Potter T.D., Colman B.R. (co-chief editors). The handbook of weather, climate, and water: dynamics, climate physical meteorology, weather systems, and measurements
Подождите немного. Документ загружается.


If we divide through by the total mass of the gas (m
j
) and note that n
j
¼ m
j
=M
j
,
where M
j
is the molecular weight of the species i, and total mass is
m ¼
P
m
j
ð13Þ
The statement of the ideal gas law (equation of state) for a mixture of gases is then
p ¼ r
R*
MM
T ¼ r
RRT ð14Þ
where
MM is the mean molecular weight of the mixture, which is given by:
MM
P
m
j
P
m
j
M
j
¼
P
M
j
N
j
ð15Þ
where N
j
is the molar fraction of a particular ga s given by N
j
¼ p
j
=p ¼ V
j
=V.
Composition of Air The current atmosphere of Earth is composed of ‘‘air,’’
which we find to contain:
1. Dry air, which is a mixture of gases described below
2. Water, which can be in any of the three states of liquid, solid, or vapor
3. Aerosols, which are solid or liquid particles of small sizes
The chemical composition of dry air is given in Table 2.
Water vapor and the liquid and solid forms of water vary in its volume fraction
from 0% in the upper atmosphere to as high as 3 to 4% near the surface under humid
conditions. Because of this variation, dry air is treated separately from vapor in
thermodynamic theory.
Among the trace constituents are carbon dioxide, ozone, chlorofluorocarbons
(CFCs), and methane, which, despite their small amounts, have a very large
impact on the atmosphere because of their interaction with terrestrial radiation
passing through the atmosphere, or in the case of CFCs because of their impact
on the ozone formation process.
Note from Table 2 that the molecular weighted average gas constant for dry air is
R
d
¼
RR ¼ 287:05 J=kg=K, which we also call the dry air gas constant.
Work by Expansion
If a system is not in mechanical equilibrium with its surroundings, it will expand or
contract. Assume that l is the surface to the system that expands infinitesimally to l
0
186 PHYSICAL ATMOSPHERIC SCIENCE
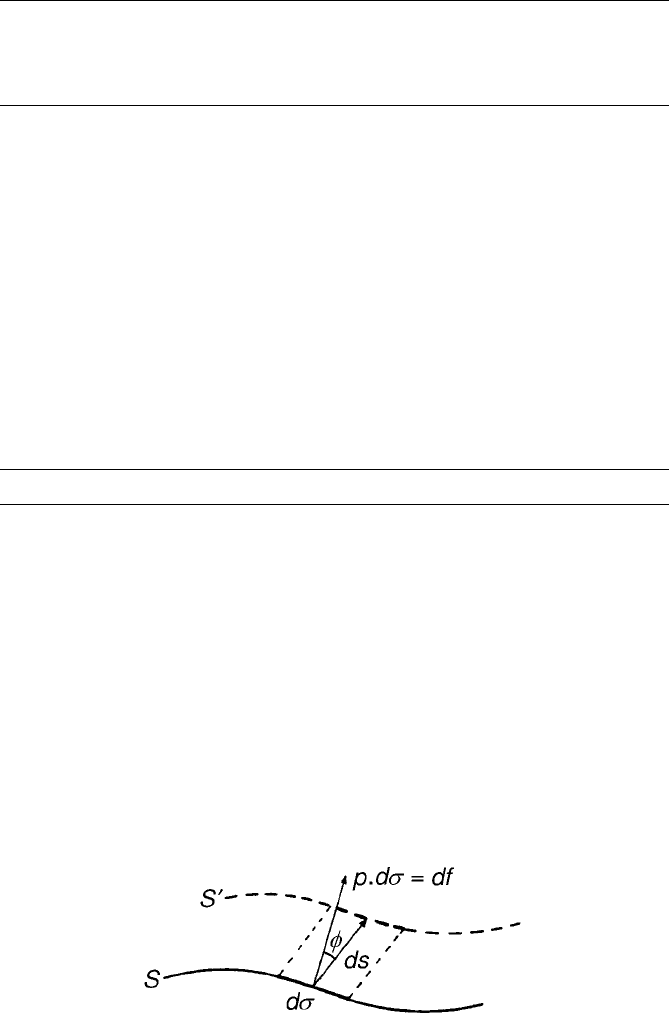
in the direction ds (see Fig. 2). Then the surface element ds has performed work
against the external pressure P. The work perfor med is
ðdW Þ
ds
¼ Pds dl cos f ¼ P
surr
dV ð16Þ
where dV is the change in volume.
For a finite expansion, then
W ¼
ð
f
i
pdV ð17Þ
TABLE 2 Composition of Dry Air near the Earth’s Surface
Gas Symbol
Molecular
Weight
Molar
(or Volume)
Fraction
Mass
Fraction
Specific
Gas
Constant
(J=kg K)
m
j
R
j
=m ð
RRÞ
(J=kg K)
Nitrogen N
2
28.013 0.7809 0.7552 296.80 224.15
Oxygen O
2
31.999 0.2095 0.2315 259.83 60.15
Argon Ar 39.948 0.0093 0.0128 208.13 2.66
Carbon Dioxide CO
2
44.010 0.0003 0.0005 188.92 0.09
Helium He 0.0005
Methane CH
4
0.00017
Hydrogen H 0.00006
Nitrous Oxide N
2
O 0.00003
Carbon Monoxide CO 0.00002
Neon Ne 0.000018
Xenon X 0.000009
Ozone O
3
0.000004
Krypton Kr 0.000001
Sulfur Dioxide SO
2
0.000001
Nitrogen Dioxide NO
2
0.000001
Chlorofluorocarbons CFC 0.00000001
Total 1.0000 1.0000
RR ¼ 287.05
Figure 2 Work of expansion (from Iribarne and Godson, 1973).
2 ATMOSPHERIC THERMODYNAMICS 187
where i, f stand for the initial and final states. Since the integrand is not a total
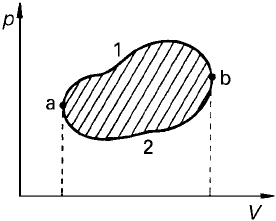
derivative, total work over a cyclic process can be nonzero. Therefore
W ¼
þ
pdV ¼
ð
b
a
PdV
1
ð
b
a
PdV
2
ð18Þ
which is defined to be positive if integrated in the clockwise sense. This is the area
enclosed by the trajectory in the graph given in Figure 3.
The work by expansion is the only kind of work that we shall consider in our
atmospheric systems. Assuming the pressure of the system is homogeneous and in
equilibrium with the surroundings, we can adopt the notation
dW ¼ PdV ð19Þ
where W is the work perfor med on the surroundings by the system.
First Law (or Principle) of Thermodynamics
The first law of thermodynamics can be simply stated: The energy of the universe is
constant. Let us consider the general concept of just what is meant by energy. We
know that if we apply force to an object over some distance and as a result we
accelerate the object, we will have performed work on the object, measured in the
energy units of force distance ¼ joules. That work will precisely equal the
increase in kinetic energy of the object, the total of which is measured by half its
mass multiplied by the square of its new velocity. Moreover, we understand that our
effort expends energy from the working substance. Depending on the method used
to accelerate the object, the energy was previously stor ed in some other form;
perhaps the ‘‘potentia l energy’’ of a compressed spring, ‘‘chemical energy’’ stored
in the muscle cells of one’s arm, or perhaps the ‘‘kinetic energy’’ of another object
that strikes the object that was accelerated. There are obviously many possibilities
for working substances featuring different types of stored energy. Energy, in a
general sense, quantifies a potential to apply a force and so perform work.
Figure 3 Work of expansion in a cycle (from Iribarne and Godson, 1973).
188
PHYSICAL ATMOSPHERIC SCIENCE
The first law requires that for any thermodynamic system an energy budget must
exist that requires any net energy flowing into a system to be accounted for by
changes in the external energy of the system or the internal energy stored within
the system. The external energy of the system includes its kinetic energy of motion
and its energy of position, relative to forces outside the system such as gravitational,
electrical, magnetic, chemical, and many other forms of potential energy between the
system and its surroundings. The same energies can also exist within the system
between the molecules, atoms, and subatomic particles composing the system and
are termed internal energies. Kinetic energy of molecular motions relative to the
movement of the center of mass of the system is measured by the system’s tempera-
ture and represen t the thermal internal energy. The other internal energies are
potential internal energies and may include a potential energy against the inter-
molecular forces of attraction (latent heat), chemical potential energy (e.g., a gas
composed of oxygen and hydrogen can potentially react), and so on.
Because we probably are not even aware of all of the forms of internal forces that
exist in a system, we make no attempt to evaluate the total internal energy. Instead,
for the purposes of classical thermodynamics, we need only consider changes in
internal energy occurring during allowable processes. For instance, for atmospheric
studies we choose to ignore nuclear reactions and even most chemical reactions. But
we cannot neglect the changes in internal potential energy due to intermolecular
forces of liquid and ice water phases in the atmosphere. The intermolecular forces
are substantial, and the energy needed to overcome them is the latent heat of
vaporization and melting. Thermodynamic energy transfers between thermal and
these internal potential energies drive the general circulation of Earth’s atmosphere!
Our limited thermodynamic discussion will ignore the treatment of some internal
energies such as surface energy of droplets, chemical energy in photochemical
processes, electrical energy in thunderstorms and the upper atmosphere, chemical
processes involving CFCs, an d other processes known to have important secondary
impacts on the evolution of the atmospheric thermodynamic system. These
processes can be added to the system when needed, following the methods described
in this chapter.
In most texts of classical thermodynamics, closed thermodynamic systems are
assumed. A closed system allows no mass exchange between the parcel (system) and
the surroundings. This approximation greatly simplifies the thermodynamic formu-
lation. Later, we can still account for molecular transfer by representing it as a
noninteracting and externally specified source of mass or energy rather than as an
explicitly interacting component of the formulation.
One such open mass flux that must be accounted for with an open system is the
mass flux of precipitation. We affix our parcel coordinate relative to the center of
mass of the dry air, which is assumed to move identically to vapor. The liquid and ice
components of the system, however, may attain a terminal velocity that allows it to
flow into and out of the parcel diabatically. As a result, we will derive thermo-
dynamic relationships for an open system, or one where external fluxes of mass
into and out of the system are allowed. To retain some simplicity, however, we will
make the assumption that external mass fluxes into and out of the system will be of
2 ATMOSPHERIC THERMODYNAMICS 189
constituents having the same state as those internal to the system. Henc e we will
allow rain to fall into our parcel, but it will be assum ed to have the same temperature
as the system. Although the effect of this assumption can be scaled to be small, there
are instances where it can be important. One such instance is the case of frontal fog,
where warm droplets falling into a cool air mass result in the formation of fog. These
moist processes will be described in detail in the next chapter.
As demanded by the first law, we form an energy budget, first for a simple one-
component system of an ideal gas:
Energy exchanged with surrounding s ¼ change in energy stored
Q W ¼ DU
P
k
DA
k
ð20Þ
where Q repres ents the flow of thermal energy into the system including radiative
energy and energy transferred by conduction, W is the work performed on the
surroundings by the system, U is the thermal internal energy, and A
k
is the kth
component of potential internal energy, summed over all of the many possible
sources of internal energy. The work, as described in the previous section, is the
work of expansion performed by the parcel on the surroundings. It applies only to
our gas system and does not exist in the same form for our liquid and ice systems. In
those systems, it should be reflected as a gravitational potential term; however, it is
typically neglected.
In general, we treat the air parcel as a closed system, whereby there are no mass
exchanges with the surroundings. This is generally a good approximation as mole-
cular transfers across the boundaries can usually be neglected or included in other
ways. One exception occurs and that is when we are considering liquid and ice
hydrometeors. If we fix our parcel to the center of mass of the dry air, then there
can be a substantial movement of liquid or ice mass into and out of the parcel.
Clearly this process must be considered with an open thermodynamic system, where
at least fluxes of liquid and ice are allowed with respect to the center of mass of the
dry air parcel. Hence we will consider the possibility that A
k
may change in part
because of exchanges of mass between the parcel and the environment, particularly
present with falling precipitation. Combining Eq. (20) with (19) for an infinitesimal
process, we obtain the form of the first law:
dQ ¼ dU þ PdV
P
k
dA
k
ð21Þ
where it should be noted that P is the press ure of the surroundings, as PdVdefines
work performed on the surroundings and dV is the change in volume of the
surroundings. Note that for the adiabatic case, dQ ¼ 0 by definition.
Because we require conservation of energy, it is evident that the change in both
internal thermal energy (dU) and internal potential energy (dA
k
) must be exact
differentials, only dependent on the initial and final states of the process and not
the path that the process takes. Hence U and A
k
must also be state variables.
190 PHYSICAL ATMOSPHERIC SCIENCE

Heat Capacities Heat capacity is a property of a substance and is defined to be
the rate at which the substance absorbs (loses) thermal energy (Q) compared to the
rate at which its temperature (T ) rises (falls) as a result. It is a property usually
defined in units of dQ=dT (energy per temperature change) or in units of dq=dT
(energy per mass per temperature change) in which case it is called specific heat
capacity. If heat is passed into a system, it may be used to either incre ase the internal
thermal or potential energy of the system or can be used to perform work. Hence
there are an infinite number of possible values for heat capacity depending on the
processes allowable. It is useful to determine the heat capacity for special cases
where the allowable processes are restricted to only one. Hence we neglect the
storage of potential internal energy and restrict the system to constant volume or
constant pressure processes. Hence,
C
v
¼
dQ
dT
V
c
v
¼
dq
dT
a
C
p
¼
dQ
dT
p
c
p
¼
dq
dT
p
ð22Þ
where C
v
and C
p
are the heat capacities at constant volume and pressure, respec-
tively, and where c
v
and c
p
are the specific heat capacities at constant volume and
pressure, respectively. It can be expected that C
p
> C
v
, since in the case of constant
pressure, the parcel can use a portion of the energy to expand, and hence perform
work on the surroundings, reducing the rise in temperature for a given addition of
heat.
Since the first law requires that the change in internal energy, U, be an exact
differential, U must also be a state variable; i.e., its value is not dependent upon path.
Hence U ¼ uðP; a; T Þ. If we accept that air can be treated as an ideal gas, then the
equation of state eliminates one of the variables, since the third becomes a function
of the other two. Employing Euler’s r ule,
dU ¼
@U
@V
T
dV þ
@U
@T
V
dT ð23Þ
Substituting Eq. (23) into the first law [Eq. (20)], we get
dQ ¼
@U
@T
V
dT þ
@U
@V
þ P
T
dV
P
k
dA
k
: ð24Þ
For a constant volume process,
C
v
¼
dQ
dT
V
¼
@U
@T
V
ð25Þ
2 ATMOSPHERIC THERMODYNAMICS 191

Hence, retur ning to Eq. (23),
dU ¼ C
v
dT þ
@U
@V
T
dV : ð26Þ
It has been shown experimentally that ð@U=@V Þ
T
¼ 0, indicating that air behaves
much as an ideal gas. Hence the internal energy of a gas is a function of temperature
only provided that C
v
is a function of temperature only. If we assume that possibil ity,
@C
v
=@V ¼ 0. Experiments show that for an ideal gas the variation of C
v
with
temperature is small, and since we hold air to be approximately ideal, it follows
that C
v
is a constant for air. The first law [Eq. (24)] is therefore written as:
dq ¼ C
v
dT þ PdV
P
k
dA
k
: ð27Þ
One can also obtain an alte rnate form of the first law by replacing dV with the ideal
gas law and then combining q
ith
Eq. (27) to yield
dQ ¼ðC
v
þ R*Þ dT VdP
P
k
dA
k
: ð28Þ
For an isobaric process, ðdP ¼ 0 Þ, one finds
C
p
¼
dq
@T
p
¼ C
v
þ R* ð29Þ
or
C
p
C
v
¼ R*: ð30Þ
Similarly for dry air, the specific heat capacities are
c
pd
c
vd
¼ R
d
ð31Þ
Equation (30) demonstrates that C
p
> C
v
. Statistical mechanics show that specific
relationships between specific heats at constant volume and at constant pressure can
be found for a gas depending on how many atoms form the gas molecule. Table 3
TABLE 3 Relationship between Specific Heats and
Molecular Structure of Gas
Monotonic gas C
v
¼
3
2
RC
p
¼ C
v
þ R ¼
5
2
R*
Diatomic gas C
v
¼
5
2
RC
p
¼
7
2
R
192
PHYSICAL ATMOSPHERIC SCIENCE

shows these relationships for diatomic and monoatomic gases. Since dry air,
composed mostly of molecular oxygen and nitrogen is nearly a diatomic gas,
c
pd
¼ 1004 J=kg=K; c
vd
¼ 717 J=kg=K: ð32Þ
Substituting Eq. (30) into Eq. (28) we obtain
dQ ¼ C
p
dT VdP
P
k
dA
k
ð33Þ
This form of the first law is especially useful because changes of pressure and
temperature are most commonly measured in atmospheric science applications.
Note that C
p
dT is not purely a change in energy, nor is Vdppurely the work.
Enthalpy For convenience, we introduce a new state variable called enthalpy,
defined as:
H U þ PV : ð34Þ
Differentiating across we obtain
dH ¼ dU þ pdVþ VdP; ð35Þ
and substituting Eq. (21), to eliminate dU:
dQ ¼ dH VdP
P
k
dA
k
: ð36Þ
Using Euler’s rule with Eq. (33), we obtain
@H
@T
p
¼ C
p
: ð37Þ
Enthalpy differs only slightly from energy but arises as the preferred energy variable
when the work term is expressed with a pressure change rather than a volume change.
The enthalpy variable exists purely for convenience (since pressure is more easily
measured than volume) and is not demanded or defined by any thermodynamic law.
Reversible Adiabatic Processes in the Atmosphere: Definition of
Potential Temperature (
u
) These types of processes are of great importance
to the atmospheric scientist. Ignoring radiative effects, or heating effects of
condensation, the dry adiabatic process represents the thermal tendencies that air
will experience as it rises and the pressure lowers or as it sinks and the pressure rises.
In the absence of condensation, this represents the bulk of the temperature change a
parcel will experience if its rise rate is fast enough to ignore radiative effects. We
defined an adiabatic process as one for which Q ¼ 0. Then for an adiabatic process,
2 ATMOSPHERIC THERMODYNAMICS 193

combining Eq. (33), the equation of state [Eq. (7)], and then integrating we obtain
T ¼ kP
k
ð38Þ
where k ¼ R*=C
p
¼ 0:29 and k is a constant of integration that can be determined
from a solution point. Equation (38) is known to atmospheric scientists as the
Poisson equation, not to be confused with the second-order partial differential
equation also known as ‘‘Poisson’s equation.’’ The Poisson equation states that,
given a relationship between temperature and pressure at some reference state, the
value of temperature can be calculated for all other pressures the system might
obtain through reversible dry adiabatic processes.
It is often convenient to define potential temperature (y) to be the temperature
that a parcel would have at 1000 kPa press ure. It follows directly from Eq. (38) that
y T
p
00
P
R=c
p
; ð39Þ
where p
00
¼ 1000 kPa is the reference pressure at which potential temperature (y)
equals temperature (T). The potential temperature proves extremely useful to meteor-
ologists. For all reversible dr y adiabatic atmospheric thermodynamic processes, y
remains constant and represents a conserved property of the flow. Later we will show
y also represents the dry entropy of the flow and that it is one in a hierarchy of
entropy variables that are conserved in flow under various permitted dry and moist
processes.
Second Law (or Principle) of Thermodynamics
The zeroth principle of thermodynamics defined temperature to be a quantity that is
determined from two bodies in thermal equilibrium. The first law stated the principle
of energy conservation during any thermodynamic process. The second law deals
with the direction of energy transfer during a thermodynamic process occurring
when two bodies are not in thermodynamic equilibrium. It is again an empirical
statement, not derived in any way from first principles but rather from observations
of our perceived universe. The second law can be stated in two ways that can be
shown to be equivalent:
1. Thermal energy will not spontaneously flow from a colder to a warmer object.
2. A ther modynamic proces s always acts down gradient in the universe to reduce
differentiation overall and hence mix things up and reduce order overall. If we
define entropy to be a measure of the degree of disorder, then the second law
states: The entropy of the universe increases or remains the same as a result of
any process. Hence, the entropy of the universe is always increasing.
Just as the first law demanded that we define the thermal variable temperature, the
second law requires a new thermodynamic variable entropy. To form a mathematical
194 PHYSICAL ATMOSPHERIC SCIENCE
treatment for entropy, we consider several special thermodynamic processes that
reveal the nature of entropy behavior and provide guidance to its form.
Mathematical Statement of the Second Law It is illustrative to examine the
amount of work performed by a parcel on its sur roundings under isothermal
conditions, implying that the internal energy of the system is held constant. This
will demonstrate an interesting behavior addressed by the second law.
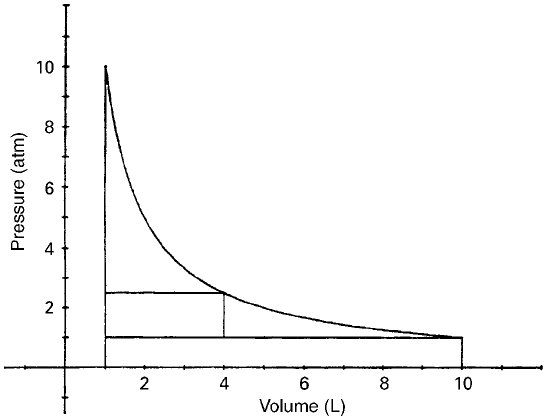
Consider a fixed mass of an ideal gas confined in a cylinder fitted with a movable
piston of variable weight. The weight of the piston and its cross-sectional area
determine the pressure on the gas. Let the entire assembly be placed in a constant
temperature bath to maintain isothermal conditions. Let the initial pressure of the gas
be 10 atm and the initial volume be 1 liter. Now consider three isothermal processes
(Fig. 4) (Sears, 1953):
Process I The weight on the piston is changed so as to produce an effective
pressure of 1 atm and since PV ¼ nR*T, its volume becomes
10 liters. The work of expansion is given by:
W
exp
¼ PDVa
ð1 atm ¼ 1013 10
2
PaÞð40Þ
Since P
surr
is constant at 1 atm, the work is 1ð10 1Þ¼9 Latm.
This work is performed on the surroundings. With respect to the
system, the energy transfer (dQ) is therefore 9 Latm.
Figure 4 Illustration of three isothermal processes.
2 ATMOSPHERIC THERMODYNAMICS 195
