Kuppan T. Heat Exchanger Design Handbook
Подождите немного. Документ загружается.


614
Chapter
I2
stress corrosion cracking of tubing in the tube sheet roller expanded region, and pitting attack
of tubing in the sludge pile [42]. Tube inserts are available with various trade names. Among
the tube inserts, a patented tube insert known as ShieWSeals for inlet-end erosion-corrosion
and Pop-A-Plug for plugging a leaking tube are discussed next.
Shield/Seals. CTI Industries, Inc., Stratford,
CT,
has developed and patented Shield/
Seals, which are inserted into the tubes to reduce wear from high-velocity water that causes
turbulence near the tube inlet. ShielddSeals are made of various think-walled (0.016-0.020
in,
0.4-0.5
1
mm) alloys with premium erosion-corrosion resistance. They are expanded to a preset
tightness inside the tube inlets by CTI’s patented mechanical expansion
or
hydraulic expansion
method.
Seals for Plugging Leaking Tubes. Patented seals known
as Pop-A-Plug for plugging
leaking tubes can serve as a new pressure boundary should a through-wall penetration develop
in the heat exchanger; they have been patented and marketed by
M/s.
Expando Seal Tools Inc.
[43]. The salient features of Pop-A-Plug are discussed next.
The patented Pop-A-Plug System uses a 6600-lb
or
17.5-ton hydraulic ram to expand a
two-piece, mechanical plug inside a leaking heat exchanger tube.
The
ram pulls a tapered
center pin through
an
externally serrated ring. As the ring expands, the serrations compress
against the tube wall. At a precise, predetermined pressure a breakaway bbpops,” sealing the
tube and separating the plug from the ram. The plugs are available
in
brass, carbon steel.
stainless steel, Monel, copper-nickel, aluminum bronze, and Inconel. Sizes fit tube inner diam-
eters from 0.400 to 1.250 in. Pop-A-Plug is shown schematically in Fig. 23.
Considerations While Using Inserts. While using metallic inserts, care should be exer-
cised to avoid formation of galvanic couple between the tube metal and the insert metal. Also.
ensure a smooth transition between the end of the tube insert and the tube surface at the
downstream to avoid turbulence, which will further enhance the erosion-corrosion
in
the down-
stream.
Stress Corrosion Cracking
Corrosion Znidving
Stressed
Materials.
When the combined effects of corrosion and stresses
are considered, they are usually divided into
two
classes [22]:
TAPERED
/-
FIN
POP-A-PLUG
Figure
23
POP-A-PLUG
to
plug a leaking tube. (Courtesy
of
Expands Seal Tools Inc., Montgomery-
ville, PA.)

Corrosion
61
5
1.
Stress corrosion cracking, involving the effects of static stresses and corrosion
2. Corrosion fatigue, involving variable stresses and corrosion
Cracks resulting from the first phenomenon are predominantly intergranular; those resulting
from the latter phenomenon are transgranular.
Environmental EfSects on Properties
of
Materials.
Certain environments drastically alter the
strength and stability of engineering structures, whereas the same structures performs satisfac-
torily in air and other environments. For example, a deep drawn brass fails spontaneously by
cracking in air containing traces
of
ammonia, a stressed mild steel may crack when exposed
to condensates of gaseous combustion products containing nitrates, and 18-8 stainless steel
above room temperature on exposure to moist environment containing traces of chlorides.
These are examples of what is called stress corrosion cracking.
Stress Corrosion Cracking.
Stress corrosion cracking (SCC) is defined as the corrosion attack
on a susceptible alloy due to combined and synergistic interaction of tensile stress and condu-
cive environment. The stress required to cause SCC is normally low and usually below the
yield stress, and it can be applied or residual stress, but it is always a tensile stress. In simple
terms, SCC requires the simultaneous Occurrence of the following three conditions:
1.
A
susceptible material
2.
A
corrosive environment
3.
Tensile stress
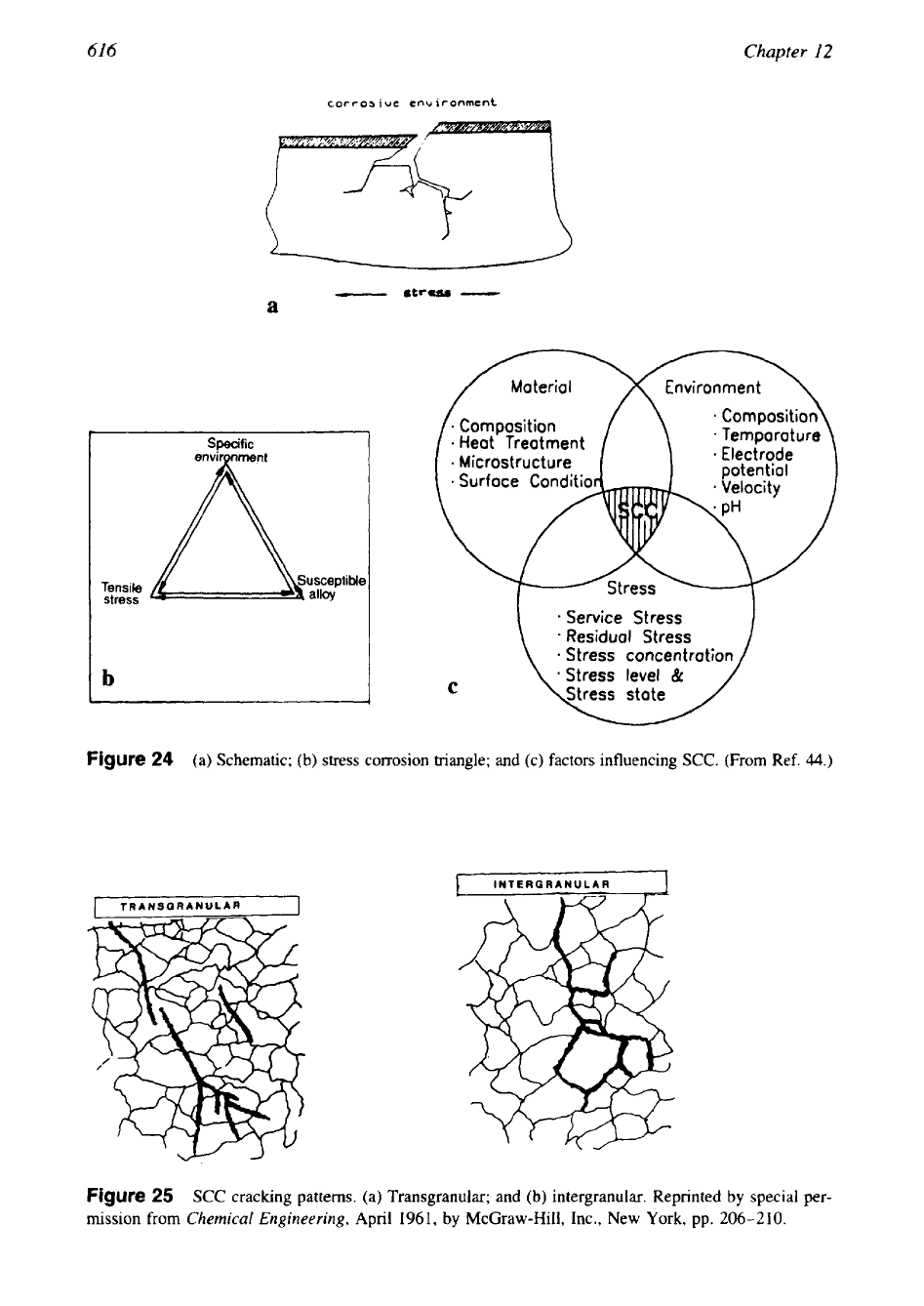
These conditions are shown schematically in Fig. 24.
Time for cracking ranges from few minutes under highly accelerated laboratory conditions
to months or even years for resistant materials exposed to a mildly corrosive environment.
SCC is much more insidious; the cracks propagate perpendicular to the tensile stress and may
be transgranular, common in stainless steels (Fig. 25a), or intergranular (Fig. 25b), as found
in
many aluminum alloys with little or no evidence of telltale corrosion products. There is little
plastic deformation
so
that the material appears to behave in a brittle fashion. The characteristic
appearance of stress corrosion cracking includes the lack of deformation and relatively small
amounts
of
general corrosion [45]. Stress corrosion cracking is the result of the interaction
of
a number of variables-mechanical, metallurgical, and environmental. The level of the corro-
sive attack depends on factors like:
1. Stress level and stress state
2.
Aggressiveness of the corrosive environment
3.
Temperature of the medium
4.
Level of cold work and the level of stress relief treatment, grain structure, surface condi-
tion, etc.
5.
Crack geometry
6.
Electrode potential
7.
Composition, metallurgical condition
Heat Exchanger Components Susceptible
to
SCC.
The most common places wherein SCC
takes place include at
(1)
tube-to-tube-sheet expanded joints,
(2)
U-tube bends,
(3)
bellows
type expansion joints, and
(4)
indented locations by the baffle plates. Items (2) and
(4)
are
shown in Fig.
26.
Tube-to-Tube-Sheet Expanded Joint.
All tube expanding methods leave residual stresses
in the tube wall. To minimize residual stress, the tube expansion should not exceed certain
percentage of the tube wall thickness, which varies from metal to metal (carbon steel 5-8%,
I
61
6
Chapter
I2
corrosive
environment
a
Specific
Figure
24
(a) Schematic;
(b)
stress corrosion triangle; and (c) factors influencing
SCC.
(From Ref.
44.)
Figure
25
SCC
cracking patterns. (a) Transgranular; and
(b)
intergranular. Reprinted
by
special per-
mission from
Chemical Engineering,
April
1961,
by
McGraw-Hill, Inc., New
York,
pp.
206-210.

Corrosion
61
7
r
CORROS
ION
PRODUCT
b
Figure
26
Some components
of
heat exchanger susceptible to
SCC.
(a)
Tube indented locations
by
the
baffle
plate;
and
(b)
regions of U-bend.
stainless steel
3-5%,
alloy steel
46%).
This value is to be controlled during rolling-in opera-
tion. The stresses are the higher for these conditions
[32]:
Bigger tube hole in relation to the tube diameter
Smaller tube diameter in relation to the bore hole
Higher deformation of the tube after being rolled into the bore hole
Rolling-in extending beyond the tube sheet (Fig.
19)
U-Bend.
Cold forming of U-bend tubes induce severe residual tensile stress in the outer
portion of the bend, which subsequently fails by stress corrosion cracking. Annealing of the
bend tubes will prevent SCC. Other areas prone to SCC are near the U-bend apex for tubes of
steam generators where
the
tube’s legs were brought closer together via the denting forces and
at nonsmooth transition region of U-bends
[42].
Various regions of U-bend tube susceptible to
SCC are shown in Fig.
26.
Thin-Walled Expansion Joints. Stress corrosion cracking occurs in
thin-walled expansion
joint elements made of austenitic stainless steel materials on the bottom of horizontal units that
are not drainable.
Classijkation
of
Stress Corrosion Cracking Failures.
Stress corrosion cracking failures may
involve
an
electrochemical mechanism, attack by a molten phase, hydrogen embrittlement, or
some other factors. According to the environment causing cracking of metals and metal alloys,
SCC can be categorized as:
Chloride cracking, e.g., austenitic stainless steel, aluminum
Caustic cracking, e.g., carbon steel
Ammonia cracking, e.g., admiralty brass

618
Chapter
12
Polythionic acid cracking, e.g., austenitic stainless steel
Sulfurous acid cracking, e.g., austenitic stainless steel
Discussion
of
Conditions Responsible
for
SCC.
Susceptible Alloys and the Environment.
Stress corrosion cracking, like most forms of
corrosion, is electrochemical and involves metals in contact with an electrolyte. The environ-
ments that cause stress corrosion cracking are specific for each metal. Except for ferritic stain-
less steel, virtually all metals and alloy systems are susceptible to SCC by a specific corrodent
under conditions of temperature, stress level, etc. Chloride ions lead to stress corrosion crack-
ing in 304 and 316 stainless steels when oxygen is present. Typical hostile environments for
few of the most widely used metals are given in Table 4. Tables of environments and alloy
combinations known to result in SCC are published by the National Association of Corrosion
Engineers (NACE) [46], the Materials Technology Institute [47], and others for the chemical
process.
Stress.
Tensile stress at the surface of the metal is an essential factor in stress corrosion
cracking. Cracking has never been found in metals under compression. The tensile stresses
may be due to internal stress caused by metal deformation near welds and bolts, deformation
caused by shrink fit, unequal cooling from high temperature, or volume changes
in
the material
caused by phase change or rearrangement of crystal structure [48], or residual stress from some
prior cold work or metal forming operation or caused by an applied stress (i.e., service-induced
external load). Residual and applied stresses are additive to evaluate the effect on cracking,
and both must be known. Welding often leaves residual stresses that lead to stress corrosion
cracking in susceptible environments.
SCC
of
Welded Joints.
Welded joints are particularly prone to SCC for three reasons [27]:
(1)
The welding operation will leave a residual tensile stress in the weld area unless effective
postweld stress relief is carried out,
(2)
stress concentrations are usually present, and (3) the
thermal cycle can produce a susceptible microstructure. SCC is overcome by shot peening after
welding to induce compressive stress on the surface, stress-relief heat treatment, and use of
alloys resistant to such cracking.
HOW
to Detect
SCC.
Some cracks may be visible to the naked eye and thus require no special
detection means. Others may be seen after surface deposits are removed, particularly under
magnification. Sophisticated microanalytical instruments such as the electron beam microprobe
analyzer, scanning electron microscope, and mass spectrometer are being increasingly applied
Table
4
Environments That Cause Stress Corrosion Cracking
Metal Environment
A1 and Al-base alloys
NaCl solution, seawater,
H202,
chloride solution, and other halide
solutions
Cu and Cu-base alloys
Ammonia and ammonium hydroxide, amines, mercury, sulfur diox-
ide,
H2S,
steam
Carbon steel
Sodium hydroxide solutions, ammonia and sodium nitrate solutions,
carbonatehicarbonate
Austenitic stainless steel
Aqueous chlorides, seawater, sulfurous and polythionic acid
Nickel and nickel-base alloys Caustic above
3
15OC, fused caustic soda, hydrofluoric acid, polythi-
onic acid
Titanium and titanium alloys
Reducing acids such as chlorides, iodides, fluorides, red fuming
ni-
tric acid, nitrogen tetroxide, methanol
Co
rros
ion
61
9
to failure analysis of power plant components [49]. Crack detection methods like magnetic
particle testing, dye penetrant testing, x-rays, ultrasonics, and eddy current testing can also be
used [48].
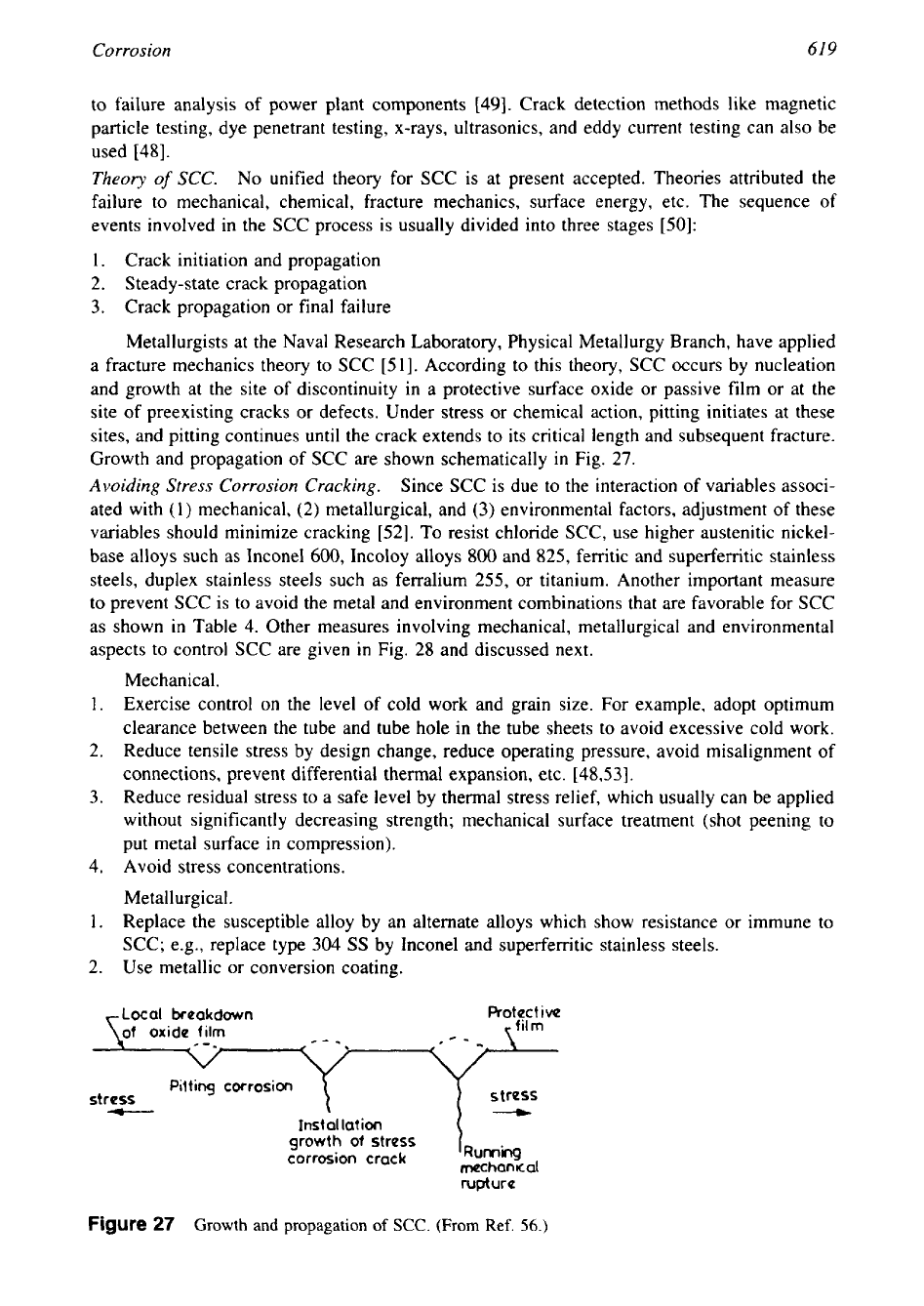
Theory
of
SCC.
No unified theory for SCC is at present accepted. Theories attributed the
failure to mechanical, chemical, fracture mechanics, surface energy, etc. The sequence
of
events involved in the SCC process is usually divided into three stages [50]:
1.
Crack initiation and propagation
2. Steady-state crack propagation
3.
Crack propagation or final failure
Metallurgists at the Naval Research Laboratory, Physical Metallurgy Branch, have applied
a fracture mechanics theory to SCC [51]. According to this theory, SCC occurs by nucleation
and growth at the site
of
discontinuity in a protective surface oxide or passive film or at the
site of preexisting cracks or defects. Under stress or chemical action, pitting initiates at these
sites, and pitting continues
until
the crack extends to its critical length and subsequent fracture.
Growth and propagation of SCC are shown schematically
in
Fig.
27.
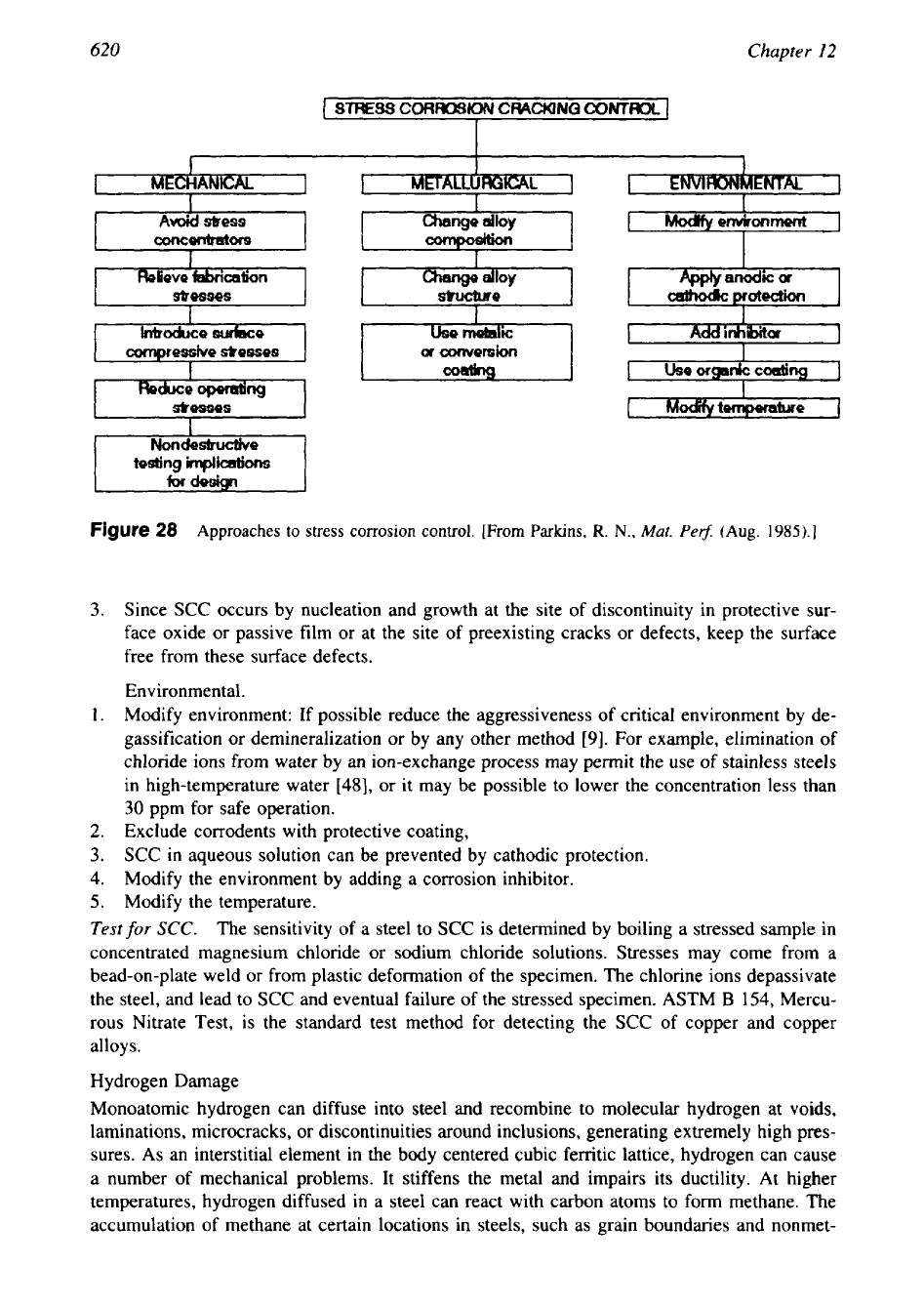
Avoiding Stress Corrosion Cracking.
Since SCC is due to the interaction of variables associ-
ated with
(1
)
mechanical, (2) metallurgical, and
(3)
environmental factors, adjustment of these
variables should minimize cracking [52]. To resist chloride SCC, use higher austenitic nickel-
base alloys such as Inconel
600,
Incoloy alloys
800
and 825, ferritic and superferritic stainless
steels, duplex stainless steels such as ferralium
255,
or titanium. Another important measure
to prevent SCC is to avoid the metal and environment combinations that are favorable for SCC
as shown
in
Table 4. Other measures involving mechanical, metallurgical and environmental
aspects to control SCC are given
in
Fig.
28
and discussed next.
Mechanical.
1.
Exercise control on the level of cold work and grain size. For example, adopt optimum
clearance between the tube and tube hole in the tube sheets to avoid excessive cold work.
2.
Reduce tensile stress by design change, reduce operating pressure, avoid misalignment of
connections, prevent differential thermal expansion, etc.
[48,53].
3.
Reduce residual stress to a safe level by thermal stress relief, which usually can be applied
without significantly decreasing strength; mechanical surface treatment (shot peening to
put metal surface
in
compression).
4.
Avoid stress concentrations.
Metallurgical.
1.
Replace the susceptible alloy by an alternate alloys which show resistance or immune to
SCC; e.g., replace type
304
SS
by Inconel and superferritic stainless steels.
2.
Use metallic or conversion coating.
:t
iw
growth
of
stress
corrosion
crack
(Aurning
mchanra,
ruptura
Figure
27
Growth and propagation
of
SCC.
(From
Ref.
56.)
620
Chapter
I2
I
STRESS
CoRRosrn
CFt9CKING
CONTROL
I
I
MECHANICAL
1
1-M
' I
I
I
cedhodcprotection
J
i
1
I
Add
inhibitor
I
I
r
Useoraaniccoadina
1
Figure
28
Approaches
to
stress corrosion control.
[From
Parkins,
R.
N.,
Mar.
Pe$
(Aug.
1985).]
3.
Since
SCC
occurs by nucleation and growth at the site of discontinuity in protective sur-
face oxide or passive film or at the site of preexisting cracks or defects, keep the surface
free from these surface defects.
Environmental.
1.
Modify environment: If possible reduce the aggressiveness of critical environment by de-
gassification or demineralization or by any other method
[9].
For example, elimination of
chloride ions from water by an ion-exchange process may permit the use of stainless steels
in high-temperature water
[48],
or it may be possible to lower the concentration less than
30
ppm for safe operation.
2.
Exclude corrodents with protective coating,
3.
SCC
in aqueous solution can be prevented by cathodic protection.
4.
Modify the environment by adding a corrosion inhibitor.
5.
Modify the temperature.
Test
for
SCC.
The sensitivity of a steel to
SCC
is determined by boiling a stressed sample in
concentrated magnesium chloride or sodium chloride solutions. Stresses may come from a
bead-on-plate weld or from plastic deformation of the specimen. The chlorine ions depassivate
the steel, and lead to
SCC
and eventual failure of the stressed specimen. ASTM
B
154,
Mercu-
rous Nitrate Test, is the standard test method for detecting the
SCC
of copper and copper
alloys.
Hydrogen Damage
Monoatomic hydrogen can diffuse into steel and recombine to molecular hydrogen at voids,
laminations, microcracks, or discontinuities around inclusions, generating extremely high pres-
sures. As an interstitial element in the body centered cubic fenitic lattice, hydrogen can cause
a number of mechanical problems. It stiffens the metal and impairs its ductility. At higher
temperatures, hydrogen diffused in a steel can react with carbon atoms to form methane. The
accumulation of methane at certain locations in steels, such as grain boundaries and nonmet-

Corrosion
621
allic inclusions, causes the irreversible loss of strength and ductility. Elevated temperature
exposure in hydrogen sometimes results in surface decarburization, followed by fissuring due
to high gas pressure at localized sites. These changes in the steel are called hydrogen attack.
The different forms of hydrogen damage are:
1. Hydrogen embrittlement
2.
Hydrogen blistering
3. Hydrogen SCC
4.
High-temperature attack
The development of hydrogen damage would presumably be hindered by anything present
in the steel that would
[54]:
1. Decrease the rate of corrosion.
2.
Decrease the rate at which the hydrogen produced by corrosion entered the steel instead
of escaping into the process stream.
3.
Speed up the passage of hydrogen through steel, thus reducing the time available for
reaction.
4.
Decrease the rate of reaction of hydrogen with carbon in the steel.
Other remedial measures include stress relief, the selection of material that does not harden
so
readily during the welding cycle, or the inhibition of the hydrogen evolution reaction causing
the trouble. Hydrogen damage due to high temperature exposure is discussed in detail in Chap-
ter 13, Material Selection, and hydrogen damage in the sour environment is discussed at the
end of this chapter.
Fretting Corrosion
Fretting corrosion is the combined wear and corrosion process that takes place at locations
where there is a relative movement between two components and the movement is restricted
to very small amplitude. At the mating surfaces, the degree of deterioration increases because
of repeated corrosion of the freshly abraded surface and the accumulation of abrasive corrosion
products between these surfaces.
A
typical example is the wear
of
the heat exchanger tubes at
the tube-baffle contacts. Various metallurgical, geometrical, and environmental factors influ-
ence the fretting wear of heat exchanger tubes. Factors that affect fretting wear include
[55]
contact load, amplitude, frequency, number of cycles, and temperature. Fretting wear of heat
exchanger tubes has been covered in Chapter 10 on flow induced vibration.
Corrosion Fatigue
Corrosion
Fatigue.
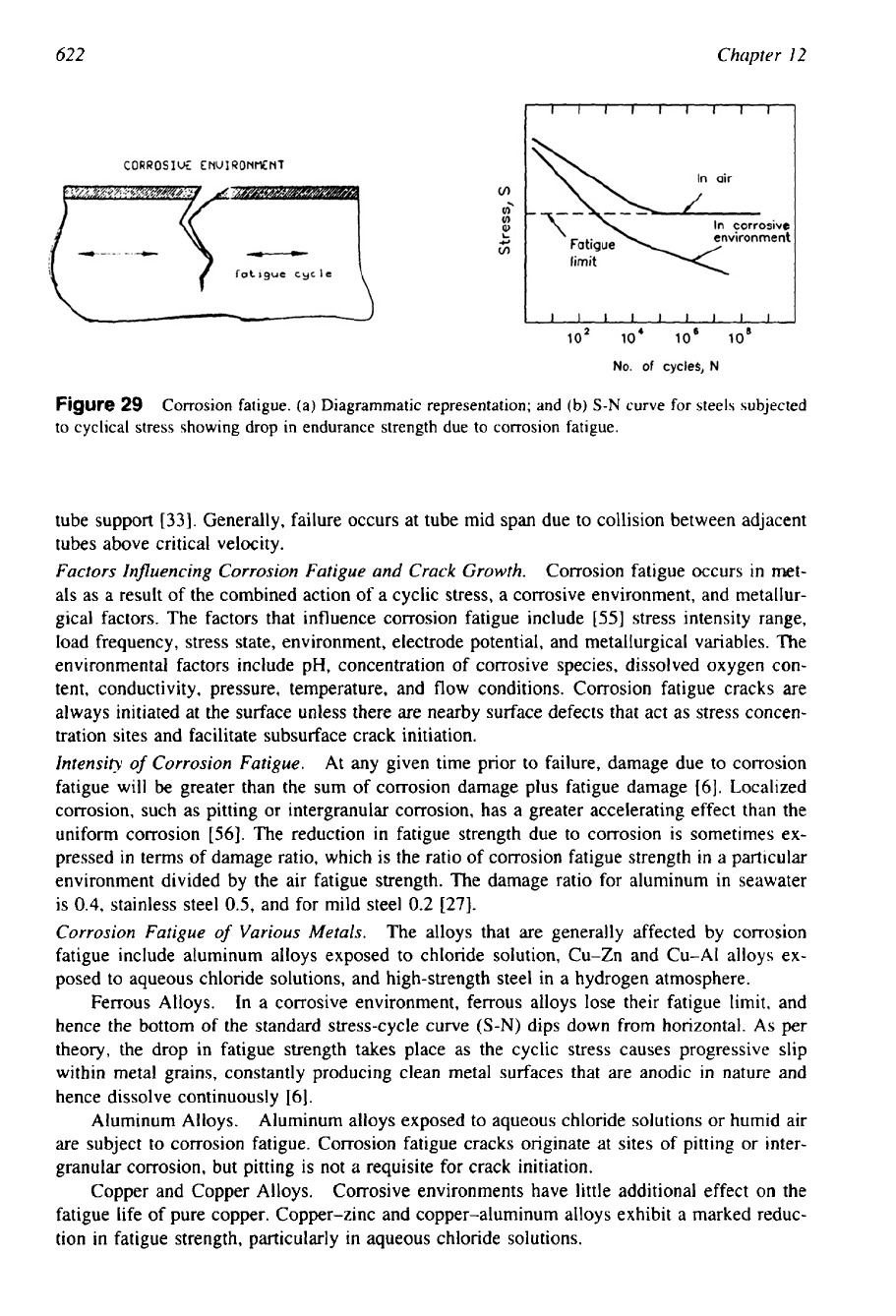
Corrosion fatigue is the reduction in the fatigue strength of a metal as a
result of exposure to a corrosive environment. When a metal is subjected to cyclic stress in a
corrosive environment, a marked drop in or elimination of the endurance limit may occur even
in a mildly corrosive environment. Surface film protected alloy is especially susceptible to
corrosion fatigue
[56].
Corrosion fatigue is shown schematically in Fig. 29a and the drop in
endurance strength in Fig. 29b. In film-protected alloys, stress reversals cause repeated crack-
ing of the otherwise protective surface film, and this allows access of the corrodent to the
unprotected metal, with resultant corrosion. The reduction in fatigue strength has a conse-
quence on the life and reliability of a component. The reduction may be
so
severe that a detail
that has been ignored at the design or inspection stage can lead to catastrophic failure in service
[27].
In heat exchangers, corrosion fatigue can occur in any tube material; it
is
caused by steam
buffeting in the case of the condenser or flow-induced vibration
in
association with inadequate
622
Choptrr
12
Figure
29
Corrosion fatigue. (a) Diagrammatic representation; and (b)
S-N
curve for steels subjected
to
cyclical stress showing drop in endurance strength due to corrosion fatigue.
tube support
[33].
Generally, failure occurs at tube mid span due to collision between adjacent
tubes above critical velocity.
Factors Influencing Corrosion Fatigue and Crack Growth.
Corrosion fatigue occurs
in
met-
als as a result of the combined action
of
a cyclic stress, a corrosive environment, and metallur-
gical factors. The factors that influence corrosion fatigue include
[55]
stress intensity range,
load frequency, stress state, environment, electrode potential, and metallurgical variables. The
environmental factors include pH, concentration of corrosive species, dissolved oxygen con-
tent, conductivity, pressure, temperature, and flow conditions. Corrosion fatigue cracks are
always initiated at the surface unless there are nearby surface defects that act as stress concen-
tration sites and facilitate subsurface crack initiation.
Zntensify
of
Corrosion Fatigue.
At any given time prior to failure, damage due to corrosion
fatigue will
be
greater than the sum of corrosion damage plus fatigue damage
(61.
Localized
corrosion, such as pitting or intergranular corrosion, has a greater accelerating effect than the
uniform corrosion
[56].
The reduction in fatigue strength due to corrosion is sometimes ex-
pressed
in
terms of damage ratio, which is the ratio of corrosion fatigue strength
in
a particular
environment divided by the air fatigue strength. The damage ratio
for
aluminum in seawater
is
0.4,
stainless steel
0.5,
and for mild steel
0.2
[27].
Corrosion Fatigue
of
Various Metals.
The alloys that are generally affected by corrosion
fatigue include aluminum alloys exposed to chloride solution, Cu-Zn and Cu-A1 alloys ex-
posed to aqueous chloride solutions, and high-strength steel in a hydrogen atmosphere.
Ferrous Alloys.
In
a corrosive environment, ferrous alloys lose their fatigue limit, and
hence the bottom of the standard stress-cycle curve
(S-N)
dips down from horizontal. As per
theory, the drop in fatigue strength takes place as the cyclic stress causes progressive slip
within metal grains, constantly producing clean metal surfaces that are anodic
in
nature and
hence dissolve continuously
[6].
Aluminum Alloys.
Aluminum alloys exposed to aqueous chloride solutions or humid air
are subject to corrosion fatigue. Corrosion fatigue cracks originate at sites of pitting or inter-
granular corrosion, but pitting
is
not
a
requisite for crack initiation.
Copper and Copper Alloys.
Corrosive environments have little additional effect
on
the
fatigue life of pure copper. Copper-zinc and copper-aluminum alloys exhibit a marked reduc-
tion
in
fatigue strength, particularly
in
aqueous chloride solutions.
Corrosion
623
Corrosion Fatigue of Welded Joints.
Under normal circumstances, welded joints are
likely to be associated with fatigue cracks associated with structural and mechanical discontinu-
ities that are usually present in the welds [27]. The toes of fillet welds provide site for crack
initiation, particularly in the presence of undercuts.
Relationship Between Corrosion Fatigue and
SCC.
Corrosion fatigue is mostly interrelated
to environmentally induced corrosive attack forms such as SCC and hydrogen embrittlement
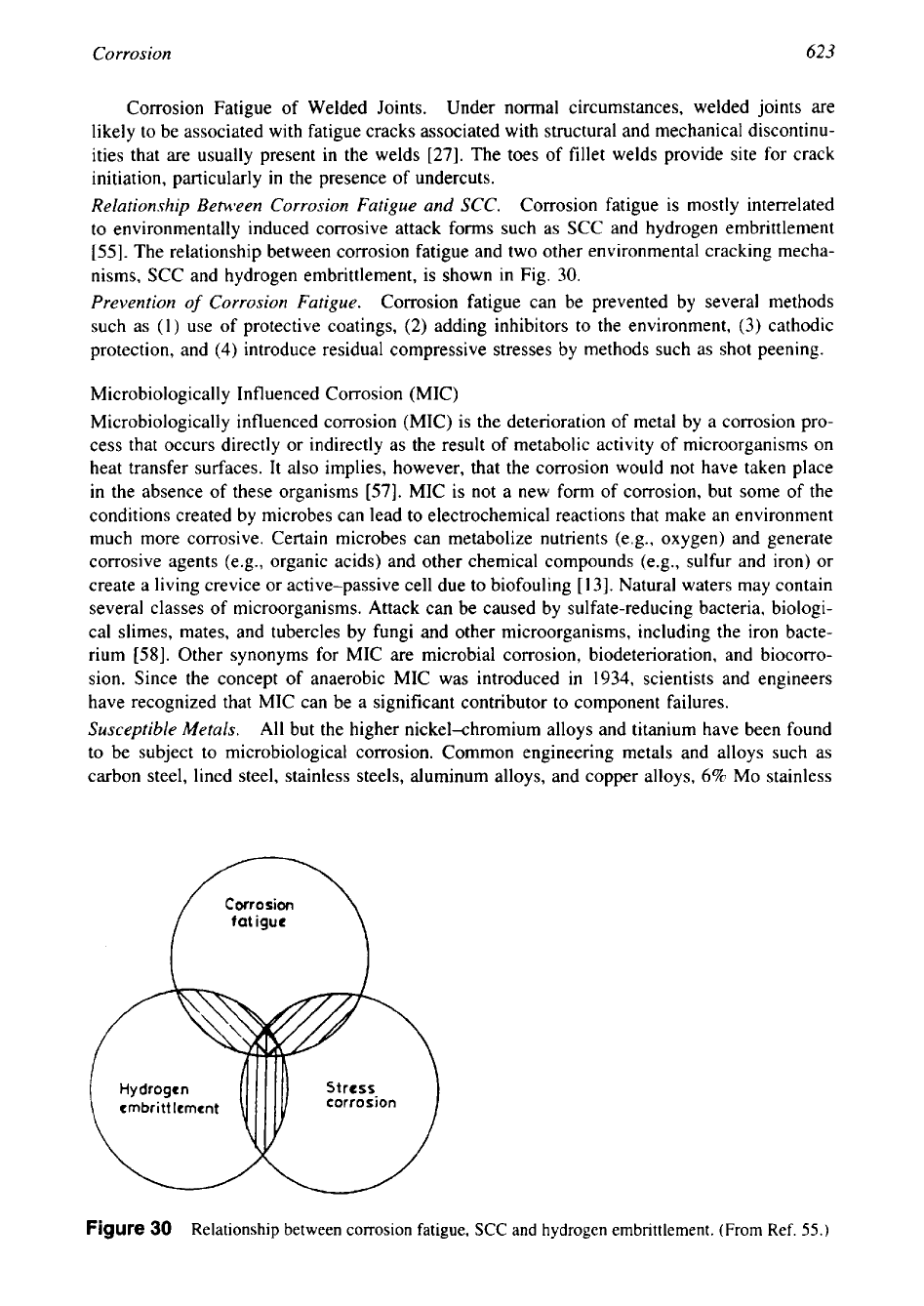
[55]. The relationship between corrosion fatigue and two other environmental cracking mecha-
nisms, SCC and hydrogen embrittlement, is shown in Fig.
30.
Prevention
of
Corrosion Fatigue.
Corrosion fatigue can be prevented by several methods
such as
(1)
use of protective coatings, (2) adding inhibitors to the environment,
(3)
cathodic
protection, and
(4)
introduce residual compressive stresses by methods such as shot peening.
Microbiologically Influenced Corrosion (MIC)
Microbiologically influenced corrosion (MIC) is the deterioration
of
metal by a corrosion pro-
cess that occurs directly or indirectly as the result of metabolic activity
of
microorganisms on
heat transfer surfaces. It also implies, however, that the corrosion would not have taken place
in the absence of these organisms [57]. MIC is not a new form of corrosion, but some of the
conditions created by microbes can lead to electrochemical reactions that make an environment
much more corrosive. Certain microbes can metabolize nutrients (e.g., oxygen) and generate
corrosive agents (e.g., organic acids) and other chemical compounds (e.g., sulfur and iron) or
create a living crevice or active-passive cell due to biofouling
[
131.
Natural waters may contain
several classes of microorganisms. Attack can be caused by sulfate-reducing bacteria, biologi-
cal slimes, mates, and tubercles by fungi and other microorganisms, including the iron bacte-
rium [58]. Other synonyms for MIC are microbial corrosion, biodeterioration, and biocorro-
sion. Since the concept of anaerobic MIC was introduced
in
1934,
scientists and engineers
have recognized that MIC can be a significant contributor to component failures.
Susceptible Metals.
All but the higher nickekhromium alloys and titanium have been found
to be subject to microbiological corrosion. Common engineering metals and alloys such as
carbon steel, lined steel, stainless steels, aluminum alloys, and copper alloys,
6%
MO stainless
Corrosion
fat
iguc
embritt lement corrosion
Figure
30
Relationship between corrosion fatigue,
SCC
and hydrogen embrittlement. (From Ref.
55.)
