Malley M.C. Radioactivity: A History of a Mysterious Science
Подождите немного. Документ загружается.

APPENDIX 2
218
Radium
Series
Atomic
weight
3·30 cms.
4·16 ,,
4·75 ,,
6·57 ,,
3·77 ,,
—
—
—
—
2000 yrs.
3·85 days
3·0 mins.
19·5 mins.
136 days
5·0 days
16·5 yrs.
1·4 mins.
26·8 mins.
1 gr.
5·7 × 10
–6
gr.
3·1 × 10
–9
,,
2·0 × 10
–8
,,
1·9 × 10
–4
,,
7·1 × 10
–6
,,
8·6 × 10
–3
,,
—
2·7 × 10
–8
,,
226
222
218
214
210
210
210
—
214
α + slow β
α
α
α
+β + γ
α
β
+ γ
slow β
β
β
+ γ
Half-value
period
Weight
per gram
of radium
Radiation
Radium Series.
Range of
α rays at
15° C.
Radium
Ra. Emanation
Radium A
Radium C
Radium F
Radium E
Radium D
Ra. C
2
Radium B
Actinium
Series
Atomic
weight
A
A
A – 4
A – 8
A – 12
A – 16
A – 16
A – 20
?
19·5 days
10·2 days
3·9 secs.
·002 sec.
36 mins.
2·1 mins.
4·71 mins.
rayless
α + β
α
α
α
slow β
α
β
+ γ
—
4·60 cms.
4·40 ,,
5·70 ,,
6·50 ,,
—
5·40 ,,
—
Half-value
period
Actinium Series.
Radiation
Range of
α rays at
15° C.
Actinium . . .
Radio-actinium
Actinium X . . .
Emanation . . .
Actinium A . . .
Actinium B . . .
Actinium C . . .
Actinium D . . .
FAMILY TREES FOR RADIOACTIVE ELEMENTS
219
orium
Series
Atomic
weight
Weight per
10
6
grams
thorium
Half-value
period
orium Series.
Radiation
Range
α rays
15° C.
2·72 cms.
3·87 ,,
4·3 ,,
5·0 ,,
5·7 ,,
4·8 ,,
8·6 ,,
—
—
—
—
1·3× 10
10
yrs.
2 yrs.
3·65 days
54 secs.
0·14 secs.
60 mins.
very short (?)
5·5 yrs.
6·2 hrs.
10·6 hrs.
3·1 mins.
232
228
224
220
216
212
212
228
228
212
208
10
9
mg.
0·15 ,,
7·4 × 10
–4
,,
1·2 × 10
–7
,,
3·1 × 10
–10
,,
7·9 × 10
–6
,,
—
0·42 mg.
5·2 × 10
–5
mg.
8·5 × 10
–5
,,
1·3 × 10
–7
,,
α
α
α
+ β
α
α
α
+ β
α
rayless
β + γ
β
+ γ
β
+ γ
orium . . .
Radiothorium
orium X . . .
. Emanation
orium A . . .
orium C . . .
β
α
. C
2
Mesothorium 1
Mesothorium 2
orium B . . .
orium D . . .
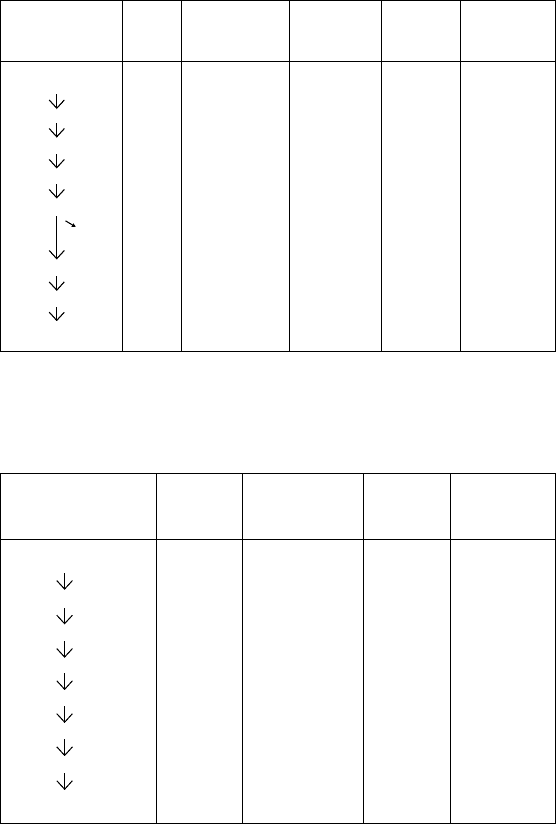
1. e Uranium Family
Radioelement and Rays
Half life
(years, days, hours, minutes, seconds)
Uranium I 4,500,000,000 y
↓ α
Uranium X
1
( orium 234) 24 d
↓ β
Uranium X
2
(Protactinium 234) 1.2 m
↓ β
The Radioactive Decay Series According to Modern Data
Source: From Ernest Rutherford, Radioactive Substances and their Radiations (Cambridge:
Cambridge University Press, 1913), 468, 518, 533, 552. Reproduced with permission of
Ernest Rutherford’s family.
(continued)

APPENDIX 2
220
1. e Uranium Family—(continued)
Radioelement and Rays Half life
(years, days, hours, minutes, seconds)
Uranium II (Uranium 234) 240,000 y
↓α
Ionium ( orium 230) 77,000 y
↓ α
Radium (Radium 226) 1,600 y
↓ α
Radium emanation (Radon 222) 3.8 d
↓ α
Radium A (Polonium 218) 3.1 m
↓ α or ↘ β
Radium B
(Lead 214)
Astatine
(Astatine 218)
27 m, 2 s
↓ β ↙ α
Radium C (Bismuth 214) 20 m
↓ β or ↘ α
Radium C'
(Polonium 214)
Radium C”
( allium 210)
0.00016 s, 1.3 m
↓ α ↙ β
Radium D (Lead 210) 22 y
↓ β
Radium E (Bismuth 210) 5.0 d
↓ β or ↘ α
Radium F
(Polonium 210)
allium
( allium 206)
140 d, 4.2 m
↓ α ↙ β
Radium G (Lead 206) Not radioactive

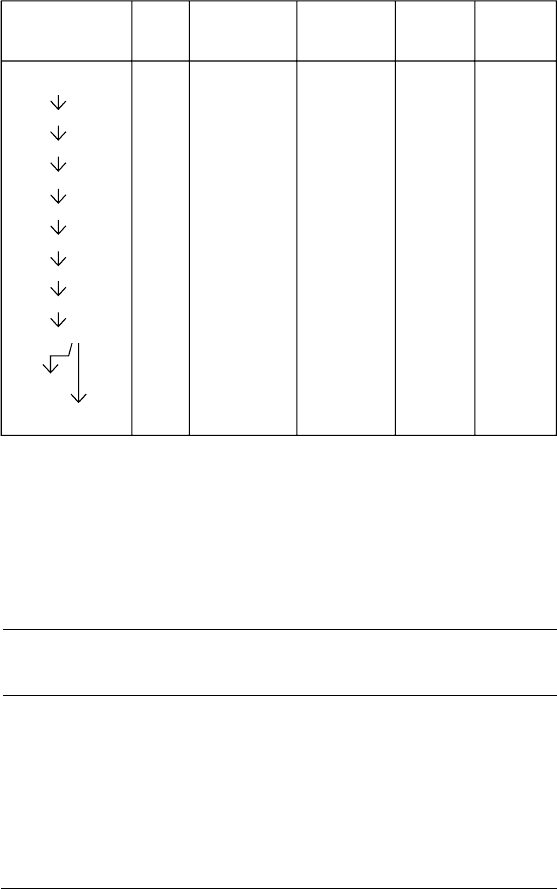
FAMILY TREES FOR RADIOACTIVE ELEMENTS
221
2. e Actinium Family
Radioelement and Rays Half life
(years, days, hours, minutes, seconds)
Actinouranium (Uranium 235) 710,000,000 y
↓ α
Uranium Y ( orium 231) 26 h
↓ β
Protactinium (Protactinium 231) 33,000 y
↓ α
Actinium (Actinium 227) 22 y
↓ β or ↘ α
Radioactinium
( orium 227)
Actinium K
(Francium 223)
19 d, 22m
↓ α ↙ β
Actinium X (Radium 223) 11 d
↓ a
Actinium emanation (Radon 219) 4.0 s
↓ α
Actinium A (Polonium 215) 0.0018 s
↓ α or ↘ β
Actinium B
(Lead 211)
Astatine
(Astatine 215)
36 m, 0.0001 s
↓ β ↙ α
Actinium C (Bismuth 211)
2.1 m
↓ β ↘ α
Actinium C’
(Polonium 211)
Actinium C”
( allium 207)
0.005 s, 4.8 m
↓ α ↙ β
Actinium D (Lead 207) Not radioactive

APPENDIX 2
222
3. e orium Family
Radioelement and Rays Half life
(years, days, hours, minutes, seconds)
orium ( orium 232) 14,000,000,000 y
↓ α
Mesothorium I (Radium 228) 5.8 y
↓ β
Mesothorium II (Actinium 228) 6.1 h
↓ β
Radiothorium ( orium 228) 1.9 y
↓ α
orium X (Radium 224) 3.7 d
↓ α
orium emanation (Radon 220) 56 s
↓ α
orium A (Polonium 216) 0.15 s
↓ α or ↘ β
orium B
(Lead 212)
Astatine
(Astatine 216)
11 h, 0.0003 s
↓ β ↙ α
orium C (Bismuth 212) 61 m
↓ β or ↘ α
orium C'
(Polonium 212)
orium C”
( allium 208)
0.0000003 s, 3.1 m
↓ α ↙ β
orium D (Lead 208) Not radioactive
Sources: Samuel Glasstone, Sourcebook on Atomic Energy. Princeton, NJ: D. Van Nostrand,
1950.
Argonne National Laboratory, EVS, Human Health Fact Sheet. Argonne National
Laboratory (Illinois), 2005.

223
APPENDIX 3
Radioactivity’s Elusive Cause
From its discovery in 1896, radioactivity mysti ed researchers because they
could not nd an energy source for the exploding atoms. No ma er what sources
they tested and which novel methods they tried, they could not change the pro-
cess of radioactivity. ey failed because radioactive elements do not use energy
from outside themselves to disintegrate.
An atom needs energy in order to disintegrate. e energy that allows certain
atoms to spontaneously decay and release excess energy comes from inside these
atoms. Atoms of naturally radioactive heavy elements can disintegrate because
they are not stable. ey have a tendency to come apart because they contain large
numbers of mutually repelling positive charges (protons) within their nuclei.
e force that causes like charges to repel and unlike charges to a ract is
called the electrostatic force. e electrostatic force creates energy when charged
particles push themselves apart or pull themselves together. It takes a good deal
of energy to overcome the electrostatic forces inside heavy nuclei and keep the
protons from ying apart.
at energy comes from a force inside the nucleus called the strong force.
e strong force holds protons and neutrons together. If the strong force holding
these particles together is greater than the electrostatic force pushing the protons
apart, the nucleus will be stable.
If an unstable nucleus casts o an alpha particle, the new element formed may
send gamma rays out from its nucleus as it assumes a more stable con guration.
e gamma radiation represents the energy di erence between di erent energy
levels of the nucleus.
Researchers were puzzled to nd cases where alpha decay occurred even
though the electrostatic forces were slightly less than the strong forces within
a nucleus. ey could not understand how an alpha particle could escape the
nucleus if it did not have enough energy to overcome the forces that kept the pro-
tons and neutrons together. ese examples seemed to violate the conservation
of energy.
APPENDIX 3
224
A theory developed in the 1920s called wave mechanics showed that, although
high energy alpha particles are most likely to be able to escape a nucleus, even
low energy particles can do so. Whether or not a particular particle escapes is a
ma er of probability, and the probability for escaping never goes to zero. ese
probabilities, calculated by using wave mechanics, determine how long it takes,
on average, for atoms of a particular radioelement to decay. Some radioelements
have average lives of less than a second, while others have lifetimes of millions or
even billions of years.
In the 1930s physicists learned that neutrons themselves can disintegrate.
is process produces beta particles. e force involved in beta particle emission
was later named the weak force, in contrast to the strong force that holds nuclei
together. In beta decay a neutron transmutes into a proton, an electron (beta
particle), and an uncharged, nearly massless particle called an anti-neutrino.
Beta particle decay is also governed by laws of probability. A er a beta particle is
ejected, a new nucleus is formed, which may emit gamma rays.
During the rst years of radioactivity scientists believed that deterministic,
mechanical causes lay underneath the probabilistic equations that described
radioactivity. ey reasoned that although probability theory could describe
radioactivity, these abstract equations did not explain it. Radioactivity’s leaders
assumed that a mechanical cause or causes for radioactivity might be found in
the future.
A er quantum mechanics was developed and interpreted in terms of proba-
bilities, many scientists decided that the probabilistic equations were themselves
the explanation of radioactivity. ere was no deeper cause for the exploding
atoms. At the subatomic level, Nature was governed by Chance.

225
APPENDIX 4
Nobel Prize Winners Included
in This Book
Names are listed according to Nobel Lectures. Physics, 1901–1921; 1922–1941;
1942–1962; and Nobel Lectures. Chemistry, 1901–1921; 1922–1941; 1942–1962
(Amsterdam: Elsevier, 1964–).
Physics
1901 Wilhelm Conrad Röntgen
1902 Pieter Zeeman
1903 Antoine Henri Becquerel, Pierre Curie, and Marie Skłodowska-Curie
1904 Lord Rayleigh (John William Stru )
1905 Philipp Eduard Anton von Lenard
1906 Joseph John omson
1911 Wilhelm Wien
1913 Heike Kamerlingh Onnes
1914 Max von Laue
1915 William Henry Bragg and William Lawrence Bragg
1917 Charles Glover Barkla
1918 Max Planck
1919 Johannes Stark
1921 Albert Einstein
1922 Niels Bohr
1926 Jean Baptiste Perrin
1927 Charles omson Rees Wilson
1935 James Chadwick
1936 Victor Franz Hess
APPENDIX 4
226
Chemistry
1904 Sir William Ramsay
1908 Ernest Rutherford
1909 Wilhelm Ostwald
1911 Marie Skłodowska Curie
1914 eodore William Richards
1921 Frederick Soddy
1935 Jean Frédéric Joliot and Irène Joliot-Curie
1943 George de Hevesy (Georg von Hevesy)
1944 O o Hahn
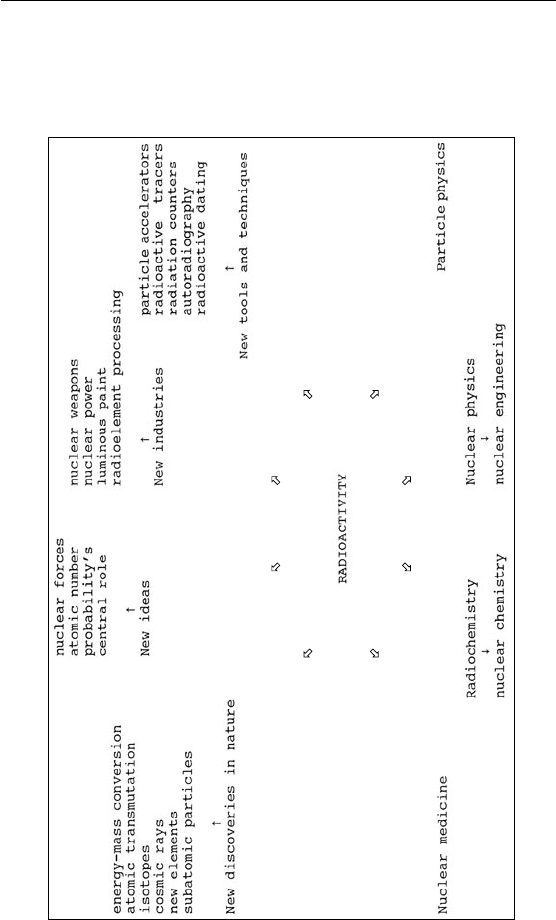
APPENDIX 5
Radioactivity’s Web of In uence
(Illustration by author)
227
