Kuppan T. Heat Exchanger Design Handbook
Подождите немного. Документ загружается.


Chapter
I2
1.5
Corrosion Kinetics
The preceding discussion deals with the criteria leading to the formation of an electrolytic cell,
which is the essential step in the corrosion process. However, the potential difference of the
galvanic couple can change with time.
As
corrosion progresses, corrosion products may accu-
mulate at the anode, cathode, or both. This reduces the speed at which corrosion proceeds. The
phenomena affecting the corrosion kinetics are referred to as polarization and passivation.
These two phenomena are also extremely important in the preventive measures that can be
used for corrosion control.
Polarization Effects
The phenomenon that controls the rate of corrosion reaction is known as polarization, which
is the ease with which anodic and cathodic reactions take place. The principle of polarization
effects is as follows.
As
soon as current begins to flow through an electrolytic cell, it produces
chemical changes at the electrodes, and these changes tend to set up a new voltaic cell with a
voltage in the opposite direction to that of the main cell voltage. This new countervoltage
is
known as polarization, and it always opposes the main voltage of the cell;
it
never reinforces
it. In simple terms, due to polarization the potentials of the metals in a corrosion cell tend
to
approach each other. The decrease in anode potential is anodic polarization, and the decrease
in cathode potential is cathodic polarization.
This
reduced voltage can drive less additional
current through the cell. It is not always true that both anodic and cathodic polarization will
take place
to
the same extent. In some cases, greater polarization is at the anode and in other
cases at the cathode. In the former case, the reaction is said to be anodically controlled. In the
latter case, it is said to be cathodically controlled.
Polarization
in
Iron-
Water System.
In the iron-water system, the reaction is cathodically
controlled because hydrogen ions are available in small quantity. In other words, cathodic
polarization limits the rate of reaction
[3].
Oxygen is a depolarizer because it decreases the
slope of one of the lines, thereby increasing the corrosion current and, in this reaction, the
amount of corrosion. A little consideration of this will indicate the importance of polarization
in limiting corrosion rate without it reaching an infinite value, which would have been the case
in the absence of any polarization. It can take the form of slow ion movement in the electrolyte,
slow combination of atoms to form gas molecules, or slow solvation
of
ions by electrolyte
[I].
Factors Afecting Polarization.
The degree of polarization is variable; some corrosion reac-
tions proceed rapidly owing to high spontaneity and low polarization, and others proceed very
slowly owing
to
high polarization even though they have a pronounced tendency to corrode as
shown by reversible emf of the corrosion cell
[4].
Factors affecting polarization include the
following
[
11:
1.
Increasing the reaction area allows the corrosion to take place more readily and hence
lowers the rate of polarization.
2.
Agitation or electrolyte movement carries away the products of corrosion reaction from
the surface and thereby provide a maximum number of ions contacting the electrodes, thus
increasing the rate of corrosion and decreasing the polarization. On the other hand, if the
cathodic reaction is activation controlled, agitation would have no effect on the corrosion
rate.
3.
Oxygen effectively depolarizes the electrode or makes the reaction go more rapidly by
removing the reaction product atomic hydrogen.
4.
Increasing the temperature increases the rate of most reactions, and therefore lowers the
polarization rate.
Corrosion
585
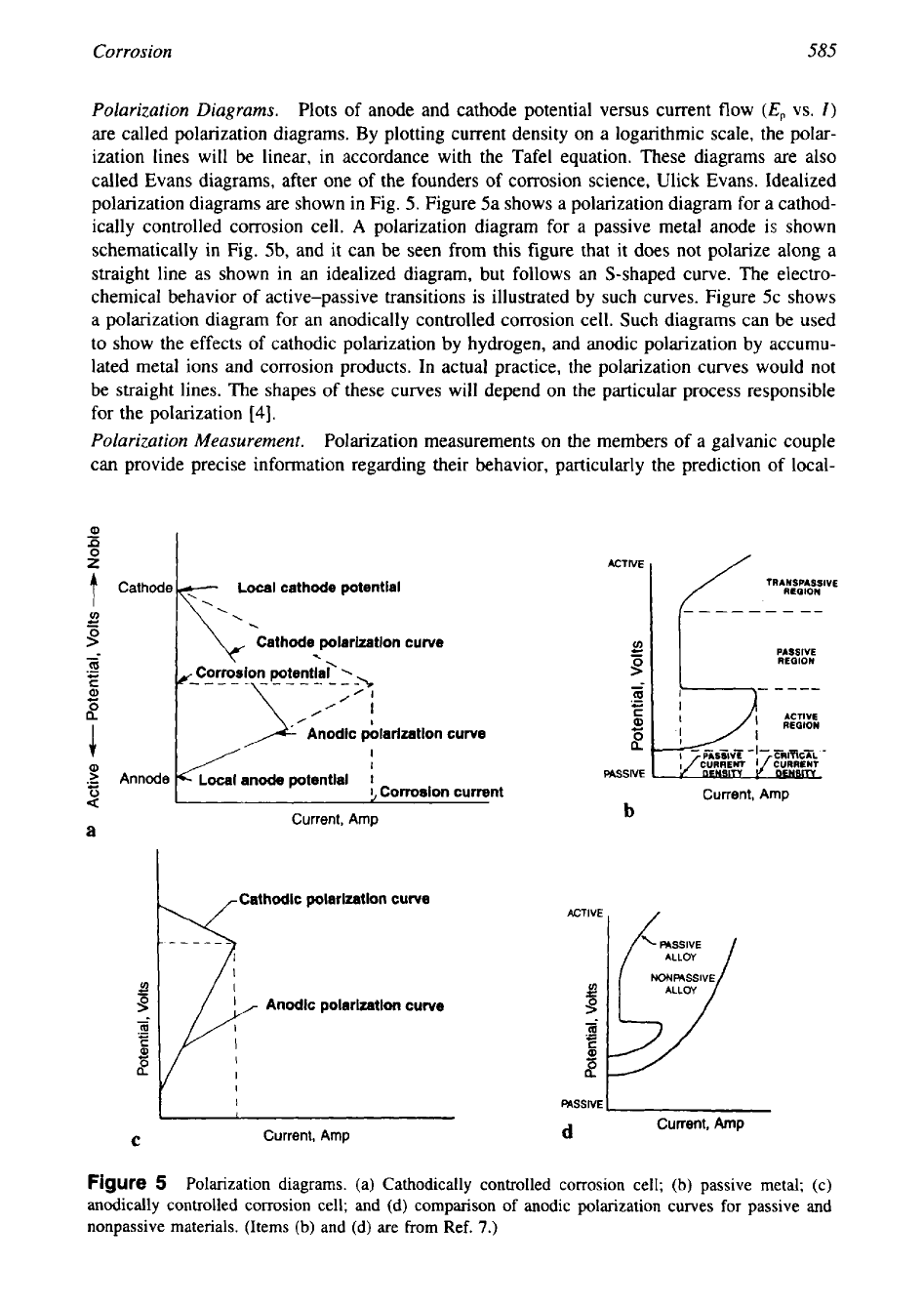
Polarization Diagrams.
Plots of anode and cathode potential versus current flow
(E,
vs.
I)
are called polarization diagrams. By plotting current density on a logarithmic scale, the polar-
ization lines will be linear, in accordance with the Tafel equation. These diagrams are also
called Evans diagrams, after one of the founders of corrosion science, Ulick Evans. Idealized
polarization diagrams are shown in Fig.
5.
Figure 5a shows a polarization diagram for a cathod-
ically controlled corrosion cell.
A
polarization diagram for a passive metal anode is shown
schematically in Fig. 5b, and it can be seen from this figure that it does not polarize along a
straight line as shown in an idealized diagram, but follows an S-shaped curve. The electro-
chemical behavior
of
active-passive transitions
is
illustrated by such curves. Figure 5c shows
a polarization diagram for an anodically controlled corrosion cell. Such diagrams can be used
to show the effects of cathodic polarization by hydrogen, and anodic polarization by accumu-
lated metal ions and corrosion products. In actual practice, the polarization curves would not
be straight lines. The shapes of these curves will depend on the particular process responsible
for the polarization
[4].
Polarization Measurement.
Polarization measurements on the members of a galvanic couple
can provide precise information regarding their behavior, particularly the prediction of local-
ACTIVE
Local cathode potential
TRANSPASSIVE
AEQION
____-----
f-
PASSIVE
9
REGION
I
0
'I
I
Anodic polarization
curve
/
I
Q,
I
anode potential
I
I,
Corrosion current
Current,
Amp
b
Current,
Amp
Cathodic polarization curve
ACTIVE
(J
NONPASSIVE
/I
U)
r
/A
Anodic polarization curve
-
9
.-
U
$2
a0
/ I
I
PASSIVE
Current,
Amp
d
Current,
Amp
Figure
5
Polarization diagrams. (a) Cathodically controlled corrosion cell;
(b)
passive metal; (c)
anodically controlled corrosion cell; and
(d)
comparison
of
anodic polarization curves
for
passive and
nonpassive materials. (Items
(b)
and (d) are from
Ref.
7.)

586
Chapter
12
ized corrosion. Polarization techniques and critical potentials are used to measure the suscepti-
bility to pitting and crevice corrosion of metals and alloys in a chloride solution
[5].
Passivation
Sometimes material corrodes, producing an adherent corrosion product that protects it from
further corrosion. Such (passivated) material corrodes very little in a specific environment,
even though it would otherwise corrode considerably
[6].
For example, a look at the galvanic
series will indicate that aluminum should corrode at a high rate. In practice, however,
it
is
found that aluminum is highly resistant to attack in most of the media except halides. This
phenomenon is known as passivation. Materials such as nickel, titanium, zirconium, chromium,
and stainless steel owe their corrosion resistance to natural passivation.
Passivity can be understood through a study of polarization diagrams (schematic) pre-
sented by Roser et al.
[7].
The anodic polarization curves of passive alloys shown
in
Fig.
5b
are distinctly different from those of nonpassive alloys. Comparison of anodic polarization
curves for passive and nonpassive materials is shown in Fig. 5d. Passivation
is
a result of
marked anodic polarization whereby a barrier of thin protective film, either metal oxide or
chemisorbed oxygen, is formed between the metal and the environment, preventing further
contact with the electrolyte. In the case of iron, when more oxygen reaches the metal surface
than can be used in the cathodic reaction, a protective passive film is able to form
131.
Thus,
the attainment of passivity is thus most important
in
avoiding accelerated corrosion. Whether
a given alloy will be passive in a given situation depends on both the anodic and cathodic
polarization effects.
Passive alloys are widely used as corrosion-resistant materials for the construction of heat
exchangers. The corrosion resistance of passive alloys depend on the chromium content, chlo-
ride and oxygen content in the environment, and the temperature
[7].
Attainment of passivity
in a given situation depends on the relative value of all factors rather than on any one of them.
For example, high chromium aids passivity, low temperature aids passivity, depassivating ions
such as chlorides hinder passivity, and oxygen aids passivity.
Behavior
of
Passive
Alloys.
Passive material corrodes very little in a specific environment,
even though
it
would otherwise corrode considerably
[6].
Conversely, alloys that commonly
exhibit passivity are invariably quite active in the nonpassive state. Some elements break down
passive films, causing the metal to corrode where the film is discontinuous. Chlorine ions, for
example, destroy the passivity of aluminum, iron, and the stainless steels, causing pitting corro-
sion. Therefore, the users of passive alloys should be particularly on guard for pitting, stress
corrosion cracking, sensitization, and oxygen starvation type corrosion
[7
1.
1.6
Factors Affecting Corrosion
of
a Material in an Environment
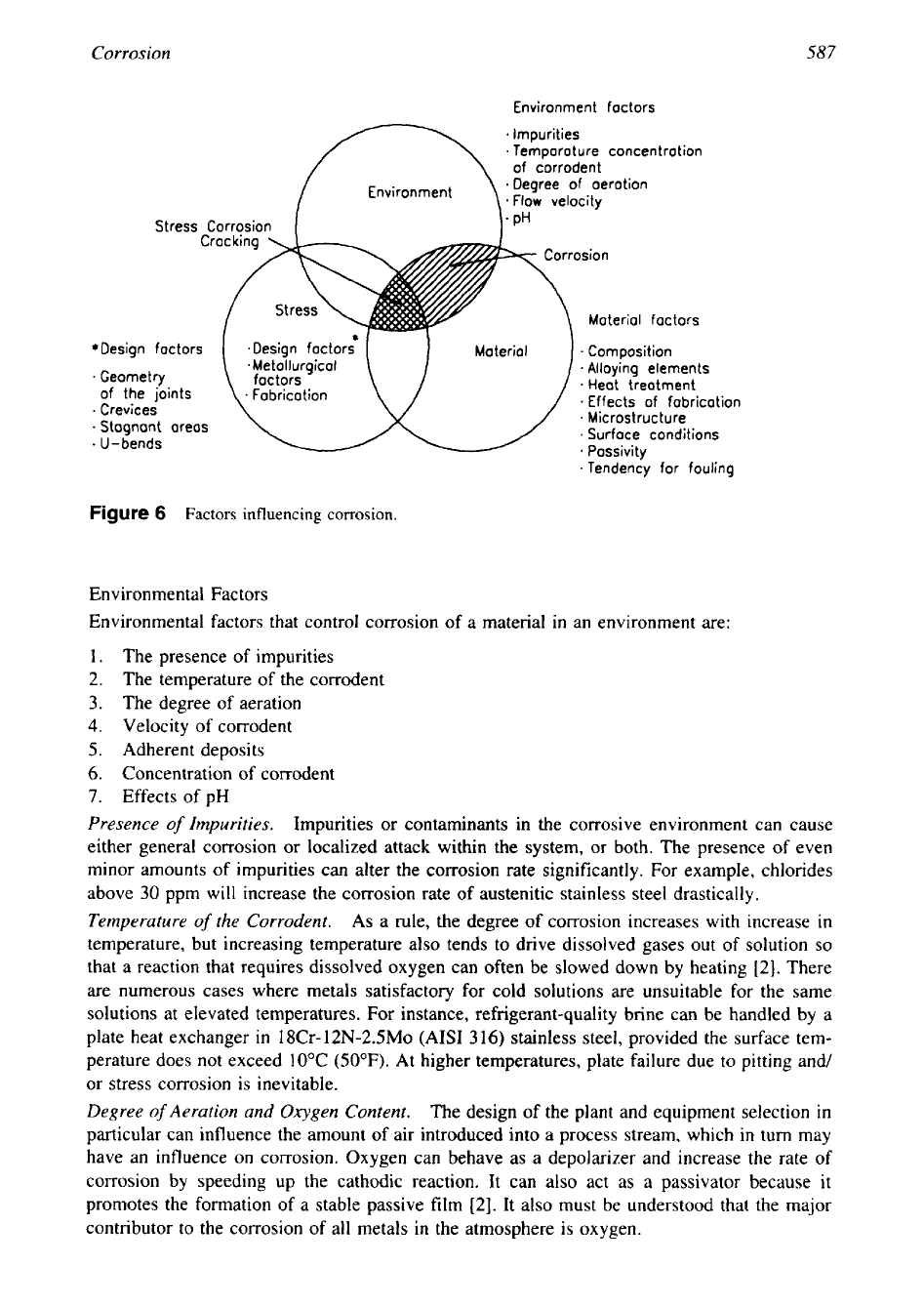
The corrosion process is affected by various parameters:
1.
Environment factors such as concentration of chemicals, pH, velocity, impurities and sus-
pended matter, and temperature of the medium.
2.
Source of heat, if any. If the environment is heated through the material being selected,
the effects of heat transfer and surface temperature may be the controlling factors.
3.
Material factors like composition, alloying elements, passivity, tendency for fouling, etc.
4.
Design conditions and geometry of the joints, like gasketed surfaces, crevices, stagnant
areas, and U-bends.
5.
Fabrication techniques: corrosion due to welding, brazing, soldering, and heat treatment.
Factors influencing corrosion is shown schematically in Fig.
6.
Only the environmental factors
are discussed next. The other factors are discussed while discussing various forms of corrosion.
Corrosion
587
Environment factors
Temporoture concentrotion
of corrodent
-
Degree of oerotion
Stress Corrosio
Material factors
Moteriol
.
Composition
Metollurgicol
-
Alloying elements
-
Geometry
factors
of the joints
-
Fabrication
*
Heat treotment
+
Effects of fobricotion
Crevices
-
Microstructure
Stognont
oreas
.
Surfoce conditions
-
U-bends
*
Possivity
.
Tendency for fouling
Figure
6
Factors
influencing corrosion.
Environmental Factors
Environmental factors that control corrosion of a material in an environment are:
1. The presence of impurities
2.
The temperature of the corrodent
3.
The degree of aeration
4.
Velocity
of
corrodent
5.
Adherent deposits
6.
Concentration
of
corrodent
7.
Effects
of
pH
Presence
of
Impurities.
Impurities or contaminants in the corrosive environment can cause
either general corrosion or localized attack within the system, or both. The presence of even
minor amounts
of
impurities can alter the corrosion rate significantly. For example, chlorides
above
30
ppm will increase the corrosion rate
of
austenitic stainless steel drastically.
Temperature
of
the Corrodent.
As a rule, the degree
of
corrosion increases with increase in
temperature, but increasing temperature also tends to drive dissolved gases out of solution
so
that a reaction that requires dissolved oxygen can often be slowed down by heating
[2].
There
are numerous cases where metals satisfactory for cold solutions are unsuitable for the same
solutions at elevated temperatures. For instance, refrigerant-quality brine can be handled by a
plate heat exchanger
in
18Cr-12N-2.5Mo (AISI
316)
stainless steel, provided the surface tem-
perature does not exceed 10°C (50°F). At higher temperatures, plate failure due to pitting and/
or stress corrosion is inevitable.
Degree
of
Aeration
and
Oxygen
Content.
The design of the plant and equipment selection
in
particular can influence the amount
of
air introduced into a process stream, which in turn may
have an influence on corrosion, Oxygen can behave as a depolarizer and increase the rate of
corrosion by speeding up the cathodic reaction. It can also act as a passivator because it
promotes the formation of a stable passive film [2]. It also must be understood that the major
contributor to the corrosion of all metals in the atmosphere is oxygen.
588
Chapter
I2
Velocity
of
Corrodent.
Velocity of the corrodent affects both the type and severity of the
corrosion and removal of fouling deposits. Corrosion is favored by too low or too high veloci-
ties. Uniform and constant flow of process fluids past heat exchanger favors less fouling and
hence less corrosion. High velocity helps to prevent the accumulation and deposition of corro-
sion products, which might create anodic sites to initiate corrosion, to maintain clean surfaces
free from fouling deposits, avoid crevices, and stagnant areas. On the other hand, too high a
velocity can destroy the protective surface film and result in erosion-corrosion, especially on
metals such as copper and aluminum alloys.
Adherent Deposits.
Deposits on the metal surface cause crevices. These act as sites for accel-
erated corrosion. Adherent deposits cause localized hot spots, which in turn contribute to high-
temperature corrosion.
Concentration
of
Corrodent.
In general, the corrosion rate increases with increasing concen-
tration, including the concentration of the aggressive chemical species such as chloride ions.
However, there are exceptions also. For example, iron is attacked vigorously
in
dilute nitric
and sulfuric acids, but the corrosion rate is drastically reduced
in
concentrated acids due to
passivation of the metal surfaces when once corrosion has begun.
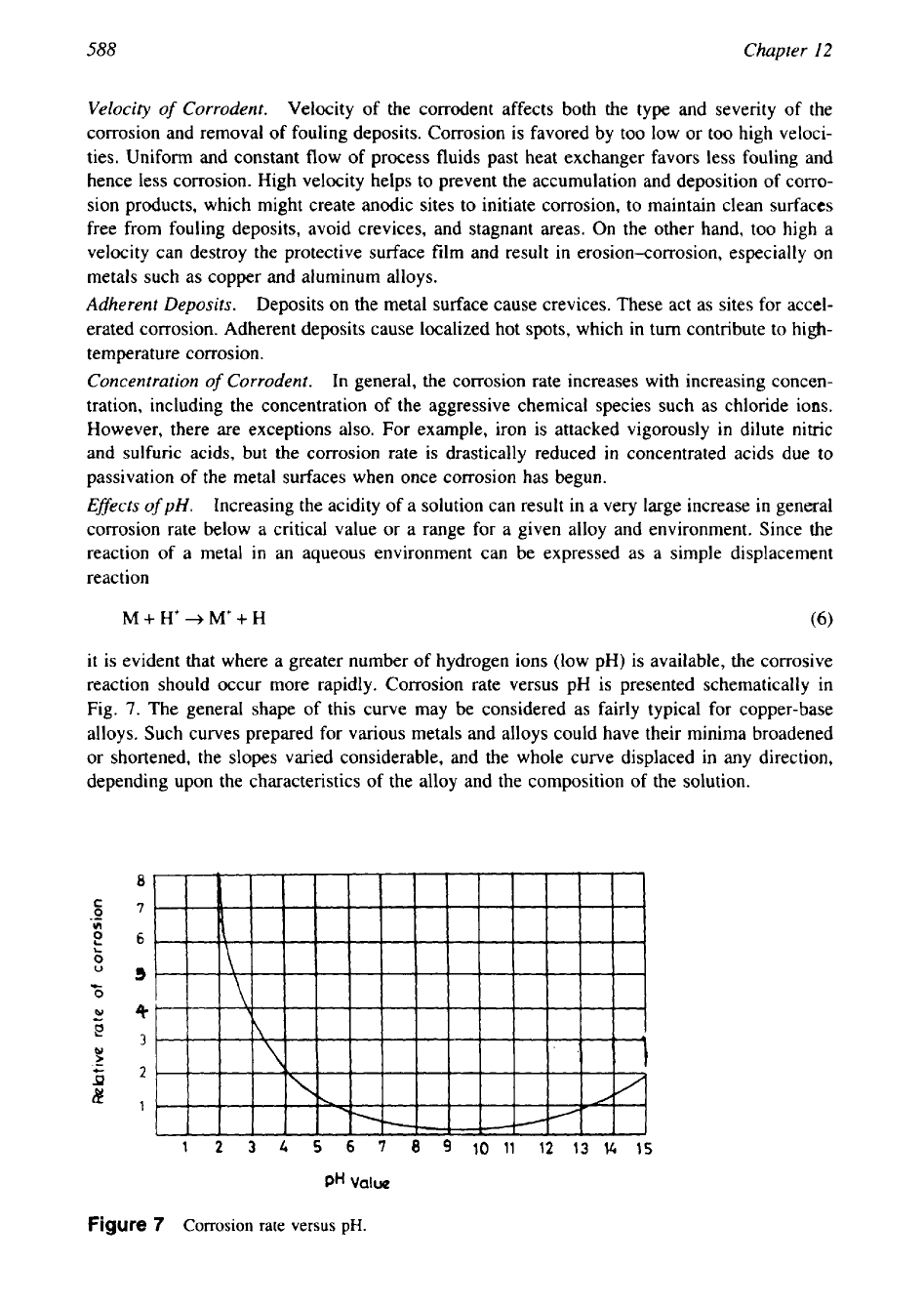
Efsects
of
pH.
Increasing the acidity of a solution can result in a very large increase in general
corrosion rate below a critical value or a range for a given alloy and environment. Since the
reaction of a metal
in
an
aqueous environment can be expressed as a simple displacement
reaction
M
+
H'
-+
M'+
H
(6)
it is evident that where a greater number of hydrogen ions (low pH)
is
available, the corrosive
reaction should occur more rapidly. Corrosion rate versus pH is presented schematically in
Fig.
7.
The general shape of this curve may be considered as fairly typical for copper-base
alloys. Such curves prepared for various metals and alloys could have their minima broadened
or shortened, the slopes varied considerable, and the whole curve displaced in any direction,
depending upon the characteristics of the alloy and the composition of the solution.
1
2
3
4
5
6
7
8
9
1011
12
1314
15
PH
Value
Figure
7
Corrosion
rate versus
pH.

Corrosion
589
2
FORMS OF CORROSION
2.1
Uniform Corrosion Versus Localized Corrosion
Corrosion attack on the metal surfaces can be either uniform or localized, where the major part
of the surface of the metal remains almost unaffected while certain localized areas are attacked
at a very high rate with rapid penetration into the section of the metal. Uniform corrosion
occurs during the corrosion of a metal in
an
acid, alkali, and during the exposure of certain
metals to natural environments like air, soil, etc. In general, uniform corrosion takes place
when the metal and environment system is homogeneous; that is, the metal is uniform in
composition and structure and the nature of the environment (composition, oxygen concentra-
tion, acidity/alkalinity), temperature, velocity, etc. are the same at all parts of the metal surface
[4]. Conversely, in many metals and environment systems, due to heterogeneities in the metal
or variations in the environment or both, corrosive attack may be localized. Various forms of
localized corrosion are pitting corrosion, crevice corrosion, intergranular corrosion, dealloying,
erosion-corrosion, etc.
2.2
Factors That Favor Localized Attack
For metals and alloys, grain boundaries, intermetallic phases, inclusions, impurities, regions
that differ in their mechanical or thermal treatments, discontinuities on metal surface such
as
cut edges or scratches, discontinuities in oxide or passive films or in applied metallic or nonme-
tallic coatings, and geometrical factors such as crevices favor localized attack
[4].
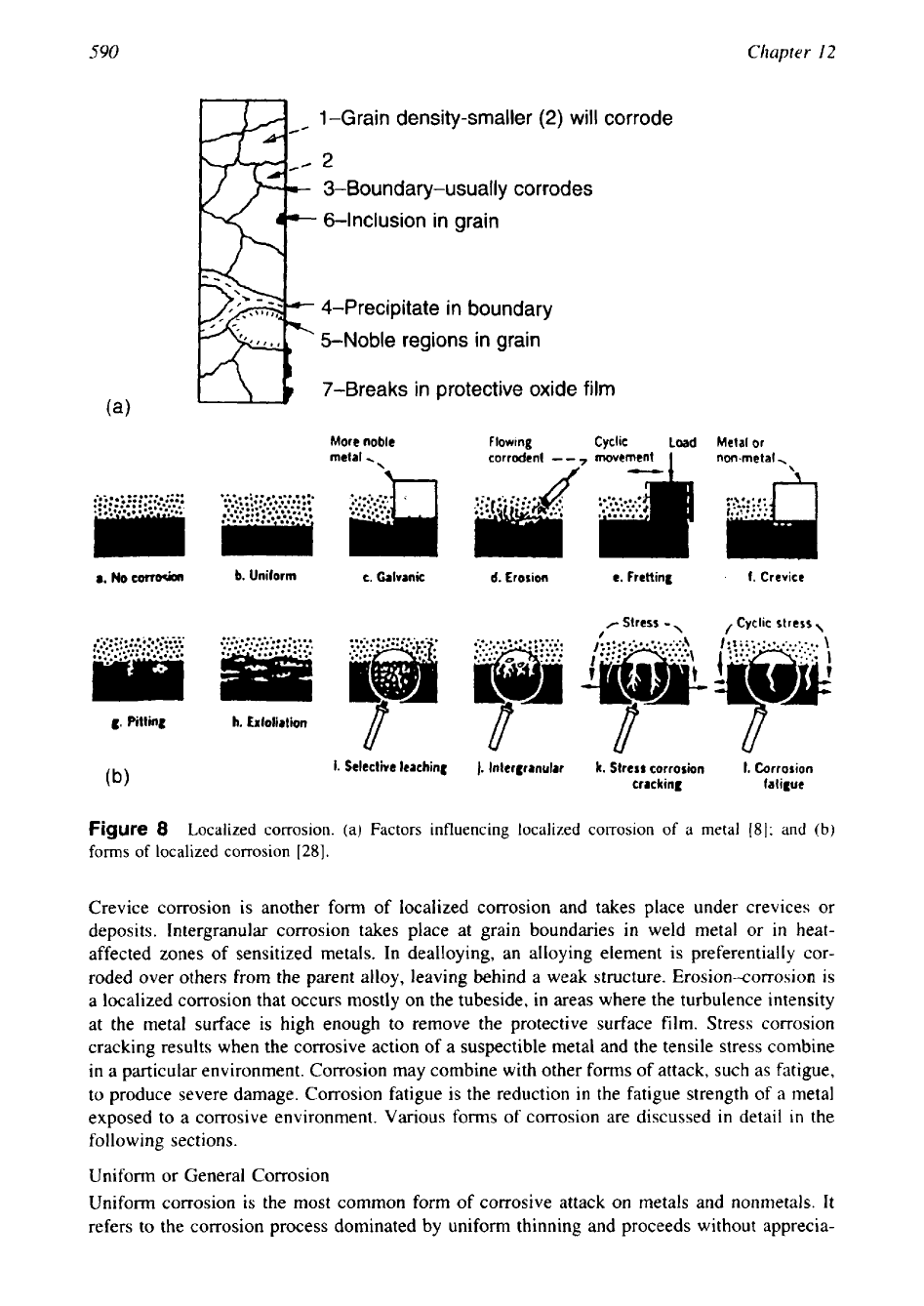
Localized
corrosion of a metal surface due to irregularities in metals is shown in Fig. 8a
[8].
2.3
Forms
of
Corrosion
Over the years, corrosion scientists and engineers have recognized that corrosion manifests
itself
in
forms that have certain similarities and therefore can be categorized into specific
groups. The most familiar and often used categorization of corrosion is probably the eight
forms presented by Fontana and Greene [9]:
1.
Uniform corrosion
2.
Galvanic corrosion
3.
Pitting corrosion
4. Crevice corrosion
5.
Intergranular corrosion
6.
Dealloying or selective leaching
7.
Erosion-corrosion
8.
Stress corrosion cracking
Hydrogen damage, although not a form of corrosion, often occurs indirectly as a result of
corrosive attack [9]. This classification of corrosion was based on visual characteristics of the
morphology of corrosion attack. Other forms of corrosion classified based on the mechanisms
of
attack rather than the visual characteristics are as follows:
Fretting corrosion
Corrosion fatigue
Microbiologically influenced corrosion
General corrosion takes place uniformly over a broad area. In galvanic corrosion, two
dissimilar metals form a galvanic couple, and the less noble metal in the couple corrodes.
Pitting is the localized attack that produces small pits, which may penetrate the metal thickness.
590
Chapter
I2
//
1
-Grain density-smaller
(2)
will corrode
3-Boundary-usually corrodes
6-lnclusion in grain
R2
4-Precipitate in boundary
41jo
%Noble regions
in
grain
7-Breaks in protective oxide film
More noble
Flowing
Cyclic
Load
Metal
or
metal.
corrodent
-
-
movement
non.metal,
-1
'.
8.
NO
COW&
b.
Uniform
c.
Galvanic
d.
Erosion
c.
Fretting
1.
Crevice
I
F
Stress
-,
,
Cyclic
stress,
g.
Pitting
h. Extolialion
U
I.
Scleclive Jcaching
i.
tnterRranulor
k.
Slrers
corrosion
1.
Corrosion
cracking
faligue
Figure
8
Localized corrosion. (a) Factors influencing localized corrosion
of
a
metal
(81;
and
(b)
forms
of
localized corrosion
[28].
Crevice corrosion is another form of localized corrosion and takes place under crevices or
deposits. Intergranular corrosion takes place at grain boundaries
in
weld metal
or
in
heat-
affected zones of sensitized metals. In dealloying, an alloying element is preferentially cor-
roded over others from the parent alloy, leaving behind a weak structure. Erosion-corrosion is
a localized corrosion that occurs mostly on the tubeside, in areas where the turbulence intensity
at the metal surface is high enough to remove the protective surface film. Stress corrosion
cracking results when the corrosive action of a suspectible metal and the tensile stress combine
in a particular environment. Corrosion may combine with other forms of attack, such as fatigue,
to produce severe damage. Corrosion fatigue is the reduction in the fatigue strength of
a
metal
exposed to a corrosive environment. Various forms
of
corrosion are discussed
in
detail
in
the
following sections.
Uniform or General Corrosion
Uniform corrosion is the most common form of corrosive attack on metals and nonmetals. It
refers to the corrosion process dominated by uniform thinning and proceeds without apprecia-

Corrosion
591
ble localized attack. Uniform corrosion is also referred as general corrosion. Uniform corrosion
results from prolonged contact with environments such as atmosphere, water, acids, alkalies,
soil, etc. It is manifested by a chemical or electrochemical reaction. Uniform corrosion of the
metal surface, as in the rusting of iron, results from anodes and cathodes rapidly interchanging
sites at random; should the anode become fixed on the surface, a localized pitting corrosion
will result instead [2]. Like other forms of corrosion, many factors influence general corrosion.
The corrosive media is the most important factor governing corrosion. Environmental factors
such as acidity, temperature, concentration, motion relative
to
metal surface, degree of oxidiz-
ing power and aeration, and presence or absence of inhibitors influence general corrosion [3].
Forms
of
Uniform
Corrosion.
Most general types of uniform corrosion are atmospheric cono-
sion and high-temperature (gaseous) corrosion. Although high-temperature attack
in
gaseous
environments may manifest itself as various forms of corrosion, it has been incorporated into
the category “general corrosion” because it
is
often dominated by uniform thinning
[
101. Bio-
logical corrosion can manifest itself in a general or a localized form. It is covered at the end
of this section.
Atmospheric Corrosion.
Atmospheric corrosion is defined as the corrosion of a material ex-
posed to the air and its pollutants rather than immersed in a liquid. Many variables influence
the atmospheric corrosion. Relative humidity, temperature, contents like sulfur dioxide, hydro-
gen sulfide, and chloride, amount of rainfall, dust, etc. influence corrosion behavior. Therefore,
in an arid atmosphere, free of contaminants, only negligible corrosion would be expected. It
also must be understood that the major contributor to the corrosion of all metals in the atmo-
sphere is oxygen.
Types of Corrosive Atmospheres.
A
common practice is to divide atmospheres into cate-
gories such as rural, industrial, marine, and indoor
[
111. Most of them are mixed and present
no clear lines of demarcation, and the type of atmosphere may
vary
with the wind pattern,
particularly where corrosive pollutants are concentrated. Except for severe marine environ-
ments, there is no need to protect against atmospheric corrosion except with protective coatings
[121.
Protection Against Atmospheric Corrosion.
Important considerations to protect against
atmospheric corrosion should involve
[
121 (1) specific plant environment around the equip-
ment,
(2)
operating temperature, and
(3)
protection against ingress of rain water into crevices.
Merrick discusses these factors in detail
[
121.
The specific plant environment around the equipment that involves releases of corrosive
gases, such as hydrogen sulfide, sulfur dioxide, or ammonia, from nearby process plant will
significantly increase atmospheric corrosion of equipment.
The operating temperature of the pressure vessels and heat exchangers has a significant
effect on atmospheric corrosion. Units that operate below the dew point experience more corro-
sion than those operating at higher temperatures.
For protection against ingress of rain water into crevices, saddles and reinforcing pads for
supports should be continuously welded to the vessel. Partial welding or stich welds will act
as a crevice. This will permit the ingress of rain water between the saddle and the vessel and
can accelerate crevice corrosion.
Control
of
Uniform Corrosion.
Uniform corrosion can be easily predicted and controlled by
Proper material selection
Small alloying additions to the base metal
Cathodic protection using sacrificial anodes
Use of inhibitors
Surface coatings

592
Chapter
12
Adding extra material known as corrosion allowances. Although uniform corrosion is obvi-
ously detrimental it is least predictable, and corrosion allowances for losses are frequently
made in the design of equipments. For corrosion-resistant materials, in general no corro-
sion allowance is given.
Rating
of
Metals Subject to
Uniform
Corrosion.
In contrast to localized forms of corrosion,
uniform corrosion is rather predictable. The uniform attack on an entire area exposed to a
corrosive environment is usually expressed in terms of an average loss of metal thickness for
a given period of time in units of mils
(1
mil
=
1/1000
in)
per year (mpy), inches per month
(ipm), or millimeter per year (mdyr). Rating of various materials against uniform corrosion
is given in Table 1.
Determination
of
General Corrosion.
General corrosion is determined by immersion tests
conducted according to ASTM G-3
1.
Eight different boiling acid and alkali solutions-20%
acetic acid,
45%
formic acid,
10%
oxalic acid,
20%
phosphoric acid, 10% sodium bisulfate,
50%
sodium hydroxide,
10%
sulfamic acid,
10%
sulfuric acid-are used to compare the perfor-
mance of different alloys in a variety of solutions rather than to simulate a particular process
industry environment. Duplicate samples are exposed for five
48-h
periods and
an
average
corrosion rate is determined.
Material Selection
for
General Corrosion.
Of the various forms of corrosion, general com-
sion is the easiest to evaluate, and hence the material selection is straightforward; if a material
shows only a general and uniform attack, a corrosion rate,
0.25
mm/yr
(10
mils/yr) or less €or
low-cost material such as carbon steel, a negligible contamination
of
the process fluids, and
availability, ease of fabrication etc., then that material favors the choice of selection. For more
costly materials such as the
300
series austenitic stainless steels and copper-and nickel-base
alloys, a maximum corrosion rate of
0.1
mdyr
(4
mils/yr) is generally acceptable
[14].
Galvanic Corrosion
When two dissimilar metals
or
alloys placed at different positions
in
electromotive series are
in contact with each other in a electrolyte, a galvanic couple is formed and results in corrosion
of one of the metals, known as the anode of the couple. In other words, galvanic corrosion
does not affect the cathode, which is known as a noble metal. This form of corrosive attack is
known as galvanic corrosion since the entire system behaves as a galvanic cell. Galvanic
corrosion can also take place even within the same group of metals due to local imperfections
or hetrogenities on the metal surfaces or due to variation in local solution chemistry. Most
often, galvanic corrosion shows up as furrows or troughs on the corroded metal at its point of
contact with the more noble metal.
For
initiation of galvanic corrosion four essential compo-
nents are required: the anode, the cathode, the electrolyte, and a metallic path between the
Table
1
Rating of Metals Against Uniform Corrosion
Corrosion Rate Rating Applications
4
mPY Excellent Very critical
5-20
mpy
Satisfactory Critical
20-50
mpy
Useful Noncritical
>50
mpy
Unsatisfactory None
mpy
=
rnil
per year.
Source:
Ref.
13.

Corrosion
593
anode and cathode, which completes the circuit. These four basic components were already
discussed.
Heat Exchanger Locations Susceptible
to
Galvanic Corrosion.
Components such as tube
sheets, water box, bolts and flanges, and supports made of less noble metals will corrode at
the following locations:
Interfaces between the tube and baffle plates
Between the tubes and tube-sheet areas
Welded joints, brazed joints, and soldered joints
Heat exchanger supports with the frame or shell bolts and fasteners if they are less noble than
the flange materials
In seawater-cooled condensers, tube materials such as copper-nickels, stainless steels, or tita-
nium are more noble than tube-sheet materials such as Muntz metal, naval brass, or aluminum
bronze; consequently, the tube sheets may suffer galvanic attack when fitted with more noble
tube materials. This is also true with tubes made of seawater resistant stainless steels like
superferritics and superaustenitics used to replace the copper alloy tubes in a Muntz metal tube-
sheet. Similarly, a cast iron water box may suffer galvanic attack because all other materials in
the condenser are more noble than cast iron. In general, weld metal corrosion can be eliminated
by using suitably balanced electrode; the remaining problems are discussed in the section on
weld-metal corrosion. Brazed joints are always at risk to galvanic corrosion because the fillers
are invariably of different composition than that of the parent metals being joined. Fortunately,
these fillers are normally noble than the metals being joined and hence the corrosion problem
is not faced. Corrosion of brazed joints and corrosion of soldered joints are discussed in detail
in Chapter 15, Heat Exchanger Manufacture, in the second part, Brazing and Soldering.
Galvanic Corrosion Sources.
Two important sources of galvanic corrosion are (1) metallurgi-
cal sources and
(2)
environmental sources.
Metallurgical Sources. Metallurgical sources are within the
metal andor in relative con-
tact between dissimilar metals. Such sources include difference in potential of dissimilar mate-
rials, distance apart in galvanic series, relative areas of anode and cathode, oxide or mill scales,
strained metal (cold work), inclusions in metal, and differences in microstructure, heat affected
zone (HAZ), and sensitization
[
15,161.
Environmental Sources. Environmental sources include conductivity
of
the fluid, concen-
tration differences in solution, changes in temperature, velocity and direction of fluid flow,
aeration, and ambient environment (seasonal changes)
[
151.
Types
of
Galvanic Corrosion.
Among the various forms of electrochemical corrosion,
(1)
bimetal corrosion,
(2)
differential aeration corrosion,
(3)
differential concentration corrosion,
and
(4)
work area corrosion belong to galvanic corrosion [17]. Items 1-3 have been discussed
earlier. Only work area corrosion is defined here.
When a metal is cold worked
so
that it is denser in one place than another, a corrosion
cell with the stressed area as an anode and the remaining area as cathode is set up if these two
areas are immersed in an electrolyte
[
171. This is called work area corrosion.
Magnitude
of
Galvanic Efsects.
The discussion given earlier deals with the criteria leading to
the formation of an electrolytic cell, which is the essential step in the corrosion process. How-
ever, the extent of corrosion attack taking place is dependent on many factors as follow:
1.
The polarization behavior
of
the metals or alloys
2.
Passivation of the alloys
