Potter T.D., Colman B.R. (co-chief editors). The handbook of weather, climate, and water: dynamics, climate physical meteorology, weather systems, and measurements
Подождите немного. Документ загружается.

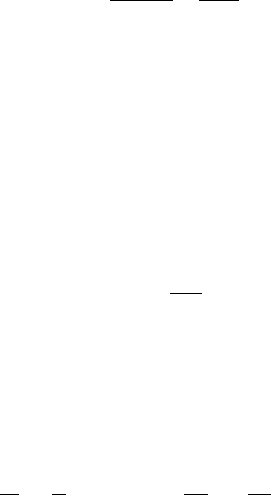
Equilibrium Between Ice and Vapor
Similarly, for equilibrium between ice and vapor, we can write
d ln e
si
dT
¼
l
iv
R
v
T
2
; ð19Þ
where we again take l
il
to be the absolute value of the difference in enthalpy between
the vapor and solid phase of water.
Computation of Saturation Vapor Pressure (e
s
and e
si
)
For a preci se integration of the Clausius–Clapeyron equation, one must consider the
latent heat variation with temperature. To a good first approximation, let temperature
be independent of latent heat and then integrate, yielding
ln e
s
¼
l
lv
R
v
T
þ const: ð20Þ
This formulation has assumed latent heat to be independent of temperature. This is a
good approximation for sublimation, but not as good for freezing or condensation.
We can improve on this by using a more precise formulation for latent heat variation
and specific heat variation with temperature with a series expansion:
ln e
s
¼
1
R
v
l
0
T
þ Da ln T þ
Db
2
T þ
Dg
6
T
2
þ
þ const : ð21Þ
where the integration constant is determined empirically.
We need not achieve this much accuracy for most meteorological considerations,
since we cannot measure vapor pressure that precisely anyway. Hence we can make
approximations that allow us to calculate e
s
or e
si
with sufficient accuracy. Here are
three approximations:
1. Solving the constant at the triple point temperat ure ðT
t
Þ, and assuming latent
heat constant, we obtain
e
s
¼ 10
ð9:40512354=T Þ
e
si
¼ 10
ð10:55532667=T Þ
2. Assuming heat capacity constant and retaining two terms in a series, we get
Magnus’s formula (liquid–vapor only):
e
s
¼ 10
ðð2937:4=T Þ4 :9283 log T þ23:5518Þ
ð22Þ
216 MOIST THERMODYNAMICS
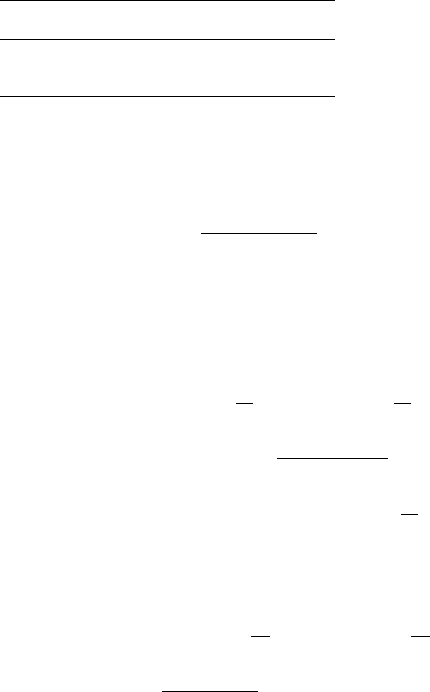
This has been simplified by Tetens (1930) and later by Murray (1966) to be
easily computed from the relationship (for pressure in millibars):
e
s
¼ 6:1078 exp
aðT 273:16Þ
T b
ð23Þ
where the constants are defined in Table 3.
3. Many atmospheric scientists use the Goff–Gratch (1946) formulation given
by:
e
s
¼ 7:95357242x10
10
exp 18:1972839
T
s
T
þ 5:02808 ln
T
s
T
70242:1852 exp
26:1205253
T
s
=T
þ 58:0691913 exp 8:039 45282
T
s
T
ð24Þ
for saturation over liquid where T
s
¼ 373:16 K. For saturation over ice:
e
s
¼ 5:57185606x10
10
exp 20:947031
T
0
T
3:56654 ln
T
0
T
2:01889049
T
0
=T
ð25Þ
where T
0
¼ 273:16 K.
Tables 4 and 5 show how the accuracy of Teten’s formula compares to Goff–
Gratch as reported by Murray (1966). Both of these forms of saturation vapor
pressure are commonly used as a basis to compute saturation vapor pressure.
5 GENERAL THEORY FOR MIXED-PHASE PROCESSES WITHIN
OPEN SYSTEMS
Often thermodynamic theory is applied to precipitating cloud systems containing
liquid and ice that often are not in equilibrium. In many thermodynamic texts,
the simplifying assumption of equilibrium is made that can invalidate the result.
TABLE 3 Constants for Teten’s Formula
Water Ice
a 17.2693882 21.8745584
b 35.86 7.66
5 GENERAL THEORY FOR MIXED-PHASE PROCESSES WITHIN OPEN SYSTEMS 217
Moreover, ice processes are often neglected, despite the existence of ice processes
within the vast majority of precipitating clouds. We will adopt the generalized
approach to the moist thermodynamics problem, developed by Dutton (1973) and
solve for the governing equations for a nonequilibrium three-phase system first and
then find the equilibrium solution as a special case.
TABLE 4 Saturation Vapor Pressure over Liquid
t (
C) Goff–Gratch (mb) Tetens (mb) Difference (%)
50 0.06356 0.06078 1:6 10
2
45 0.1111 0.1074 1:6 10
2
40 0.1891 0.1842 1:6 10
2
35 0.3139 0.3078 1:7 10
2
30 0.5088 0.5018 2:1 10
2
25 0.8070 0.7993 4:5 10
2
20 1.2540 1.2462 2:8 10
2
15 1.9118 1.9046 5:8 10
2
10 2.8627 2.8571 1:9 10
3
5 4.2149 4.2117 5:2 10
4
0 6.1078 6.1078 1:8 10
7
5 8.7192 8.7227 1:9 10
4
10 12.272 12.2789 2:2 10
4
15 17.044 17.0523 1:8 10
4
20 23.373 23.3809 1:1 10
4
25 31.671 31.6749 3:7 10
5
30 42.430 42.426 2:5 10
5
35 56.237 56.221 7:1 10
5
40 73.777 73.747 9:5 10
5
45 95.855 95.812 9:7 10
5
50 123.40 123.35 7:5 10
5
TABLE 5 Saturation Vapor Pressure over Ice
t (
C) Goff–Gratch (mb) Tetens (mb) Difference (%)
50 0.03935 0.03817 9:4 10
3
45 0.07198 0.07032 8:8 10
3
40 0.1283 0.1261 8:5 10
3
35 0.2233 0.2205 8:5 10
3
30 0.3798 0.3764 9:2 10
3
25 0.6323 0.6286 1:3 10
2
20 1.032 1.028 1:2 10
1
15 1.652 1.648 4:1 10
3
10 2.597 2.595 9:9 10
4
5 4.015 4.014 1:7 10
4
0 6.107 6.108 6:3 10
5
218 MOIST THERMODYNAMICS
Besides the nonequilibrium phase changes, the formation of liquid and ice hydro-
meteors create a heterogeneous system having components of gas, solids, and
liquids, each of which may be moving at different velocities. We must therefore
forego our traditional assumption of an adiabatic system and instead consider an
open system. In doing so, we fix our coordinate system relative to the center of the
dry air parcel. Although we can assume that vapor will remain stationary relative to
the dry air parcel, we must consider movements of the liquid and solid components
of the system relative to the parcel, hence implying diabatic effects. We therefore
generalize the derivation of Eq. (4) to include these effects. It will be convenient to
work in the system of specific energies (energy per mass).
We will assume that our system is composed of several constituents, each of mass
m
j
. Then the total mass is
m ¼
P
j
m
j
: ð26Þ
We account for both internal changes ðd
i
Þ, which result from exchanges between
phases internal to the parcel, and external changes ðd
e
Þ, which result from fluxes of
mass into and out of the parcel. The total change in mass is thus
dm
j
¼ d
i
m
j
þ d
e
m
j
: ð27Þ
By definition and for mass conservation, we require
d
i
m ¼
P
j
d
i
m
j
¼ 0; and
d
e
m ¼
P
j
d
e
m
j
¼ d
e
m
l
þ d
e
m
i
:
As discussed above, external mass changes are restricted to liquid or ice consti-
tuents, while gas constituents are assumed not to move relative to the system. An
exception can be made, although not considered here, for small-scale turbulent
fluxes relative to the parcel. From our original discussion of the first law, we can
write the generalized form of Eq. (4) in a relative mass form as:
dH ¼ dQ þ VdPþ
P
j
h
j
dm
j
ð28Þ
where h
j
¼ð@H
j
=@m
j
Þ. We found earlier that the terms involving h
j
eventually result
in the latent heat term.
We can split the heating function, dQ ¼ Q
i
þ Q
e
. Here Q
i
is the diabatic change
due to energy flowing into or out of the system that does not involve a mass flow. Q
e
accounts for diabatic heat fluxes resulting from mass fluxes into or out of the system.
The diabatic heat source of conduction between the parcel and an outside source
would be accounted for in Q
i
if no mass back and forth flow into and out of the
parcel is not explicitly represented. Even the case of turbulent heat transfer into and
out of the parcel would not affect Q
i
unless the explicit fluxes of mass in and out
were represented. The heat flux associated with the movement of precipitation
featuring a different temperature than the parcel, into and out of the parcel, would
also be represented by Q
e
; as it would appear as a different enthalpy for the preci-
5 GENERAL THEORY FOR MIXED-PHASE PROCESSES WITHIN OPEN SYSTEMS 219
pitation constituent, and be accounted for by the d
e
m
j
term. As is conventional, we
will assume Q
e
¼ 0. Now;
Q
i
¼ d
i
H Vdp
P
j
h
j
d
i
m
j
; and ð29Þ
Q
e
¼ d
e
H
P
j
ðh
j
hÞ d
e
m
j
: ð30Þ
We can similarly split the enthalpy tendency into its internal and external parts:
dH
i
¼ Q
i
þ Vdp
P
j
h
j
d
i
m
j
; and ð31Þ
dH
e
¼
P
j
ðh
j
hÞ d
e
m
j
: ð32Þ
Ignoring the noninteracting free energies, Gibbs’ relat ion for the mixed-phase
system is written in ‘‘per mass’’ form as:
TdS¼ dH Vdp
P
j
h
j
dm
j
P
j
m
j
dm
j
; ð33Þ
where m
j
is the chemical potential per mass rather than per mole as we first defined
it. We can now divide the entropy change between internal and external changes, and
employing Eq. (29)
Td
i
S ¼ Q
i
P
j
h
j
d
i
m
j
P
j
m
j
d
i
m
j
; and
Td
e
S ¼
P
j
m
j
d
e
m
j
;
ð34Þ
for the external change. Since, m
j
¼ g
j
¼ h
j
Ts
j
, and Eq. (30), then
Td
e
S ¼
P
j
h
j
d
e
m
j
P
j
Ts
j
d
e
m
j
: ð35Þ
Now we apply our results to the particular water–air system. Consider a mixture
composed of m
d
grams of dry air, m
v
grams of vapor, m
l
grams of liquid water, and
m
i
grams of ice. We require
d
i
m
d
¼ d
e
m
d
¼ 0;
d
i
m
v
þ d
i
m
l
þ d
i
m
i
¼ 0; and
d
e
m
v
¼ 0:
ð36Þ
220 MOIST THERMODYNAMICS

The internal enthalpy equation [Eq. (31) and internal entropy Eq. (36)] can then
be written for this mixed-phase system:
d
i
H ¼ Q
i
þ Vdpþ
P
j
h
j
d
i
m
j
; and ð37Þ
d
i
S ¼
Q
i
T
P
j
h
j
d
i
m
j
T
P
j
m
j
d
i
m
j
T
: ð38Þ
For the air–water system Eq. (38) becomes
d
i
S ¼
l
iv
d
i
m
v
þ
l
il
T
d
i
m
i
a
iv
T
d
i
m
v
þ
a
il
T
d
i
m
i
þ
Q
i
T
; ð39Þ
where the a
iv
and a
il
are the specific affinities of sublimation and melting. They are
defined as:
a
iv
m
l
m
i
;
a
il
m
l
m
i
:
The affinity terms take into account the entropy change resulting if the two inter-
acting phases are out of equilibrium. They would be expected to be nonzero, for
instance, if rain drops are evaporating in an environment where the air is subsatu-
rated. Under equilibrium conditions, they would vanish. An example is cloud drops
growing by condensation in an environment where the vapor pressure is equal to the
saturation vapor pressure over liquid. The affinity is the Gibbs free energy available
to drive a process and unavailable to perform work. Notice the symmetry between
the affinity declarations and the latent heat declarations.
If we apply Eq. (39) to adiabatic melting, condensation, and sublimation, we
obtain
Tðs
l
s
i
Þ¼l
il
þ a
il
;
Tðs
v
s
l
Þ¼l
lv
þ a
lv
; and
Tðs
v
s
i
Þ¼l
iv
þ a
iv
:
ð40Þ
We can state that the total entropy is equal to the sum of the entropy in each system
component:
S ¼ m
d
s
d
þ m
v
s
v
þ m
l
s
l
þ m
i
s
i
; ð41Þ
where the dry air entropy is
s
d
c
pd
ln T R
d
ln p
d
: ð42 Þ
5 GENERAL THEORY FOR MIXED-PHASE PROCESSES WITHIN OPEN SYSTEMS 221

We have required that the only external fluxes are those of precipitation. Hence,
we find
d
i
S ¼ dS d
e
S;
¼ dS s
l
d
e
m
l
s
i
d
e
m
i
;
¼ dðm
d
s
d
þ m
v
s
v
Þþs
i
d
i
m
l
þ s
i
d
i
m
i
þ m
l
ds
l
þ m
i
ds
i
¼
A
lv
T
d
i
m
v
A
il
T
d
i
m
i
þ
Q
i
T
:
ð43Þ
Combining Eqs. (36) and (40) with (4) we obtain:
dm
d
s
d
þ
m
v
l
lv
T
d
i
w
i
l
il
T
þ m
v
d
A
lv
T
m
i
d
A
il
T
þðm
v
þ m
l
þ m
i
Þ ds
l
¼
Q
i
T
: ð 44Þ
If we now assume that the liquid is approximately incompressible, then
ds
l
¼ c
l
ðdT=TÞ. Defining mixing ratio of the jth constituent as:
r
j
m
j
m
d
; ð45Þ
and divide Eq. (44) by m
d
. Then we obtain the following general relation:
dc
pd
ln T R
d
ln p
d
þ
r
v
l
lv
T
d
i
r
i
l
il
T
þ r
v
d
A
lv
T
r
i
d
A
il
T
þðr
v
þ r
l
þ r
i
Þc
l
dT
T
¼
q
i
T
: ð 46Þ
The differentials account for the interrelationship between temperature change, pres-
sure change, and liquid phase change. Additional diabatic heating tendencies by
radiative transfer, molecular diffusion, and eddy diffusion can be accounted for with
the internal heating term q
i
. Neglected are the effects of chemical reaction on
entropy, alth ough the heating effect can be included in the heating term. Generally,
these effects are small and can be neglected.
What is included are not only the latent heating effects of the equilibrium reaction
but the effects on heating resulting from the entropy change in nonequilibrium
reactions. Hence, since entropy change removes energy from that available for
work, nonequilibrium heating from phase change modifies the enthalpy change
resulting from phase change. The affinity terms ac count for these effects.
Notice that both affinity terms are inexact differentials, implying that their effect
is irreversible. The heat storage term [last term on the left-hand side (LHS)] is also
222 MOIST THERMODYNAMICS
an exact differential if we neglect the variation of c
l
and we assume the total water,
defined as:
r
T
¼ r
v
þ r
l
þ r
i
; ð47Þ
is a constant. If there is a change, i.e., a diabatic loss or gain of moisture by
precipitation, then the differential is inexact and the process is irreversible. This
term also contains all of the net effects of diabatic fluxes of moisture for systems
that are otherwise in equilibrium between phases. The heating term, q
i
, is purely
diabatic and defines a diabatic thermal forcing on the system such as by radiative
transfer or molecular diffusion and turbulence.
Note that, although the first term on the LHS is written as a total derivative, the
external derivative of the quantity in brackets is in fact zero. Hence all reversible
terms appear as internal derivatives while the reversible terms contain both internal
derivatives for moist adiabatic process and external derivatives for diabatic flux
terms.
6 ENTHALPY FORM OF THE FIRST–SECOND LAW
We now derive what is perhaps a more commonly used form of Eq. (46). Whereas
Eq. (46) is in a form representing entropy change, it can be rewritten as an enthalpy
change. To do so we make some manipulations.
First, we make the following assumptions:
1. Neglect the curvature effects of droplets.
2. Neglect solution effects of droplets.
These assumptions are really quite unimportant for the macrosystem of fluid parcels.
However, the assumptions would be critical when discussing the microsystem of the
droplet itself, since these effects strongly influence nucleation and the early growth
of very small droplets. With these assumptions, the chemical potential of the vapor is
defined:
m
l
¼ m
o
þ R
v
T ln e
v
; ð48Þ
where e
v
is the atmospheric partial pressure of the vapor. The chemical potential of
the liquid and ice are defined as the chemical potential of vapor, which would be in
equilibrium with a plane pure surface of the liquid or ice and are given by:
m
l
¼ m
o
þ R
v
T ln e
s
; ð49Þ
m
i
¼ m
o
þ R
v
T ln e
si
; ð50Þ
6 ENTHALPY FORM OF THE FIRST–SECOND LAW 223

where e
s
and e
si
are the saturation vapor pressures of the ice and liqu id defined by the
temperature of the liquid or ice particle. This temperature need not be the same as
the vapor temperature T for this equation.
Combining the equation of state applied to vapor and to dry air, it can be shown
that:
d ln pðR
d
þ r
v
R
v
Þ¼R
d
d ln p
d
þ r
v
R
v
d ln e
v
: ð51Þ
Combining Kirchoff’s equation (5), with Eqs. (46) to (51) and (18) and (19),
c
pm
d ln T R
m
d ln p þ
l
lv
T
dr
v
l
il
T
d
i
r
i
¼
q
i
T
ð52Þ
where c
pm
¼ c
p
þ r
v
c
vp
þ r
i
c
i
þ r
l
c
l
is the effective heat capacity of moist air and
R
m
¼ R
d
þ r
v
R
v
is the moist gas constant (not to be confused with the gas constant
of moist air).
Although Eq. (52) appears different from Eq. (46), it contains no additional
approximations other than the neglect of the curvature and solution effects implicit
in the assumed form of che mical potential. It is simpler and easier to solve than the
other form because the affinity terms and latent heat storage terms are gone. Note,
however, that there are some subtle inconveniences. In particular, each term is an
inexact differential that means that they will not vanish for a cyclic process. This
makes it more difficult to integrate Eq. (52) analytically. Nevertheless, it is a conve-
nient form for applications such as a numerical integration of the temperat ure change
during a thermodyn amic process.
Some of the effects of precipitation falling into or out of the system are included
in Eq. (52) implicitly. To see this look at the change in vapor. It is a total derivative
because only internal changes are allowed. The ice change, on the other hand, is
strictly written as an internal change. Hence it is the internal change that implies a
phase change, and knowing the ice phase change and liquid phase change, the liquid
phase change is implicitly determined since the total of all internal phase changes are
zero. Since, by vir tue of the assumption that a heterogenous system is composed of
multiple homogenous systems, we assumed that hydrometeors falling into or out of
the system all have the same enthalpy as those in the system itself, there is no
explicit effect on temperat ure.
This assumption can have important implications. For instance, frontal fog forms
when warm rain droplets fall into a cold parcel, hence providing an external flux of
heat and moisture through the diabatic movement of the rain droplet relative to the
parcel. We neglect this effect implicitly with eq. (52). By requiring
d
e
H ¼
P
j
d
e
ðm
j
h
j
Þ¼
P
j
h
j
d
e
m
j
, we only considered the external changes due to
an external flux of water with the same enthalpy of the parcel. The neglect of these
effects is consistent with the pseudo-adiabatic assumption that is often made. That
assumption assumes condensed water immediately disappears from the system, and
so the heat storage effects within the system and for parcels falling into or out of the
system can be neglected. So far, the pseudo-adiabatic assumption has only been
224 MOIST THERMODYNAMICS

partially made because we still retain the heat storage terms of the liquid and ice
phases within the syst em. It is unclear whether there is an advantage to retaining
them only par tially.
7 HUMIDITY VARIAB LES
Water vapor, unlike the ‘‘dry’’ gases in the atmosphere exists in varying percent ages
of the total air mass. Obviously, defining the amount of vapor is critical to under-
standing the thermodynamics of the water–air system. We have developed a number
of variables to define the vapor, liquid, and ice contents of the atmosphere. Below is
a list of the variables used to define water content.
Vapor Pressure (e
v
)
Vapor pressure represents the partial pressure of the vapor and is measured in
pascals. The saturation vapor pressure over a plane surface of pure liquid water is
defined to be e
s
while the vapor pressure exerted by a plane surface of pure ice is e
si
.
Mixing Ratio and Specifi c Humidity
We have already introduced mixing ratio to be r
v
¼ r
v
=r
d
, and employing the
equation of state, we can relate mixing ratio to vapor pressure:
r
v
¼
Ee
v
p e
v
ð53Þ
where E M
d
=M
v
. We define specific humidity to be the ratio of q
v
¼ r
v
=r.It
follows that
q
v
¼
r
v
1 þ r
v
Then, similar to Eq. (53) we can write for specific humidity:
q
v
¼
Ee
v
p
1
ð1 þðr
v
=EÞÞ
ð54Þ
To a reasonable approximation, one can show that e
v
p and hence:
q
v
r
v
E
e
v
p
ð55Þ
7 HUMIDITY VARIABLES 225
