions. This compli-
cation is averted by describing the parcel's behavior in terms of a
pseudo-
adiabatic process,
in which the system is treated as open and condensate is
removed (added) immediately after (before) it is produced (destroyed). Be-

5.4
Thermodynamic Behavior Accompanying Vertical Motion
131
cause the water component accounts for only a small fraction of the system's
mass, the pseudo-adiabatic process is nearly identical to a reversible saturated
adiabatic process.
A pseudo-adiabatic change of state may be constructed in two legs:
1. Reversible saturated adiabatic expansion (compression), which results
in the production (destruction) of condensate of mass
dmr
and a com-
mensurate release (absorption) of latent heat to (from) the gas phase
2. Removal (addition) of condensate of mass
dmc
Since the phase transformation occurs adiabatically and reversibly, this process
is isentropic:
ds - O.
Also, since just enough vapor condenses to maintain chemical equilibrium
(r = rc),
the change of water vapor mixing ratio is given by
Then (5.22) implies
dr = dr c.
('rc
dln
0 = -d cpT} "
(5.25)
With the removal of condensate, (5.25) describes the change of potential tem-
perature of the gas phase in terms of the transformation of the water compo-
nent. A decrease of rc, such as accompanies ascent and expansional cooling,
increases 0, whereas an increase of
rc,
such as accompanies descent and com-
pressional warming, decreases 0.
Integrating (5.25) obtains
0 exp(Irc~
- const, (5.26)
\ cpT,]
which describes a family of paths in the state space of moist air, one analogous
to the family of adiabats described by Poisson's equation (2.30) for dry air.
Just as the latter was used to introduce the potential temperature, which is
preserved for an adiabatic process, (5.26) may be used to introduce another
state variable, which is preserved for a pseudo-adiabatic process. Evaluating
(5.26) at a reference state of zero pressure (toward which r~ approaches zero
faster than does the parcel's temperature) yields
0e ('re t
~ = exp c-~ ' (5.27)
which defines the
equivalent potential temperature Oe.
According to (5.26), 0e
is constant during a pseudo-adiabatic process. Condensation is accompanied
by a reduction of
rc
and an increase of 0, but the two vary in such proportion
as to preserve 0 e. The same holds for vaporization. In physical space, the

132 5
Transformations of Moist Air
material line in Fig. 5.4 advances to isentropes of higher 0, due to the release
of latent heat, but it remains coincident with the isopleth of 0e with which it
coincided initially. The equivalent potential temperature reflects the maximum
temperature a moist air parcel can assume through adiabatic compression
and the release of latent heat: namely, if it was displaced to the top of the
atmosphere, where all of the moisture condensed and released its latent heat,
if the condensate produced subsequently precipitated out, and if the parcel
was then brought down adiabatically to the surface.
Like 0, 0 e "--
Oe(P, T,
r)
is a state variable. Just as 0 is conserved along an
adiabat in state space and below the LCL, 0e is conserved along a
pseudo
or
saturated adiabat
in state space and above the LCL. Because an adiabatic
process involving no transformation of phase is also pseudo-adiabatic, 0e is
conserved under unsaturated conditions as well. However, the definition (5.27)
can be applied only under saturated conditions because only then does r =
rc,
as is impficit in the derivation of 0e. Alternatively, because it is conserved,
0e can be calculated with r in place of
rc
if T is replaced by the parcel's
temperature at the LCL, where it just becomes saturated.
5.4.3 The Saturated Adiabatic Lapse Rate
The temperature of a dry air parcel decreases with its altitude at the dry adi-
abatic lapse rate Fa. To a good approximation, the same holds for a moist
air parcel under unsaturated conditions because the trace abundance of water
vapor modifies thermal properties of air only slightly. Under saturated condi-
tions, the adiabatic description of air breaks down due to the release of latent
heat that accompanies transformation of water from one phase to another.
Latent heat exchanged with the gas phase then offsets cooling and warming
that accompanies adiabatic expansion and compression.
An approximate description of how the temperature of a saturated parcel
changes with altitude can be derived from the first law with the aid of (5.19).
From (2.35) the first law for the gas phase can be expressed
6q
cpd
In
T - Rd
In p - -~--. (5.28)
If the parcel is unsaturated, (5.28) recovers the dry adiabatic lapse rate (2.33).
If it is saturated, the heat transferred to the gas phase is given by
6q - -tdr c.
(5.29)
Then (5.28) becomes
l
cpd
In
T - Rd
In p =
--~dr c,
where l is treated as constant. Hydrostatic equilibrium (1.16) reduces this to
cpdT + gdz - -ldr c.
(5.30)
5.4
Thermodynamic Behavior Accompanying Vertical Motion
133
Strictly, the saturation mixing ratio depends on both pressure and temper-
ature, so
() (3rc)
3rc dT + dp.
drc= --~-f p \,gp r
However, the strong dependence on temperature passed on from the Clausius-
Clapeyron equation through (5.7) is the dominant influence on r~. If its de-
pendence on pressure is ignored, the change of saturation mixing ratio can be
written
drc
drc = --d-~ dT,
where T and z are understood to refer to the displaced parcel. Then (5.30)
reduces to
drr
cpdT + gdz - -l-d- ~ dT
or
( drc)
Cp +
l-d- f dT + gdz = O.
In terms of the dry adiabatic lapse rate, this can be expressed
1 + ~ dr + Fa dz
= 0. (5.31)
cp -d-T
Then the saturated parcel's temperature decreases with altitude according to
dT F d
dz 1
-[ - l--drc
Cp dT
= Vs, (5.32)
which defines the
saturated adiabatic lapse rate.
Unlike Fa, F, varies with the parcel's altitude due to the nonlinear depen-
dence on T of
r c.
However, since
(drc/dT)
> 0, (5.32) implies
F s < F a, (5.33)
so a parcel's temperature decreases with altitude slower under saturated con-
ditions than under unsaturated conditions. This property of saturated vertical
motion follows from the release of latent heat to the gas phase, which offsets
cooling associated with adiabatic expansion. Although variable, the saturated
adiabatic lapse rate has a value of Fs ~ 6.5 K
km -1
for conditions represen-
tative of the troposphere. This is close to the global-mean lapse rate of the
troposphere (Fig. 1.2). No accident, this correspondence follows from dynam-
ical processes that are developed in Chapters 6 and 7.
134
5
Transformations of Moist
Air
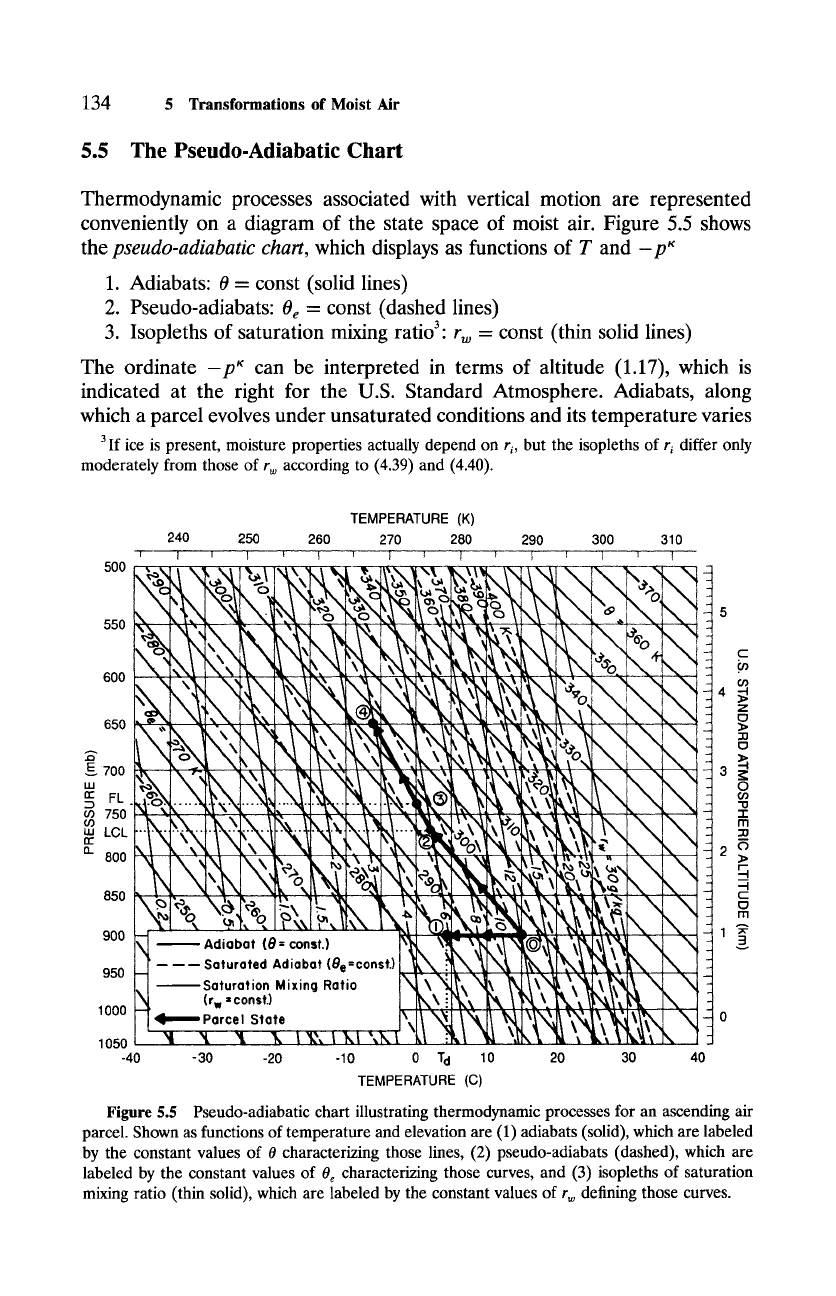
5.5 The Pseudo-Adiabatic Chart
Thermodynamic processes associated with vertical motion are represented
conveniently on a diagram of the state space of moist air. Figure 5.5 shows
the
pseudo-adiabatic chart,
which displays as functions of T and -p~
1. Adiabats: 0 = const (solid lines)
2. Pseudo-adiabats:
0 e --
const (dashed lines)
3. Isopleths of saturation mixing ratio 3. r w = const (thin solid lines)
The ordinate -p~ can be interpreted in terms of altitude (1.17), which is
indicated at the right for the U.S. Standard Atmosphere. Adiabats, along
which a parcel evolves under unsaturated conditions and its temperature varies
3If ice is present, moisture properties actually depend on r i, but the isopleths of
r i
differ only
moderately from those of rw according to (4.39) and (4.40).
500
TEMPERATURE (K)
240 250
260 270 280 290
300 310
-1-- i ~ =: [ ] i ] r ........ ]--r----~--T--T--T [ r--I--
5
550
9oo , 3 ""
so,o,o,., co,,.,,
,,,,,,
a',>
950; SaturatiOn(r. = c on st.) M i xin(j Rat io ~ ~? ~? k'~ L~,-~, '~ ~,N~i~,, ~_~ ~" ~
1000 ~ ~ Parce I State \\ \ ~/~1~\ ~1~ ~~\~ \~ ~: N 0
-40 -30 -20 -10 0 T d 10 20 30 40
TEMPERATURE (C)
Figure
5.5 Pseudo-adiabatic chart illustrating thermodynamic processes for an ascending air
parcel. Shown as functions of temperature and elevation are (1) adiabats (solid), which are labeled
by the constant values of 0 characterizing those lines, (2) pseudo-adiabats (dashed), which are
labeled by the constant values of 0 e characterizing those curves, and (3) isopleths of saturation
mixing ratio (thin solid), which are labeled by the constant values of r w defining those curves.

5.5 The Pseudo-Adiabatic Chart
135
with height at the dry adiabatic lapse
rate F d,
appear as straight lines in this
representation (2.34). Pseudo-adiabats, along which a parcel evolves under
saturated conditions and its temperature varies with height at the saturated
adiabatic lapse rate Fs, are curved~but only weakly. Consistent with (5.33),
pseudo-adiabats have a slope with respect to height that is everywhere smaller
than that of adiabats. Both adiabats and pseudo-adiabats are labeled in Kelvin,
which correspond to the constant values of 0 and 0e characterizing those paths.
The use of the pseudo-adiabatic chart is best illustrated with an example.
Consider conditions leading to the formation of a cumulus cloud. The cloud
is fed by air ascending in a moist thermal that is driven by surface heating
(Fig. 5.4). At the surface, which is located at 900 mb, air has a temperature of
To = 15~ and a mixing ratio r0 = 6.0 g kg -1. Because air inside the thermal
originates at the surface, thermodynamic properties inside the cloud, as well
as beneath the cloud, can be inferred from the evolution of an air parcel that
is initially at the ground. Once the parcel's initial state (state 0) has been
located on the pseudo-adiabatic chart, individual properties follow by allowing
the system to evolve along certain paths in the state space of the parcel, which
are indicated in Fig. 5.5.
SURFACE RELATIVE HUMIDITY
The saturation mixing ratio at the surface follows from the isopleth of rw
passing through state 0, which gives
rw0 = 12 g kg -1.
Thus, the initial relative humidity of surface air is
6
RH~
= 12 = 50%.
SURFACE POTENTIAL TEMPERATURE
The parcel's initial potential temperature is determined by the adiabat pass-
ing through state 0, which is characterized by the constant value
0 = 00 = 297 K.
SURFACE
DEW POINT
The dew point of surface air follows from isobaric cooling out of state 0.
During that process, the parcel's state evolves along the isobar p = 900 mb,
which cuts across isopleths of saturation mixing ratio toward lower values
of r w. Eventually, state 1 is reached, at which r w = r = 6 g kg -1 and the
parcel is saturated. The temperature at which this occurs defines the dew
point temperature of surface air
Tao
= 4~

136
5
Transformations of Moist
Air
The foregoing process is responsible for the formation of ground fog. The
dew point spread of surface air, which is ll~ in this example, provides an
indirect measure of the base of convective clouds, since it reflects the amount
of adiabatic cooling necessary to achieve saturation (Problem 5.25).
CUMULUS CLOUD BASE
The base of convective clouds corresponds to the LCL of surface air. The
latter may be determined by displacing the parcel upward adiabatically. During
that process, the parcel's state evolves along the adiabat passing through state 0
(0 = 297 K), which also cuts across isopleths of saturation mixing ratio toward
lower values of rw. Eventually, state 2 is reached, at which rw = r and the
parcel is again saturated. The level at which this occurs corresponds to the LCL
PLCL = 770 mb.
The parcel's temperature at this level, which is 13 K colder than its initial tem-
perature, is only 2 K colder than the dew point temperature, reflecting the weak
pressure dependence of
rc.
EQUIVALENT POTENTIAL TEMPERATURE AT THE SURFACE
The equivalent potential temperature is determined once the LCL is lo-
cated. Through state 2 and along subsequent states, passes a saturated adiabat
that defines 0e for the parcel. Because 0e is conserved under both saturated
and unsaturated conditions, that value is also the equivalent potential temper-
ature of the parcel below the LCL, so
0e0 = 315 K
at 900 mb. Note, even though it is conserved throughout,
0 e
must be inferred
at and above the LCL, for reasons discussed in Sec. 5.4.2.
FREEZING LEVEL OF SURFACE AIR
At the LCL, the parcel's temperature is greater than 0~ so the freezing
level (FL) lies inside the cloud. Hence, lower portions of the cloud contain
water droplets, whereas higher portions contain ice particles. 4 Conditions in-
side the cloud can be determined by displacing the parcel above the LCL. The
parcel's state then evolves along the saturated adiabat passing through state 2.
Cutting across isopleths of saturation mixing ratio toward lower rw and across
adiabats toward higher 0, the 0e = 315 K saturated adiabat eventually reaches
state 3, where the temperature is 0~ That condition is achieved at the level
PrL = 740 mb.
4Ice forms only in the presence of a special type of aerosol particle, referred to as a
freezing
nucleus
(Chapter 9). Since freezing nuclei are comparatively rare, many cloud droplets are actually
"supercooled,"
that is,
they remain liquid
at temperatures below 0~

5.5 The Pseudo-Adiabatic Chart
137
LIQUID WATER CONTENT AT THE FREEZING LEVEL
Because the parcel is saturated, its mixing ratio must equal the saturation
mixing ratio at the freezing level. The isopleth of saturation mixing ratio
passing through state 3 gives for the mixing ratio there
rFL -- 5.5
gkg -1.
If no precipitation occurs, the total water content of the parcel is preserved.
Therefore, the liquid water content at the freezing level is given by
r t
= 0.5 gkg -1.
Approximately 10% of the parcel's moisture has condensed by this altitude.
TEMPERATURE INSIDE CLOUD AT 650 MB
At 650 mb, the parcel has evolved along the 0e = 315 K saturated adiabat
to state 4, where its temperature is
T650 ----
_6~
Were the parcel perfectly dry, it would have continued to evolve above 770 mb
along the 315 K adiabat. Without the release of latent heat to offset adiabatic
cooling, the parcel's temperature would then have decreased more rapidly,
resulting in a temperature at 650 mb of -12~
MIXING RATIO INSIDE CLOUD AT
650
MB
Because the parcel remains saturated, the isopleth of saturation mixing
ratio passing through state 4 gives for the mixing ratio at 650 mb
r650 - 4.0 g kg- 1.
By this level, one-third of the parcel's moisture has transformed into conden-
sate.
The foregoing example illustrates the strong constraint on water vapor im-
posed by thermodynamics and hydrostatic stratification. Inside convective tow-
ers, which transport moisture upward from its source at the earth's surface, the
abundance of vapor that can be supported decreases with altitude (Fig. 5.3).
By 500 mb, less than 30% of the surface mixing ratio of water inside the
parcel described above remains as vapor. According to (1.24), the absolute
humidity p~ decreases even faster. Thus, less than 15% of the absolute con-
centration of water vapor remains after the parcel has been displaced to the
middle troposphere. It is for this reason that deep convection increases the
column abundance of water vapor in Fig. 1.16 only modestly. Much of s
resides in the lowest 1 to 2 km, where water vapor is distributed more uni-
formly.
Through this mechanism, water vapor transported vertically inside convec-
tive towers is systematically extracted. Condensation leads to a decrease with

138 5
Transformations of Moist Air
altitude of water vapor mixing ratio and, through precipitation, a similar de-
crease of total water content. Outside convective towers, air is even drier
because it has not recently been in contact with the reservoir of moisture at
the Earth's surface or it has been dehydrated inside convective towers in the
aforementioned manner. The same mechanism operates on large scales inside
sloping convection, like that involved in synoptic weather systems. By limit-
ing the vertical transport of water vapor, thermodynamics in combination with
hydrostatic stratification confines moisture to a shallow neighborhood of the
earth's surface and has preserved water on the planet.
Suggested Reading
A complete discussion of the Gibbs-Dalton law, along with its implications
for multi-component systems, is given in Thermodynamics (1970) by Keenan.
Atmospheric Thermodynamics (1981) by Iribarne and Godson provides a de-
tailed treatment of moisture-dependent air properties, including those under
saturated conditions, and a complete survey of thermodynamic charts.
The Principles of Chemical Equilibrium (1971) by Denbigh discusses the ther-
modynamics of condensed phases.
Problems
The pseudo-adiabatic chart in Appendix F is to be used only for those prob-
lems in which it is explicitly indicated.
5.1. A downslope wind in North America is called a chinook, which is an
Indian term meaning "snow eater." A chinook often occurs with the
mountains blanketed in clouds but with clear skies leeward. Consider
the following synoptic situation: Moist air originating in the eastern Pa-
cific is advected over the western United States where it is forced over
the continental divide. On the windward side, the surface lies at 800 mb,
where the temperature and mixing ratio are 20~ and 15 g kg -1, respec-
tively. If the summit lies at 600 mb and if any condensate that forms
precipitates out, determine (a) the surface air temperature at Denver,
which lies at 830 mb, (b) the mixing ratio at Denver, and (c) the relative
humidity at Denver.
5.2. A refrigerator having an interior volume of 2.0 m 3 is sealed and switched
on. If the air initially has a temperature of 30~ and a relative humidity
of 0.50, determine (a) the temperature at which condensation forms
on the walls, (b) how much moisture will have condensed when the
temperature reaches 2~ and (c) how much heat must be rejected to
the surroundings to achieve the final state in part (b).
5.3. A cold front described by the surface
z = h(x - ct)

Problems
139
5.4.
5.5.
5.6.
5.7.
5.8.
5.9.
5.10.
5.11.
moves eastward with velocity c and undercuts warmer air ahead of it.
Far ahead of the front, undisturbed air is characterized by the temper-
ature and mixing ratio profiles
T~(z)
and
ro~(z),
respectively. If air is
lifted in unison over the frontal surface, for a given location x, derive an
expression for (a) the variation of temperature with altitude above the
frontal surface but beneath the LCL as a function of time, (b) the vari-
ation of mixing ratio with altitude above the frontal surface but beneath
the LCL as a function of time, and (c) an expression for the altitude of
the LCL if, far from the frontal zone, pressure decreases with altitude
approximately as e -z/n, with H -- const.
The development in Sec. 5.1 for a mixture of dry air and water con-
siders the presence of only one condensed phase. Why and under what
conditions is this permissible?
State the number of thermodynamic degrees of freedom for (a) moist
unsaturated air and (b) moist saturated air. (c) Explain the numbers in
parts (a) and (b) and why they differ.
Compare 0 and 0~ for saturated conditions at 1000 mb and a temperature
of (a) 10~ (b) 20~ and (c) 30~
Warm moist air leaves an array of cooling towers at a power plant situ-
ated at 825 mb. A cloud forms directly overhead. If the ambient temper-
ature profile is isothermal, with T = 5~ and if the initial temperature
and mixing ratio of air leaving the towers are 30~ and 25 g kg -1, respec-
tively, determine (a) the relative humidity immediately above the towers,
(b) the dew point immediately above the towers, (c) the virtual poten-
tial temperature immediately above the towers; compare this value to
the potential temperature and discuss it in relation to typical differences
between 0 and 0~, (d) the pressure at the cloud base, (e) the equivalent
potential temperature at 800 mb, (f) the mixing ratio at 700 mb, and
(g) the pressure at cloud top. A pseudo-adiabatic chart is provided in
Appendix E
Room temperature on a given day is 22~ while the outside temperature
at 1000 mb is 2~ Calculate the maximum relative humidity that can
be accommodated inside without room windows fogging, if the windows
can be treated as having a uniform temperature.
(a) Use the same conditions as in Problem 5.8, but for an aircraft cabin
that is pressurized to 800 mb. (b) As in part (a), but noting that the
interior pane is thermally isolated from the exterior pane.
Demonstrate that the pressure dependence in (5.15) is negligible.
Evaporation is an efficient means of cooling air and, through contact
with other media, of rejecting heat to the environment. An evaporative
cooler processes ambient air at 35~ and 900 mb to produce room air
