Kuppan T. Heat Exchanger Design Handbook
Подождите немного. Документ загружается.


604
Chapter
12
case of passive metals like aluminum or the stainless steels, oxygen starvation within the
crevice usually destroys the passive film responsible for the corrosion resistance, and forms a
passive-active cell. The passive-active cell exhibits a greater potential difference and hence
induces higher current than occurs within crevices formed by nonpassive metals like iron and
copper 121.
Mechanism
of
Crevice Corrosion.
Crevice corrosion takes place when a small volume of
solution gets into a crack or a small opening. It stays there, stagnant, and its composition
changes by the corrosion process
so
that its composition is different from the bulk solution. It
may be oxygen depleted (oxygen concentration cell), enriched in metal ions (metal ion concen-
tration cell), or enriched in chloride ions or at a lower pH than the rest of the solution. The
corrosion rate increases as the crevice mouth narrows and
as
the external cathode area is
increased. Crevices are particularly detrimental when alternate wetting and drying occurs, be-
cause corrosive liquids retained in the crevices are concentrated by evaporation [27,28].
To
avoid crevice corrosion, the crevice must be wide open to allow the free movement
of
electro-
lyte. It usually occurs in gaps just a few micrometers wide, not in wide gaps or grooves
[6].
Heat Exchanger Locations Prone to Crevice Corrosion.
Areas prone to crevice corrosion
in
a shell and tube heat exchanger (STHE) and plate heat exchangers (PHE) are:
Shell and tube heat exchanger: clearance between the rolled tubes and the tube sheet, open
welds at tube sheet, beneath deposits, water box gaskets, bolt holes, nuts, washer, disbon-
ded water box linings, etc.
Plate heat exchanger: beneath gaskets, plate contact points, and beneath deposits.
Crevice Corrosion Versus
Pitting
Corrosion.
The mechanism of propagation of pits and crev-
ice corrosion is identical; however, the mechanisms of initiation differ [24]. Crevice corrosion
is initiated by differential concentration of oxygen or ions in the electrolyte, whereas pitting
is
initiated (on plane surfaces) by metallurgical factors and structural factors only [29]. These
may include discontinuities in a protective film or coating, or compositional variations such
as
inclusions. The level of crevice corrosion occurring at crevices such as under deposits or
gaskets or at joints between two metals is significantly greater than that of pitting on open
surfaces. These two forms of corrosion are compared with reference to austenitic stainless
steels in Chapter 13, Material Selection and Fabrication, in the section
on
austenitic stainless
steel.
Crevice Corrosion Control.
Like all forms of localized attack, crevice corrosion does not
take place in all metal and corrodent combinations. Passive metals are more susceptible
to
it
than others. These materials can be alloyed to improve resistance to crevice corrosion.
This
approach, together with designing to minimize crevices and maintenance practices to keep
surfaces clean, is used to overcome crevice corrosion. Various practices recommended for
safeguards against the occurrence of crevice corrosion include the following:
1.
Structural designs should avoid any and all crevices. This is especially true for passive
metals like aluminum, stainless steels, and various nickel-base alloys. Unavoidable crev-
ices should be filled by weld metal or with nonconducting sealants or cements [2].
2.
In a new equipment, specify butt welding joints and emphasize the necessity for complete
penetration of the weld metal to guard against even minute crevices.
3.
During the design stage avoid sharp corners, stagnant areas, or other sites favorable to
the accumulation of precipitates or wherein the solute or
O2
concentration cell takes place.
For example, projection of tubes beyond the tube sheet as shown in Fig. 19 can lead to
crevice corrosion. Figure 19a shows the original design with protrusion of tubes and
rolling-in operation that produced a crowned tube sheet. This created crevices between
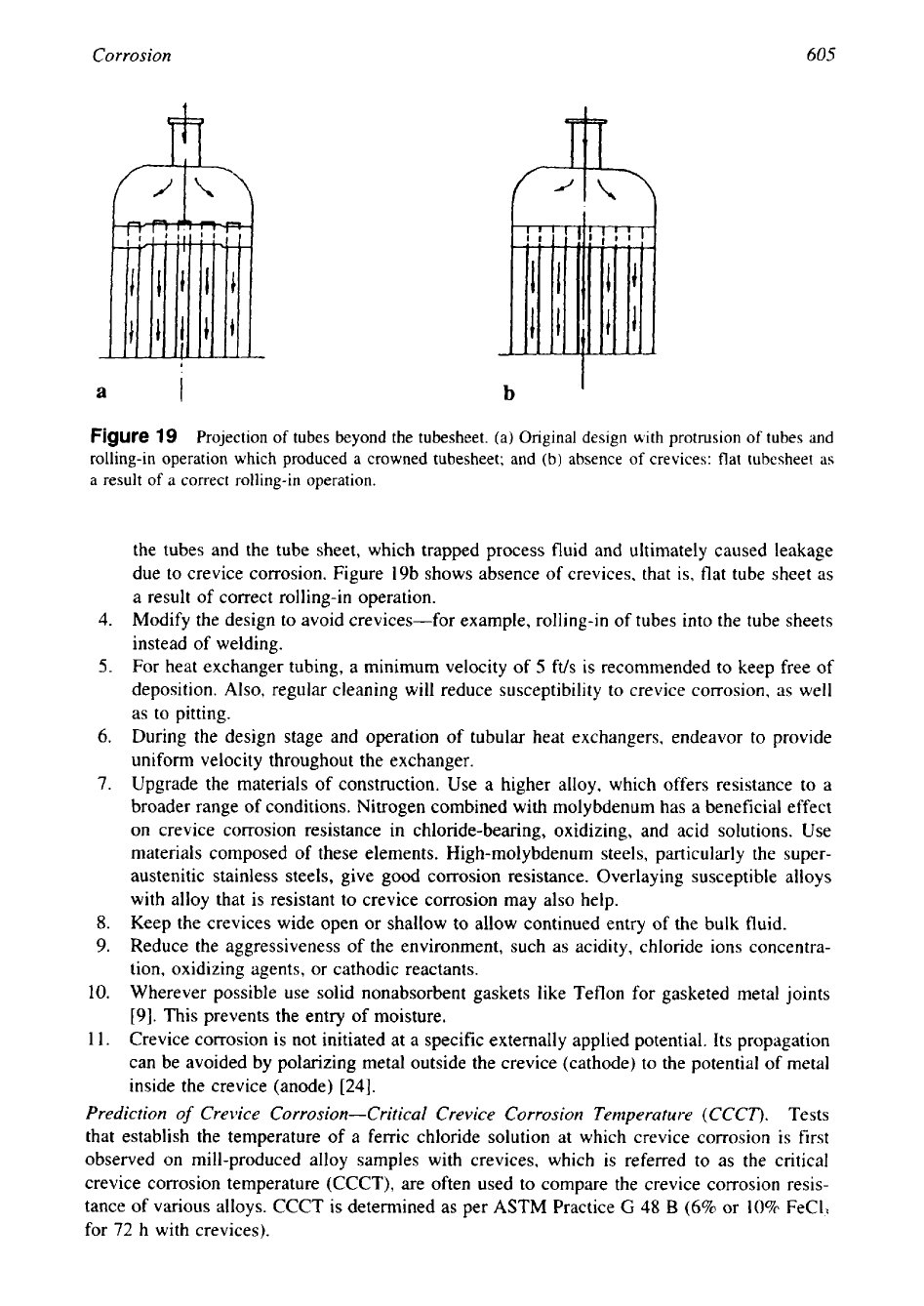
Corrosion
605
1
a
I
Figure
19
Projection
of
tubes beyond the tubesheet. (a) Original design with protrusion
of
tubes and
rolling-in operation which produced a crowned tubesheet; and (b) absence
of
crevices: flat tubesheet as
a result of
a
correct rolling-in operation.
the tubes and the tube sheet, which trapped process fluid and ultimately caused leakage
due to crevice corrosion. Figure 19b shows absence of crevices, that is, flat tube sheet as
a result of correct rolling-in operation.
4.
Modify the design to avoid crevices-for example, rolling-in of tubes into the tube sheets
instead of welding.
5.
For heat exchanger tubing, a minimum velocity of
5
ft/s is recommended to keep free of
deposition.
Also,
regular cleaning will reduce susceptibility to crevice corrosion, as well
as to pitting.
6.
During the design stage and operation of tubular heat exchangers, endeavor to provide
uniform velocity throughout the exchanger.
7.
Upgrade the materials of construction. Use a higher alloy, which offers resistance
to
a
broader range
of
conditions. Nitrogen combined with molybdenum has a beneficial effect
on crevice corrosion resistance in chloride-bearing, oxidizing, and acid solutions. Use
materials composed of these elements. High-molybdenum steels, particularly the super-
austenitic stainless steels, give
good
corrosion resistance. Overlaying susceptible alloys
with alloy that is resistant to crevice corrosion may also help.
8.
Keep the crevices wide open or shallow to allow continued entry of the bulk fluid.
9.
Reduce the aggressiveness of the environment, such as acidity, chloride ions concentra-
tion, oxidizing agents, or cathodic reactants.
10.
Wherever possible use solid nonabsorbent gaskets like Teflon for gasketed metal joints
[9].
This prevents the entry of moisture.
11.
Crevice corrosion is not initiated at a specific externally applied potential. Its propagation
can be avoided by polarizing metal outside the crevice (cathode) to the potential of metal
inside the crevice (anode)
[24].
Prediction
of
Crevice Corrosion-Critical Crevice Corrosion Temperature (CCCT).
Tests
that establish the temperature
of
a ferric chloride solution at which crevice corrosion is first
observed on mill-produced alloy samples with crevices, which is referred to as the critical
crevice corrosion temperature (CCCT), are often used to compare the crevice corrosion resis-
tance of various alloys. CCCT is determined as per ASTM Practice
G
48
B
(6%
or 10% FeC13
for
72
h with crevices).

606
Chapter
I2
Intergranular Corrosion
A
localized and preferential form of corrosion attack in a narrow region along the grain bound-
aries or closely adjacent regions without appreciable attack on the grains is called intergranular
corrosion. Due to this form of corrosion, the metal looses its strength and metallurgical corro-
sion. Intergranular corrosion generally takes place because the corrodent preferentially attacks
the grain boundary phase or a zone adjacent to it that has lost an element necessary for adequate
corrosion resistance. The depletion of a particular alloying element along the grain boundaries
is usually caused by improper heat treatment or heat from welding or any other high-tempera-
ture operation that causes the precipitation of certain alloying element at the grain boundary.
Conversely, alloys that do not form second-phase microconstituents at grain boundaries.
or
those in which the constituents have corrosion potentials similar to the matrix, are not suscepti-
ble to intergranular corrosion. It should be noted that the problem of sensitization seldom
occurs in thin sheet metal
[27].
Susceptible
Alloys.
In austenitic stainless steels (18-8) this form
of
attack is most common.
Other susceptible alloys include ferritic, superferritic, and duplex stainless steels. Nonferrous
metals such as nickel
200
and nickel-base alloys like Inconel alloys
600
and 601, Incoloy
alloys
800
and
SOOH,
and Hastelloys
B
and C are susceptible to intergranular corrosion
[
141.
Sensitization of Austenitic Stainless Steels.
During heating of austenitic stainless steels
between 800 and 1500°F (450 and 815°C) while the metal is subjected to welding, heat treat-
ment, or high-temperature exposure, chromium carbides (Cr&) are precipitated along the
grain boundaries. This precipitation causes the steel to loose chromium below 11% and makes
the zone susceptible to corrosion, and this is known as sensitization. Compared to the rest of
the grain, the chromium-depleted region is anodic, and severe attack occurs adjacent to the
grain boundary
if
the metal comes into contact with an electrolyte. In the extreme case, whole
grains become detached from the materials, which are considerably weakened.
Infergranirlar Corrosion Mechanism.
Intergranular corrosion is an electrochemical corrosion
that takes place as the result of local cell action in the grain boundaries. A galvanic cell is
formed due to potential difference between second-phase microconstituents and the depleted
solid solution from which the constituents are formed. The carbide precipitate and the grain
matrix are cathodic to the locally depleted grain boundary region. The high cathode to anode
area ratio results
in
rapid corrosion of the grain boundary material and the metal disintegrates
[271.
Weld Decay.
During welding there will be a region of the HAZ at either side of the weld
bead, which is inevitably sensitized, and these regions are susceptible to intergranular corro-
sion. This phenomenon is invariably termed weld decay, which is an unfortunate choice of
words since, as discussed earlier, welding alone does not cause sensitization of stainless steels
~71.
Control
of
Intergranular Corrosion in Austenitic Stainless Steel.
There are three basic reme-
dies for combating intergranular corrosion of austenic stainless steel:
Heat treatment. Employ
a
suitable high-temperature solution heat treatment, commonly
known as quench annealing or solution annealing. This involves heating the steel to
1976°F
(
1080°C) followed by rapid cooling. High-temperature heating causes decomposi-
tion of the Cr& and homogenization
of
the chromium by diffusion. Rapid cooling is
necessary to prevent the reformation of the carbide.
Low-carbon steel. Ensure that the steel contains insufficient carbon to
form
Crz7Chand
resulting alloy depletion. Such steels contain less than
0.03%
C and are called extra-low-
carbon (ELC) steels, signified by the suffix L (e.g., type 304L and 316L). Type 304L

Corrosion 607’
(O.O3%C max), the low-carbon version of type 304, is now used extensively in applications
calling for resistance to intergranular attack in the welded condition. In a similar manner,
type 316L is the low-carbon version of type 316. Lowering the carbon content bellow
0.03%
is normally achieved by argon oxidation process (AOD) and other modern steel
meltinghefining processes.
3.
Stabilization. It is possible to stabilize
an
austenitic stainless steel, such as the 18Cr-8Ni,
by adding a potent carbide-forming element such as niobium (also known as columbium)
or niobium plus tantalum or titanium. These elements fix the carbon
so
that it is unable to
form Cr&,. The added elements are called stabilizers. It is usual to add titanium or nio-
bium at about 5-10
x
%C in order to ensure that no chromium carbides are formed. Typi-
cal stainless steels produced by adding stabilizers are type
347
(columbium stabilized) and
type 32
1
(titanium stabilized).
Intergranular
Corrosion
in
Ferritic Stainless Steels.
Ferritic stainless steels are susceptible
to intergranular corrosion after being heated to 1700-1800°F (925-982°C) due to welding or
improper heat treatment. It appears that sensitization of ferritic stainless steel occurs under a
wider range
of
conditions than for austenitic steels. This problem is overcome by alloying with
titanium and/or columbium to form the carbides of these elements.
Test
for
Detecting Susceptibility
of
Austenitic Stainless Steels to Intergranular Corrosion.
ASTM A262, Standard Practices for Detecting Susceptibility to Intergranular Attack in Stain-
less Steels, is followed for evaluating austenitic stainless steel HAZ for sensitization. In this
method, if the grain boundaries of the metallographic specimen
are
not preferentially attacked
during etching, the material is said to be “nonsensitized.” On the other hand, if a preferentially
attacked microstructure is developed, other tests must be performed
to
confirm sensitization of
the microstructure. Detailed test methods for various stainless steel grades are given in Chapter
13,
Material Selection and Fabrication.
Dealloying or Selective Leaching
Dealloying or selective leaching (sometimes called parting)
is
a corrosion process
in
which an
alloying element
is
preferentially corroded over others from the parent alloy, leaving behind a
weak structure. The corrosion
is
detrimental largely because it leaves a porous metal with poor
mechanical properties. Dealloying takes place in specific alloy and environment combinations.
Combinations of alloys and environments subject to dealloying and elements preferentially
removed are given in Table
3.
The most common example is dealloying of zinc
in
brass
Table
3
Combinations
of
Alloys and Environments Subject to Dealloying
and Elements Preferentially Removed
[30]
Alloy Environment Element
~ ~~~~~~~~~~~~~
Brasses Waters under stagnant condi- Zinc
tions
Aluminum bronzes Hydrofluoric acid, acids contain- Aluminum
ing chloride ions
Silicon bronzes High-temperature steam and Silicon
acidic species
Copper-nickels High heat flux and low water Nickel
velocity
Monels Hydrofluoric and other acids Copper
in
some
acids and
nickel in others

608
Chapter
12
(copper-zinc alloys), known as dezincification. Specific categories
of
the dealloying process
normally carry the name of the alloying element that is selectively leached out in their titles,
such as dezincification, denickelification, dealuminification, decobaltification, or desiliconifica-
tion.
Factors Influencing Dealloying.
Dealloying is influenced by many critical factors; in general,
factors that increase general corrosion will promote dealloying. However specific accelerating
factors may be further classified into one of the three categories:
(1)
metallurgical, (2) environ-
mental, and
(3)
water chemistry. These factors are explained while discussing dezincification
of brasses.
Dealloying of zinc from brass containing more than
15%
Zn (e.g., yellow brass
70%
Cu:
30% Zn) under conditions of slow-moving water or stagnant water or under deposits is known
as dezincification. Alloys susceptible to dezincification include
C
23000,
C
26000,
C
26800,
C
27000,
C
28000,
C
36500,
C
44300 (uninhibited), and
C
46400.
Dezincification Types.
Two major types of dezinification are
(1)
layer and
(2)
plug type. The
names are taken from the characteristic corrosion product morphologies.
In layer type dezincification, the component surface is converted to corrosion product
to
roughly uniform depth. The alloying generally increases with increasing temperature, and with
increasing chloride content of the cooling water. Since the corrosive wear is normally slight,
visual observation is difficult; only microscopic examination will reveal the damage.
When dealloying is generally restricted to localized areas such as beneath deposits, at hot
spots. or
in
stagnant regions, it is called “plug type” dealloying. Plug type dezincification
produces small pockets of plugs of almost pure copper.
Factors Influencing Dezincification.
Metallurgical.
Metallurgical factors cover the classes
of
copper alloys like brass, alumi-
num bronzes, and cupronickel susceptible to dealloying and the alloying elements parted from
them. This has been given in Table
3.
One important metallurgical factor influencing dezincifi-
cation is known as beta-phase attack.
For beta-phase attack, in the two-phase (alpha and beta) alloys such as Muntz and naval
brasses, dezincification may be concentrated initially on the beta phase and may be sufficient
to weaken the metal. If the attack spreads to both alpha and beta phases, complete dezincifica-
tion may result with the formation of a layer of porous copper 1311.
Environment. Stagnant conditions, deposits, high heat flux, crevice condition, and
stresses accelerate dealloying. Porous or granular deposits further enhance the attack.
In common with many other types of corrosion and chemical reactions, an increase in
temperature accelerates the rate
of
dezincification of brasses. At lower temperature, the rate of
dezincification
is
low, and this explains the long life obtained from naval brass and Muntz
metal piping, tubing, plates, sheets, etc. used in contact with cooling brine [31].
In terms of water chemistry, soft water, high concentration of dissolved carbon dioxide,
acidic or high-pH conditions, and high chlorine concentrations promote dezincification.
Waters that deposit magnesium, calcium or iron silicates, or silica scale are protective to
the underlying brass. Dense adherent calcium and iron carbonate scales are also effective in
reducing or preventing dezincification. But thick scales are objectionable for well-known rea-
sons
[31].
Preivntion
of
Dealloying-General.
Dealloying is overcome by material substitution, surface
cleanliness. and chemical treatment. The most common method of preventing dealloying
is
to

Co
r
ro
s
ion
609
ensure that the tubes are kept clean and free of deposits by screening methods and cleaning
procedures. Stagnant conditions should also be avoided.
Prevention
of
Dezincifcation.
Though dezincification can be minimized by reducing the ag-
gressiveness of the environment, (e.g., oxygen removal) or by cathodic protection [9], it is
mostly overcome by substituting alloys immune to or less susceptible to this form of attack.
The resistance of brass is greatly increased by the addition of a little arsenic, antimony, and
phosphorus, and the resulting alloy is known as inhibited admiralty brass. As a result, inhibited
grades of brasses are routinely used in condensers. Typical alloys resistant to dezincification
include:
Admiralty brass, developed by addition of tin
Inhibited admiralty brass, developed by the addition of inhibitors such as phosphorus, anti-
mony, or arsenic
Alternate alloys like aluminum brass (inhibited), cupronickel, and titanium. Among the copper
alloys resistant to dealloying, Cu-Ni alloys are considerably more resistant than Cu-Zn
alloys.
Erosion-Corrosion
Erosion-corrosion, a form of localized corrosion, takes place due to the movement of a fluid
over a material surface. It takes place mostly on the tube side with water flowing through it.
The corrosion damage involves both mechanical and chemical factors that allow the corrosion
to proceed unhindered. The relative importance of mechanical wear (erosion) and corrosion is
often difficult to assess and varies greatly from one situation to the other [28]. The role of
erosion is usually attributed to the removal of protective surface film or adherent corrosion
products by the fluid shear stress under high turbulence conditions.
Erosion-corrosion is usually accelerated when the fluid is entrained with air or abrasive
solid particles, such as sand, but erosion-corrosion can also occur in filtered, bubble-free water
[26]. The nature and properties of the protective films that form on some metals or alloys are
very important from the standpoint of resistance to erosion-corrosion. A hard, dense, adherent,
and continuous film would provide better protection than one that is easily removed by me-
chanical means or hydraulic force [9]. Erosionxorrosion is normally restricted to copper and
certain copper-base alloys and aluminum alloys. In this section, erosion-corrosion is discussed
in two different forms:
(1)
erosion-corrosion, and
(2)
erosive wear.
Parameters
Influencing
Erosion-Corrosion.
Erosion-corrosion
is
influenced by two parame-
ters:
(1)
turbulence and parameters related to process fluids, such as fluid velocity, impinge-
ment attack, level of suspended particles, aeration, bubble level, local partial pressure, cavita-
tion, etc., and (2) flow geometry. Turbulence increases with increasing velocity
so
that higher
velocities favor the initiation of erosion-corrosion. Turbulence intensity is higher at tube inlets
than downstream, resulting in the phenomenon of inlet-end erosion-corrosion. Similarly, on
the shell side, the peripheral tubes located beneath the inlet nozzle without impingement plate
protection are most affected by turbulence. Erosion-corrosion also occurs adjacent to a partial
blockage in a tube where local velocities through the restricted opening are high or in the
turbulent region just downstream of the blockage.
Pattern of Erosion-Corrosion. Erosion-corrosion mostly exhibits a directional property
and is characterized by directional grooves, waves, valleys, gullies, holes, etc. [9], or pits in
the shape
of
horseshoes at the site where the protective surface film is damaged
[32],
or
crescent-shaped indentations facing upstream of the water flow that are often influenced by
the local flow conditions; consequently, this form of attack has been sometimes referred as
61
0
Chapter
I2
“horseshoe,” “star,” “crescent,” and “slot” attack
[26].
A
typical pattern of erosion-corrosion
is shown in Fig. 8b (item d).
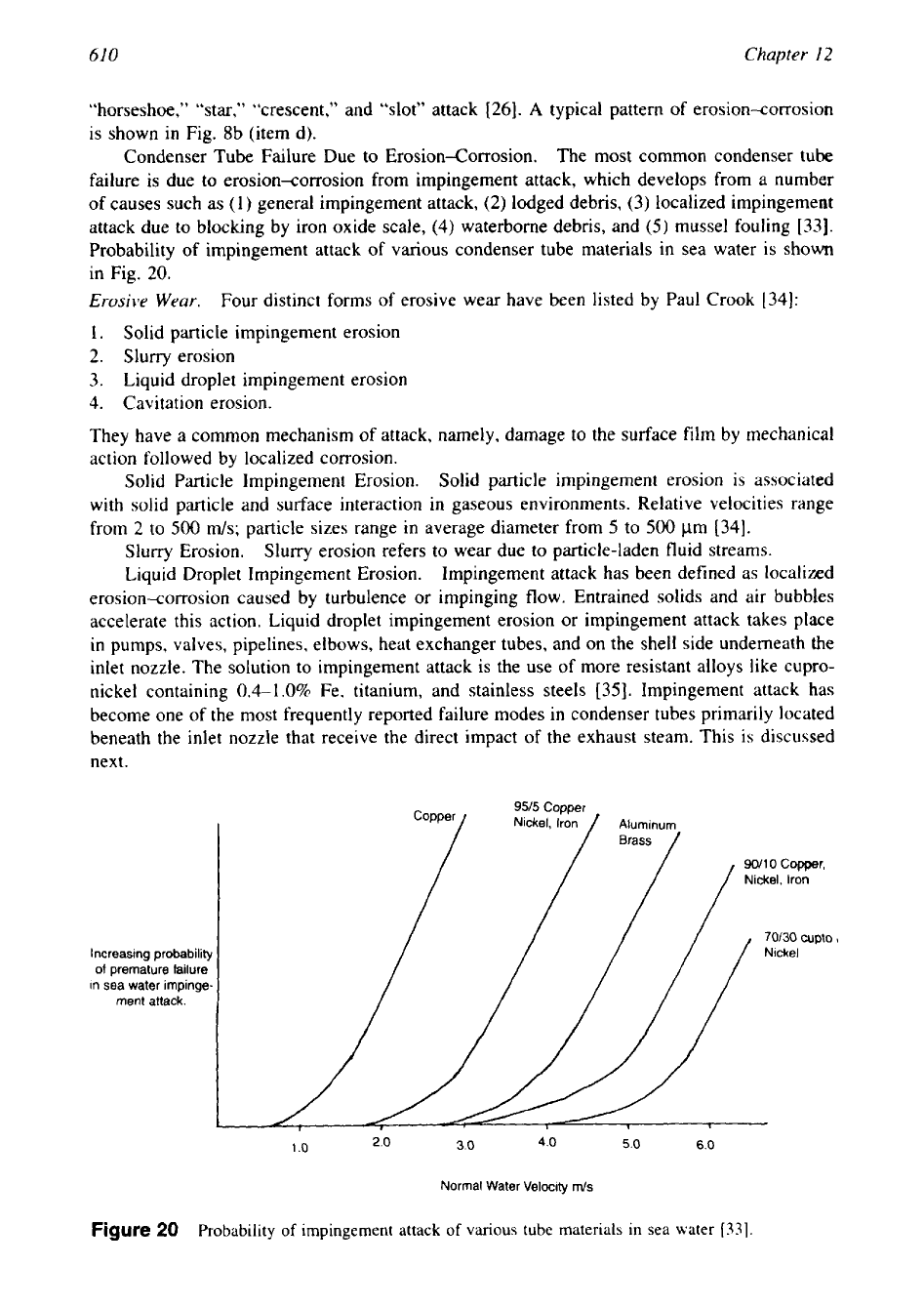
Condenser Tube Failure Due to Erosion-Corrosion. The most common condenser tube
failure is due to erosion-corrosion from impingement attack, which develops from a number
of causes such as (1) general impingement attack,
(2)
lodged debris,
(3)
localized impingement
attack due to blocking by iron oxide scale,
(4)
waterborne debris, and
(5)
mussel fouling [33].
Probability
of
impingement attack of various condenser tube materials in sea water is shown
in Fig.
20.
Erosive
Wear.
Four distinct forms of erosive wear have been listed by Paul Crook [34]:
1.
Solid particle impingement erosion
2.
Slurry erosion
3.
Liquid droplet impingement erosion
4.
Cavitation erosion.
They have a common mechanism of attack, namely, damage to the surface film by mechanical
action followed by localized corrosion.
Solid Particle Impingement Erosion.
Solid particle impingement erosion is associated
with solid particle and surface interaction in gaseous environments. Relative velocities range
from
2
to
500
ds;
particle sizes range in average diameter from
5
to
500
pm
[34].
Slurry Erosion. Slurry erosion refers to wear due to particle-laden fluid streams.
Liquid Droplet Impingement Erosion.
Impingement attack has been defined as localized
erosion-corrosion caused by turbulence or impinging flow. Entrained solids and air bubbles
accelerate this action. Liquid droplet impingement erosion or impingement attack takes place
in pumps, valves, pipelines, elbows, heat exchanger tubes, and on the shell side underneath the
inlet nozzle. The solution to impingement attack is the use
of
more resistant alloys like cupro-
nickel containing 0.4-1
.O%
Fe, titanium, and stainless steels
[35].
Impingement attack has
become one
of
the most frequently reported failure modes in condenser tubes primarily located
beneath the inlet nozzle that receive the direct impact
of
the exhaust steam. This
is
discussed
next.
95J5
Copper
90/10
Copper,
Nickel, iron
70/30
Cupto
I
increasing probability Nickel
of premature failure
in
sea water impinge-
I
/ /
/ /
1
.O
2
.o
3.0
4.0
5.0
6.0
Normal Water Velocity
m/s
Figure
20
Probability
of
impingement attack
of
various
tube materials in sea water
[33].

Corrosion
61
1
Impingement Attack or Steam-Side Erosion of Condenser.
Impingement attack,
so
called
steam-side erosion, has been discussed in detail in ref. 26. The problem arises when water
droplets entrained in the steam enter the condenser and impact on the tubes at high velocity.
While corrosion may play a small part in the overall process, purely mechanical processes are
considered more important.
To
resist impingement attack, stainless steel and titanium tubes are
superior to copper alloys. For this reason, it is common practice to install stainless steels or
titanium tubes, at least in the peripheral sections of condensers where high erosion resistance
is required. When thin-walled titanium tubes are used in the peripheral sections. adequate tube
support must be provided to avoid the flow-induced vibration (FIV) problems. Properly de-
signed condenser necks will reduce the collection and development of big droplets, which are
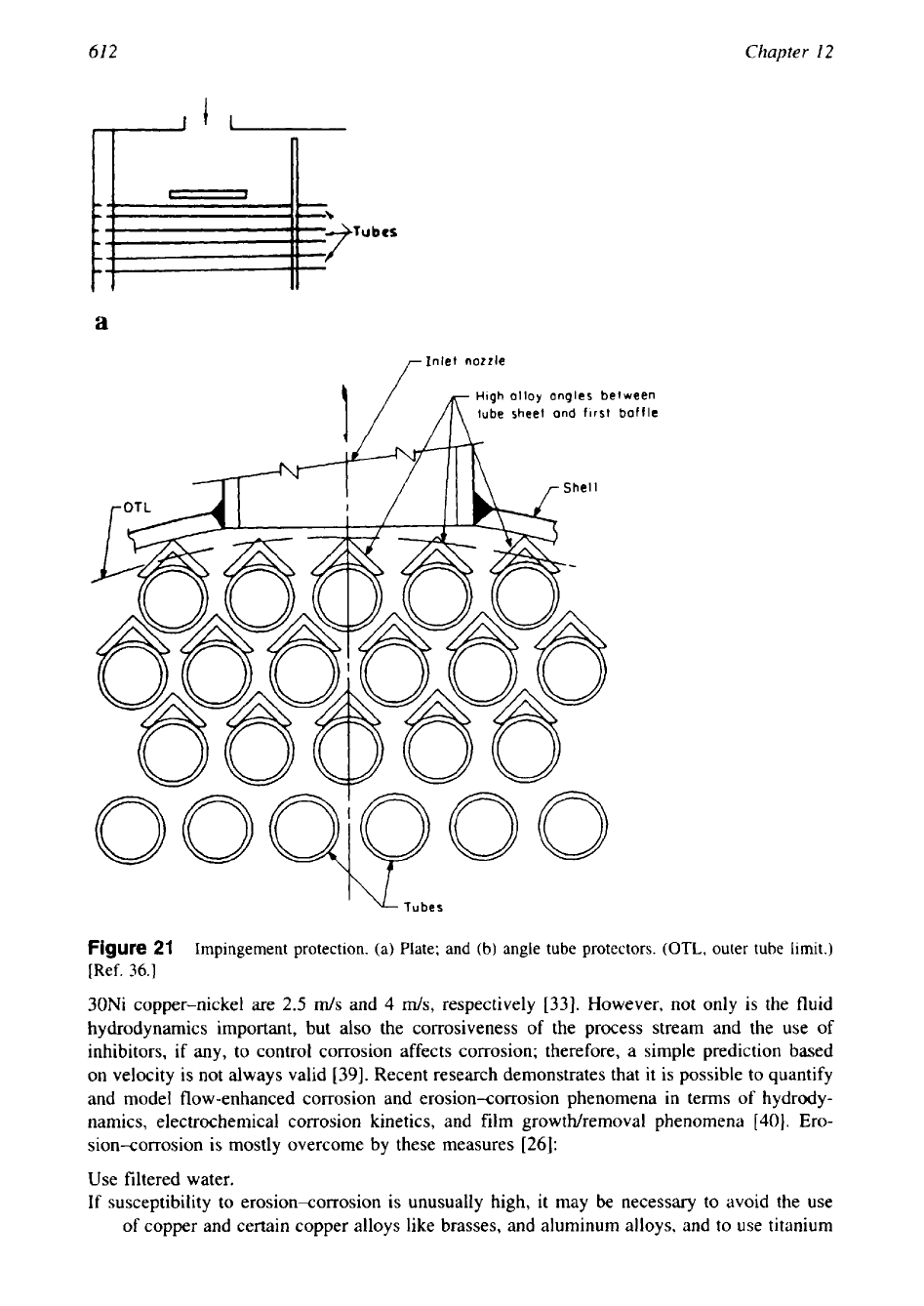
especially harmful. Other popular methods of controlling impingement attack are shown Fig.
21.
Figure 21a shows the installation of impingement plate protection, and Fig. 21b shows
clip-on or angle tube protectors made from a more erosion-resistant material
[36].
TEMA
[37]
Guidelines to Limit Impingement Attack. On the shell side, an impingement
plate or other means to protect the tube bundle against impinging fluids shall be provided when
entrance line values of
pV’
exceed the following (where
V
is the velocity of the fluid in ft/s
and
p
is fluid density in lb/ft’):
1.
Noncorrosive, nonabrasive, single-phase fluids,
1500
2.
All other liquids, including a liquid at its boiling point,
500
In no case shall the shell or bundle entrance or exit area produce a value
of
pV2
in excess of
4000.
On the tube side, consideration shall be given to the need for special devices to prevent
erosion of the tube ends under the following conditions:
1.
Use of an axial inlet nozzle.
2.
Liquid
pV’
is in excess of 6000.
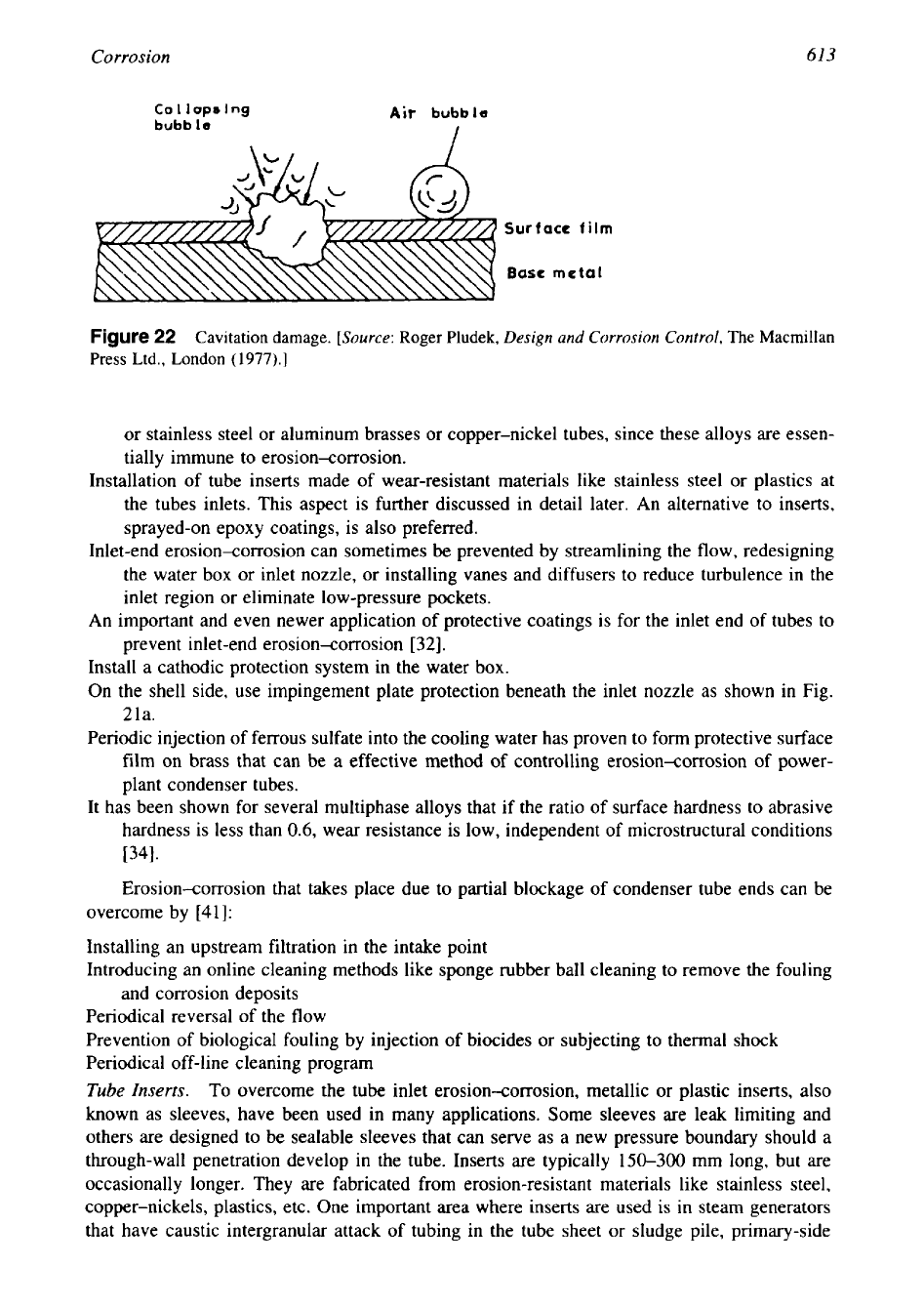
Cavitation.
Cavitation damage, sometimes referred to as cavitation corrosion or cavitation
erosion, is a form of localized corrosion combined with mechanical damage that occurs
in
turbulent flow or high-velocity fluids. It takes the form of areas or patches of pitted or rough-
ened surface
[38].
Cavitation is caused by rapid formation and collapse of vapor bubbles (i.e.,
voids or cavities), which exert high pressure forces at the metal surface; these high-pressure
forces can deform the underlying metal and remove protective surface films and form pits on
surfaces. The occurrence of cavitation damage is shown schematically in Fig. 22. The bubbles
are created
as
a result of turbulence or when pressure in liquid falls below its vapor pressure.
Collapse is caused by subsequent pressure increase. Among various factors, the surface finish
plays an important rule
in
the formation of bubbles. Smooth surfaces are beneficial since they
reduce the number of sites for bubble formation
[28].
Control of Cavitation Erosion.
Cavitation damage involves both physical as well as elec-
trochemical processes. Cavitation resistance increases as the grain size of a metal becomes
smaller. Among the alloys, stainless steels
is
more resistant to cavitation damage because of
its ductility, toughness, high corrosion fatigue limit, homogeneity, fine grain size, and ability
to work harden
(38).
Preventing cavitation damage requires the use of the most resistant alloys
and designing the system to avoid turbulence and cavitation.
Control
of
Erosion-Corrosion.
Current engineering practice limits fluid velocities in tubes,
pipes, and heat exchangers to some arbitrary value based on empirical tests or field experience
[39].
The maximum allowable velocity is known as the threshold velocity or critical velocity.
Below the critical velocity, the impingement attack does not occur, and above it,
it
increases
rapidly. For example, the critical velocity for the protective film on aluminum brass and 70Cu-
61
2
Chapter
12
-1,
a
r
Inlet nozzle
High
alloy
angles be
t
ween
1/
m
tube sheet and
first
baffle
'
\L.
Tubes
Figure
21
Impingement protection.
(a)
Plate; and (b) angle tube protectors.
(OTL,
outer tube
imit.)
[Ref.
36.1
30Ni copper-nickel are
2.5
m/s
and
4
ds,
respectively
[33].
However, not only
is
the fluid
hydrodynamics important, but also the corrosiveness of the process stream and the use
of
inhibitors, if any, to control corrosion affects corrosion; therefore,
a
simple prediction based
on velocity is not always valid
[39].
Recent research demonstrates that it is possible to quantify
and model flow-enhanced corrosion and erosion-corrosion phenomena in terms
of
hydrody-
namics, electrochemical corrosion kinetics, and film growthhemoval phenomena
[40].
Ero-
sion-corrosion is mostly overcome by these measures
[26]:
Use filtered water.
If
susceptibility to erosion-corrosion is unusually high, it may
be
necessary to avoid the use
of copper and certain copper alloys like brasses, and aluminum alloys, and to use titanium
Corrosion
61
3
CO
11
ops
lng
Air
bubble
bubb
1
e
J
Figure22
Cavitation damage.
[Source:
Roger Pludek,
Design and
Corrosion Control,
The
Macmillan
Press Ltd., London
(1977).]
or stainless steel or aluminum brasses or copper-nickel tubes, since these alloys are essen-
tially immune to erosion-corrosion.
Installation of tube inserts made of wear-resistant materials like stainless steel or plastics at
the tubes inlets. This aspect is further discussed in detail later. An alternative to inserts,
sprayed-on epoxy coatings, is also preferred.
Inlet-end erosion-corrosion can sometimes be prevented by streamlining the flow, redesigning
the water box or inlet nozzle, or installing vanes and diffusers to reduce turbulence in the
inlet region or eliminate low-pressure pockets.
An important and even newer application of protective coatings is for the inlet end of tubes to
prevent inlet-end erosion<orrosion
[32].
Install a cathodic protection system in the water box.
On the shell side, use impingement plate protection beneath the inlet nozzle as shown in Fig.
21a.
Periodic injection of ferrous sulfate into the cooling water has proven to form protective surface
film on brass that can be a effective method of controlling erosion-corrosion of power-
plant condenser tubes.
It has been shown for several multiphase alloys that if the ratio of surface hardness to abrasive
hardness is less than
0.6,
wear resistance is low, independent of microstructural conditions
Wl.
Erosion-corrosion that takes place due to partial blockage of condenser tube ends can be
overcome by
[41]:
Installing an upstream filtration in the intake point
Introducing an online cleaning methods like sponge rubber ball cleaning to remove the fouling
and corrosion deposits
Periodical reversal of the flow
Prevention of biological fouling by injection of biocides or subjecting to thermal shock
Periodical off-line cleaning program
Tube
Inserts.
To overcome the tube inlet erosion-corrosion, metallic or plastic inserts, also
known as sleeves, have been used in many applications. Some sleeves are leak limiting and
others are designed to be sealable sleeves that can serve as a new pressure boundary should a
through-wall penetration develop in the tube. Inserts are typically 150-300 mm long, but are
occasionally longer. They are fabricated from erosion-resistant materials like stainless steel,
copper-nickels, plastics, etc. One important area where inserts are used is in steam generators
that have caustic intergranular attack of tubing in the tube sheet or sludge pile, primary-side
