Kuppan T. Heat Exchanger Design Handbook
Подождите немного. Документ загружается.


Chapter
12
Arm
of
cathe
Ratio=
*
Area
of
anad.
Figure
9
Galvanic corrosion due to area effect.
3.
Potential difference between the metals or alloys, that is, the distance effect
4.
Area effect, that is, the geometric relationships such as relative surface area
Items
1
and
2
have been discussed earlier and the remaining points are discussed next.
Distance Effect. Enhanced corrosion takes place
when the metals in galvanic couple are
placed further apart
in
the galvanic series. This is due to high electrochemical current density,
In
some cases, the separation between the two metals or alloys
in
the galvanic series gives an
indication of the probable magnitude of the corrosive effect.
Area Effect.
Another important factor in galvanic corrosion is the area effect.
As
the
ratio of the cathode to anode area increases, the corrosion rate of the anode metal is rapidly
accelerated (Fig.
9).
On the other hand, if the area of anode is large compared to the cathode
area. the corrosion of the anode is
so
widely distributed that the amount of metal loss
in
terms
of its thickness may be
so
small that it may be ignored. These observations suggest the well-
established rule in corrosion engineering that when dissimilar metals are coupled, the anode
area must be maintained as large as possible compared to cathode area. For example, the
nuts
and bolts that are critical to the flanged joints must always
be
noble (cathodic) to the larger
area of the flange. The nuts and bolts (Fig. 10) corrode at a rate well below normal at the
Corroded
Zones
Brass
Bolt
E:
lectro
Iyte
Figure
10
Nuts
and
bolts
(cathodic) in a flanged joint (anodic).
(From
Ref.
3.)

Corrosion
595
negligible expense of the flange, which corrodes at only slightly above normal rate over a
large area.
Tools
to Determine the Degree
of
Galvanic Corrosion.
The degree of galvanic corrosion is
normally known from the following two tools. They are the electromotive force (emf) series
and the galvanic series.
Electromotive Force (EMF) Series.
The electromotive force series is a list
of
elements
arranged according to their standard electrode potentials, with the sign being positive for ele-
ments whose potentials are cathodic to hydrogen and negative for those anodic to hydrogen.
In this listing, hydrogen is used as an arbitrary reference element, and metals such as gold and
platinum have large positive values indicating little tendency for corrosion attack. Since the
emf series is of little value to the practical corrosion scientist or technologist,
it
is necessary
to develop some alternate system by which the relative corrodibility
of
a galvanic couple may
be assessed
[4].
Such a listing is known
as
the galvanic series.
Galvanic Series.
Whether a given metal or alloy is naturally anodic or cathodic with
respect to another metal is judged by the galvanic series, which consists
of
an arrangement of
metals and alloys in accordance with the measured potentials
in
flowing seawater at velocities
ranging from
2.5
to
4
ds.
A
typical tabulation is given in Table
2.
With certain exceptions,
Table
2
Galvanic Series in Seawater at 25°C (77°F)
Anodic (least noble) end or active end
Cartridge or yellow brass C27000
Magnesium
Admiralty brass C44300, C44400, C44500
Zinc
Aluminum bronze C60800, C61400
Galvanized iron
Red brass C23000
Aluminum alloy 5052H
ETP copper C
1
1000
Aluminum alloy 3004
Silicon bronze C65 100, C65500
Aluminum alloy 3003
Copper-nickel,
10%
Aluminum alloy
1
100
Copper-nickel, 30%
Aluminum alloy 6053T
Nickel 200 (passive)
Alclad aluminum alloys
Inconel alloy 600 (passive)
Aluminum alloys, 2
1
17
Monel alloy 400
Aluminum alloys, 201 7T
Stainless steel type 4 10 (passive)
Aluminum alloys, 2024T
Stainless steel type 430 (passive)
Low-carbon steel
Stainless steel type 304 (passive)
Low-alloy steel
Stainless steel type 3 16 (passive)
Cast iron
E-Brite alloy
Stainless steel type 410 (active)
AL-29-4C alloy
Stainless steel type 430 (active)
AL-6XN alloy
50-50
Lead-tin solder
Inconel alloy 825
Stainless steel type 304 (active)
Inconel alloy 625, alloy 276
Stainless steel type 316 (active)
Hastelloy alloy C
Lead
Silver
Tin
Titanium
Muntz metal C28000
Graphite
Manganese bronze A-C67500
Zirconium
Naval brass: C46400, C46500, C46600, C46700,
Tantalum
Nickel 200 (active)
Gold
Inconel alloy
600
(active)
Platinum
Hastelloy alloy
B
Cathodic (most noble) end
Source:
Adapted from
Refs.
3,
13,
and
18.

596
Chapter
12
this series is broadly applicable in other natural waters and in uncontaminated atmospheres.
The galvanic series allows one to determine which metal or alloy in a galvanic couple is more
active. The metals closer to the active end of the series will behave as the anode and will
corrode, whereas those closer to the noble end will behave as the cathode and will be protected.
The metals and alloys that are next to each other have little tendency to galvanic corrosion
when connected together,
so
it is relatively safe to use such combinations in jointed assemblies,
Galrwnic Corrosion Control.
Design is a major factor in preventing or minimizing galvanic
corrosion. Typical measures to control galvanic corrosion are:
1.
Choose the combination of metals as close together as possible in the galvanic series,
unless the more noble metal is easily polarized.
2.
Avoid the unfavorable area effect, that is, small area
of
anode and a large cathode area,
Otherwise small components such as bolts and fasteners should be of more noble metal.
3.
Insulate
or
break the circuit between the two metals by applying coatings, introducing
gaskets, nonmetallic washers, etc., and make sure that metal-to-metal contact is not re-
stored
in
service.
4.
Add corrosion inhibitors to decrease the aggressiveness of the environment or to control
the rate of cathodic andor anodic reaction.
5.
Maintain coatings. Coating is the most common method for combatting corrosion.
6.
Cathodic protection is one
of
the recommended methods of protecting the anode. Use
a
sacrificial anode such as Zn, Al, or Mg that is anodic to both the structural metals. Exam-
ples include sacrificial cladding (Alclad) applied
in
the internal aluminum tube surfaces
of
radiators to give protection to the tube core alloy, and planting of Zn anode on the water
box of condensers to protect both the tubes and the tube-sheet.
It
is important to note that
overprotection of the titanium tube-sheet may result
in
the hydriding of the titanium tubes.
Pitting Corrosion
Instead of a uniform corrosion
in
a corrosive environment, a metal very often suffers localized
attack resulting in pitting. Pitting usually occurs on metals that are covered with a very thin
adherent protective surface film that formed on the metal surface during a surface treatment
process or produced by reaction with an environment
[
191. Thus, pitting corrosion occurs
on
aluminum, titanium, stainless steels, nickel, and their alloys, in which surface film develops.
Pitting takes place when there is a breakdown of protective surface film. The protective film
is an oxide layer in the case of carbon steel and ferritic stainless steel, whereas it is a passive
film
in
the case of austenitic stainless steel. Once pits are initiated, they may continue to grow
by
a
self-sustaining or autocatalytic process; that is, the corrosion process within a pit produces
conditions that are both stimulating and self-propagating [9]. Pitting is the most aggressive
form of corrosion and leads to premature failure due to perforation of the surfaces. Pitting
corrosion is shown schematically
in
Fig.
11.
Parameters Responsible
for
Pitting
Corrosion.
Pitting corrosion is caused by factors such
as
[20]
(
1
)
metallurgical and structural factors,
(2)
environmental factors, and
(3)
polarization
phenomena. Other causes for pitting are the attachment of microorganisms, presence of corro-
sion products, deposits, etc. These parameters are explained next.
Metallurgical and Structural Factors.
The following metallurgical and structural factors
act as nucleation sites for initiation of pitting corrosion:
1.
The breakdown of passive film, mill scale
[21],
or applied coating.
2.
Defect structures.
3.
A compositional heterogeneity; inhomogeneities in the alloys caused by segregation of
alloys or by cold working
[7].
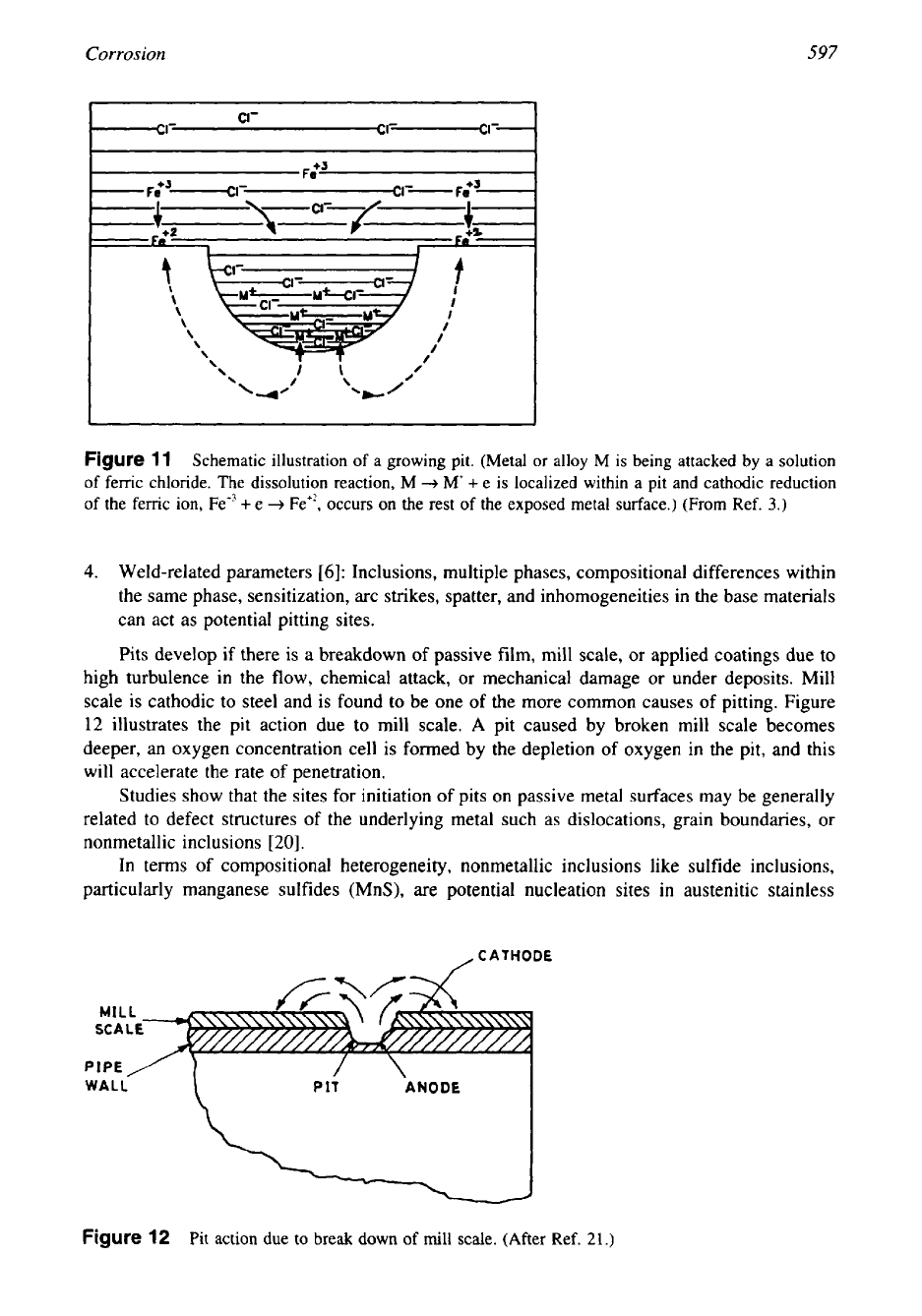
Corrosion
597
Figure
11
Schematic illustration of
a
growing
pit.
(Metal
or
alloy M is being attacked
by
a solution
of femc chloride.
The
dissolution reaction, M
-+
M
+
e
is localized within a pit and cathodic reduction
of the femc
ion,
Fe”
+
e
+
Fe”. occurs on the rest of the exposed metal surface.)
(From
Ref.
3.)
4.
Weld-related parameters [6]: Inclusions, multiple phases, compositional differences within
the same phase, sensitization, arc strikes, spatter, and inhomogeneities
in
the base materials
can act as potential pitting sites.
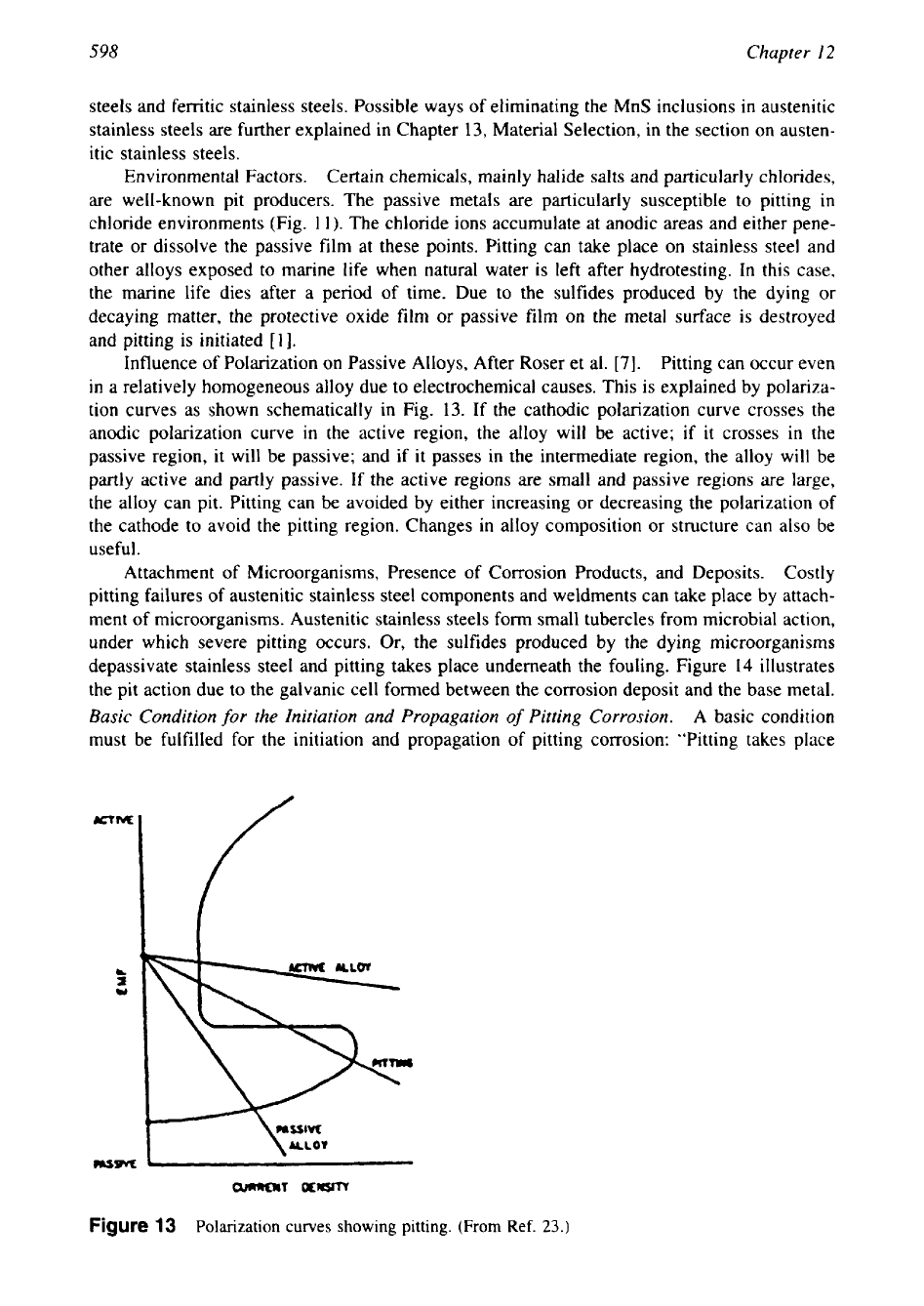
Pits develop
if
there is a breakdown of passive film, mill scale, or applied coatings due to
high turbulence
in
the
flow,
chemical attack, or mechanical damage or under deposits. Mill
scale is cathodic to steel and is found to be one
of
the more common causes of pitting. Figure
12
illustrates the pit action due to mill scale.
A
pit caused by broken mill scale becomes
deeper, an oxygen concentration cell is formed by the depletion of oxygen in the pit, and this
will accelerate the rate
of
penetration.
Studies show that the sites for initiation of pits on passive metal surfaces may be generally
related to defect structures of the underlying metal such as dislocations, grain boundaries, or
nonmetallic inclusions
[20].
In
terms of cornpositional heterogeneity, nonmetallic inclusions like sulfide inclusions,
particularly manganese sulfides (MnS), are potential nucleation sites
in
austenitic stainless
,CATHODE
MIL
SCA
PIPE
WALL
PIT
ANODE
Figure
12
Pit
action
due
to
break down of
mill
scale. (After Ref.
21.)
598
Chapter
12
steels and ferritic stainless steels. Possible ways of eliminating the MnS inclusions in austenitic
stainless steels are further explained in Chapter 13, Material Selection, in the section on austen-
itic stainless steels.
Environmental Factors. Certain chemicals, mainly halide salts and particularly chlorides,
are well-known pit producers. The passive metals are particularly susceptible to pitting in
chloride environments (Fig. 11). The chloride ions accumulate at anodic areas and either pene-
trate or dissolve the passive film at these points. Pitting can take place on stainless steel and
other alloys exposed to marine life when natural water is left after hydrotesting. In this case,
the marine life dies after a period of time. Due to the sulfides produced by the dying or
decaying matter, the protective oxide film or passive film on the metal surface is destroyed
and pitting is initiated
[
11.
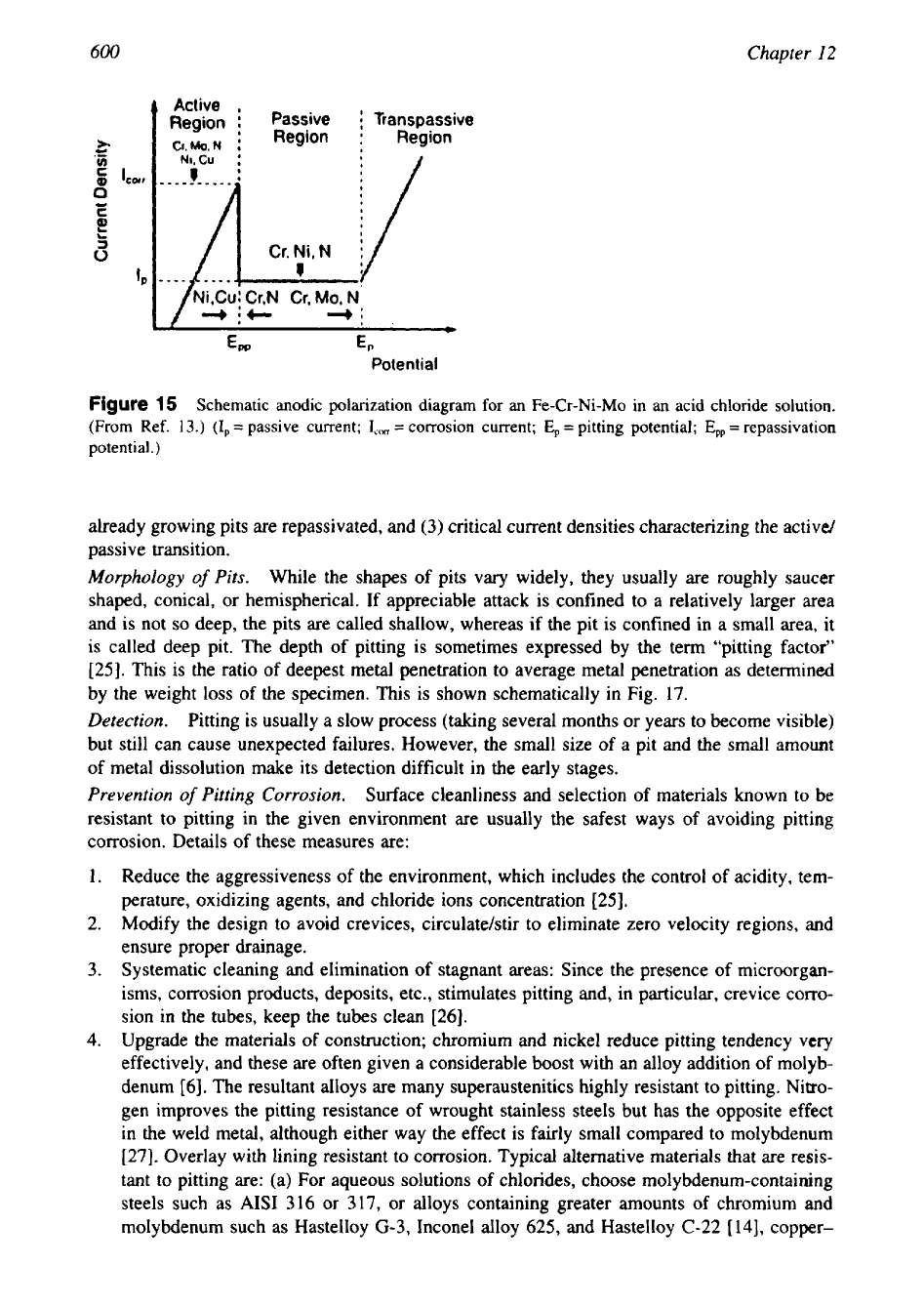
Influence of Polarization on Passive Alloys, After Roser et al.
[7].
Pitting can occur even
in a relatively homogeneous alloy due to electrochemical causes. This is explained by polariza-
tion curves as shown schematically in Fig. 13. If the cathodic polarization curve crosses the
anodic polarization curve
in
the active region, the alloy will be active; if it crosses
in
the
passive region, it will be passive; and if it passes in the intermediate region, the alloy will be
partly active and partly passive. If the active regions are small and passive regions are large,
the alloy can pit. Pitting can be avoided by either increasing or decreasing the polarization
of
the cathode to avoid the pitting region. Changes in alloy composition or structure can also
be
useful.
Attachment of Microorganisms, Presence of Corrosion Products, and Deposits. Costly
pitting failures of austenitic stainless steel components and weldments can take place by attach-
ment
of
microorganisms. Austenitic stainless steels form small tubercles from microbial action,
under which severe pitting occurs. Or, the sulfides produced by the dying microorganisms
depassivate stainless steel and pitting takes place underneath the fouling. Figure
14
illustrates
the pit action due to the galvanic cell formed between the corrosion deposit and the base metal.
Basic Condition
for
the Initiation and Propagation
of
Pitting Corrosion.
A basic condition
must be fulfilled for the initiation and propagation of pitting corrosion: “Pitting takes place
(From Ref.
23.)

Corrosion
599
HYOROGEN
ION
HYDROGEN
F
JLV
FITHOOE
Figure
14
Pitting due
to
deposit (galvanic cell).
(From
Ref.
21.)
when the anodic breakdown potential of the metal surface film is equal to or less than the
corrosion potential under a given set of conditions.”
Mechanisms and Theories
of
Pitting
Corrosion.
Nucleation and Growth. The
modern theory of pitting presupposes the formation of a pit
at a minute area of a metal surface that suffers a breakdown in passivity
[22].
This is known
as the pit nucleation stage. The passive film breakdown is followed by formation of an electro-
lytic cell, which leads to growth and propagation of a pit rather than to spreading along the
entire surface. The anode of this cell is a minute area of active metal, and the cathode is a
considerable area of passive metal
1231.
The large cathode to anode area ratio accounts for the
considerable flow
of
current with rapid corrosion at the anode. Pits once initiated can propagate
inside the pits. They stop propagating if the metal is polarized to (or below) the potential of
metal inside the pits, which in the extreme is that of the active (nonpassive) state
[24].
Growth and Propagation of Pit.
A
pit develops in stages: original attack, propagation,
termination, and reinitiation
[
191.
Termination will occur with increase in internal resistance
of
the local cell.
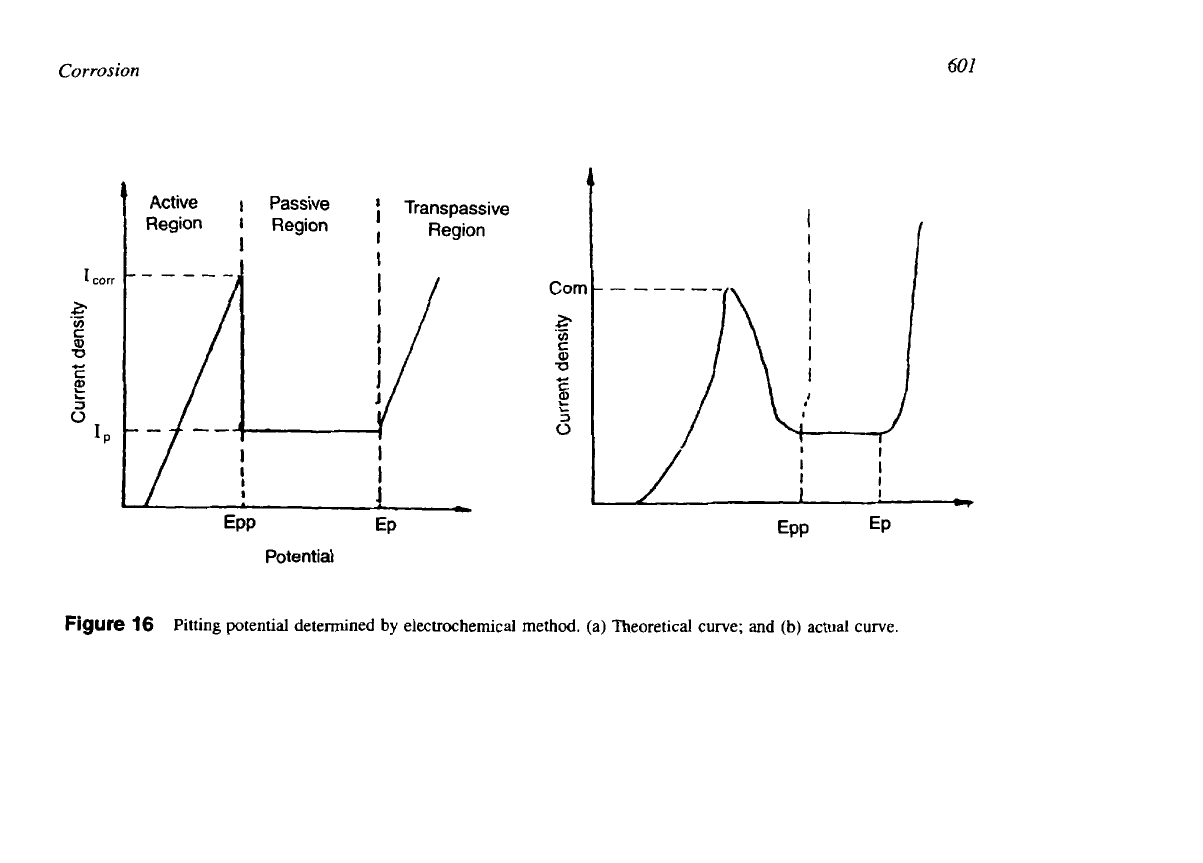
Pitting Potential.
For passive metals, pits are initiated at or above a specific potential. The
potential at which pit initiation occurs is called the pitting potential. Resistance to pitting
increases with the pitting potential. The pitting potential is
an
important criterion for evaluation
of the stability of a passive film in an environment. The value of pitting potential depends on
the material and its composition, the environment, and the concentration of aggressive ions,
pH of the solutions, temperature, and history of heat treatment operation
[24].
The effects of
various alloying elements on the polarization behaviour of
AL-6
x
N
(Fe-Cr-Nt-Mo) alloy
when exposed to an acid chloride solution like HCl are shown in Fig.
15.
Pitting potential can
be readily evaluated by lab test.
Determination of Pitting Potential.
Pitting potential is determined by electrochemical
techniques, which consist of measuring current and potential potentiostatically either stepwise
or by applying a constant potential sweep rate in a standard chloride-containing solution. The
recorded values of the current and potential are plotted.
A
theoretical curve obtained by electro-
chemical method is shown schematically in Fig.
16
[
131.
From such a curve the following
values are obtained and used to characterize alloys with respect to pitting and crevice corrosion:
(1)
pitting potential
E,,
where pits start to grow,
(2)
repassivation potential
E,.,,,,
below which
600
Chapter
12
Active
,
Region
;
Passive
i
Transpassive
C,,Mo,N
i
Region
:
Region
I
E,
E,
Potential
Figure
15
Schematic anodic polarization diagram for an Fe-Cr-Ni-Mo in an acid chloride solution.
(From Ref.
13.)
(Ip
=
passive current;
I,,,
=
corrosion current;
E,
=
pitting potential;
E,,
=
repassivation
potential.)
already growing pits are repassivated, and
(3)
critical current densities characterizing the active/
passive transition.
Morphology
of
Pits.
While the shapes of pits vary widely, they usually are roughly saucer
shaped, conical, or hemispherical. If appreciable attack is confined to a relatively larger area
and is not
so
deep, the pits are called shallow, whereas if the pit is confined in a small area, it
is called deep pit. The depth of pitting is sometimes expressed by the term “pitting factor”
[25].
This is the ratio of deepest metal penetration to average metal penetration as determined
by the weight loss of the specimen.
This
is shown schematically
in
Fig.
17.
Detection.
Pitting is usually a slow process (taking several months or years to become visible)
but still can cause unexpected failures, However, the small size of a pit and the small amount
of metal dissolution make its detection difficult in the early stages.
Prevention
of
Pitting Corrosion.
Surface cleanliness and selection of materials known
to
be
resistant to pitting in the given environment are usually the safest ways of avoiding pitting
corrosion. Details of these measures are:
1.
Reduce the aggressiveness of the environment, which includes the control
of
acidity, tem-
perature, oxidizing agents, and chloride ions concentration
[25].
2.
Modify the design to avoid crevices, circulate/stir to eliminate zero velocity regions, and
ensure proper drainage.
3.
Systematic cleaning and elimination of stagnant areas: Since the presence of microorgan-
isms, corrosion products, deposits, etc., stimulates pitting and, in particular, crevice corro-
sion
in
the tubes, keep the tubes clean
[26].
4.
Upgrade the materials of construction; chromium and nickel reduce pitting tendency very
effectively, and these are often given a considerable boost with an alloy addition
of
molyb-
denum
[6].
The resultant alloys are many superaustenitics highly resistant to pitting. Nitro-
gen improves the pitting resistance of wrought stainless steels but has the opposite effect
in the weld metal, although either way the effect is fairly small compared to molybdenum
[27].
Overlay with lining resistant to corrosion. Typical alternative materials that are resis-
tant to pitting are: (a) For aqueous solutions of chlorides, choose molybdenum-containing
steels such as AISI
316
or
317,
or alloys containing greater amounts of chromium and
molybdenum such as Hastelloy
G-3,
Inconel alloy 625, and Hastelloy
C-22
[
141,
copper-
601
Corrosion
Active
I
Passive
Transpassive
Region
I
Region
Region
1
.----
I
Con
A
I
/
/
I
I
Potential
Figure
16
Pitting potential determined
by
electrochemical method. (a) Theoretical curve;
and
(b)
actual curve.

602
Chapter
12
P
Pit
t
ing
factor
=
-
d
Figure
17
Pitting
factor. (From Ref.
25.)
nickel, Monel, or titanium. (b) In seawater and stagnant natural water applications, use
materials such as copper-nickel, aluminum bronze, inhibited admiralty brass, titanium,
superferritics, and duplex stainless steels. (c) Proven lower cost nonmetallic coatings,
lin-
ings, or cladding can be helpful. (d) The rating of certain stainless steel materials for
pitting corrosion is discussed in Chapter
13,
Material Selection, and
in
the section on
stainless steels.
Avoid the metals and corrosive combinations that have the pitting tendencies
[
141:
1. A1 and A1 alloys: electrolytes containing ions of heavy metals such as copper, lead, and
mercury.
2. Plain carbon and low-alloy steels: waters containing dissolved
0,
or sulfate-reducing
bac-
teria.
3.
Austenitic stainless steel weldment: exposed to stagnant natural waters that are infected
with iron andor manganese bacteria.
HOM~
to
Gauge Resistance to Pitting.
The susceptibility of passive metals to pitting corrosion
is
usually investigated by experimental techniques:
(I)
simple immersion tests, (2) electro-
chemical methods, and
(3)
using empirical correlation for stainless steels. The methods are
described next.
Critical Pitting Corrosion Temperature (CPT).
The formation of visible pits
in
specimens
exposed to aqueous chloride containing solutions, at different temperatures, is frequently used
as a measure of pitting resistance. The temperature at which the pits begin to form is known
as the critical pitting temperature (CPT). This is determined as per ASTM G48.
ASTM G48 is a laboratory test method for determining the resistance of stainless steels
and related alloys to pitting and crevice corrosion. The method uses a ferric chloride (FeC13)
solution. The concentration of ferric chloride is usually
6%,
but sometimes 10% is used instead
and sometimes in an acidic mixture of chlorides and sulfates [4% NaCl
+
1% Fe2(S0,),
+
0.01
M
HCl]. The resulting indices are known as critical pitting corrosion temperature (CPT or
CPCT) and critical crevice corrosion temperature (CCT or CCCT). These are the minimum
temperatures at which these types of localized attack start in the FeC13 solution. CCT
is
deter-
mined with crevices.
To determine the CPT, a coupon of stainless steel is exposed for 24 h in the corrosive
solution at a fixed temperature and then examined visually for pits on the rolled surface (edge
pits are counted). If no pits exists, the temperature is increased by 2S°C and the coupon is
reimmersed. By stepwise increase in temperature of 2.5OC, the pits initiated are to be noted
for each step.
Corrosion
603
Pitting Potential.
Another laboratory test that is frequently used for ranking stainless
steel is pitting potentials as measured using
an
electrochemical apparatus in a standard chloride
containing solution. The pitting potential indicates the relative susceptibility of
an
alloy to
localized corrosion. Resistance to pitting increases with the pitting potential.
Pitting Index Number (PREJ. Because
the resistance of a stainless steel against pitting
and crevice corrosion is primarily determined by the amount of chromium, molybdenum, and
nitrogen in it, an index for comparing the resistance to these types of attack is often evaluated
in terms
of
these elements. The index is called the pitting resistance equivalent number (PRE
or PREN). It is defined, in weight percent, using the following equation:
PREN
=
%Cr
+
3.3%Mo
+
16%N
for austenitic and duplex stainless steel
=
%Cr
+
3.3%Mo
for ferritic stainless steel
=
%Cr
+
3.3%Mo
+
30%N for
6%
MO superaustenitic stainless steel
The higher the PREN number, the better the performance of an alloy in chloride environments.
For example the PRE number for 6%
MO
superaustenitic alloys containing nitrogen is 43,
Alloy
2205
has 35, while for type 316L it is
24.
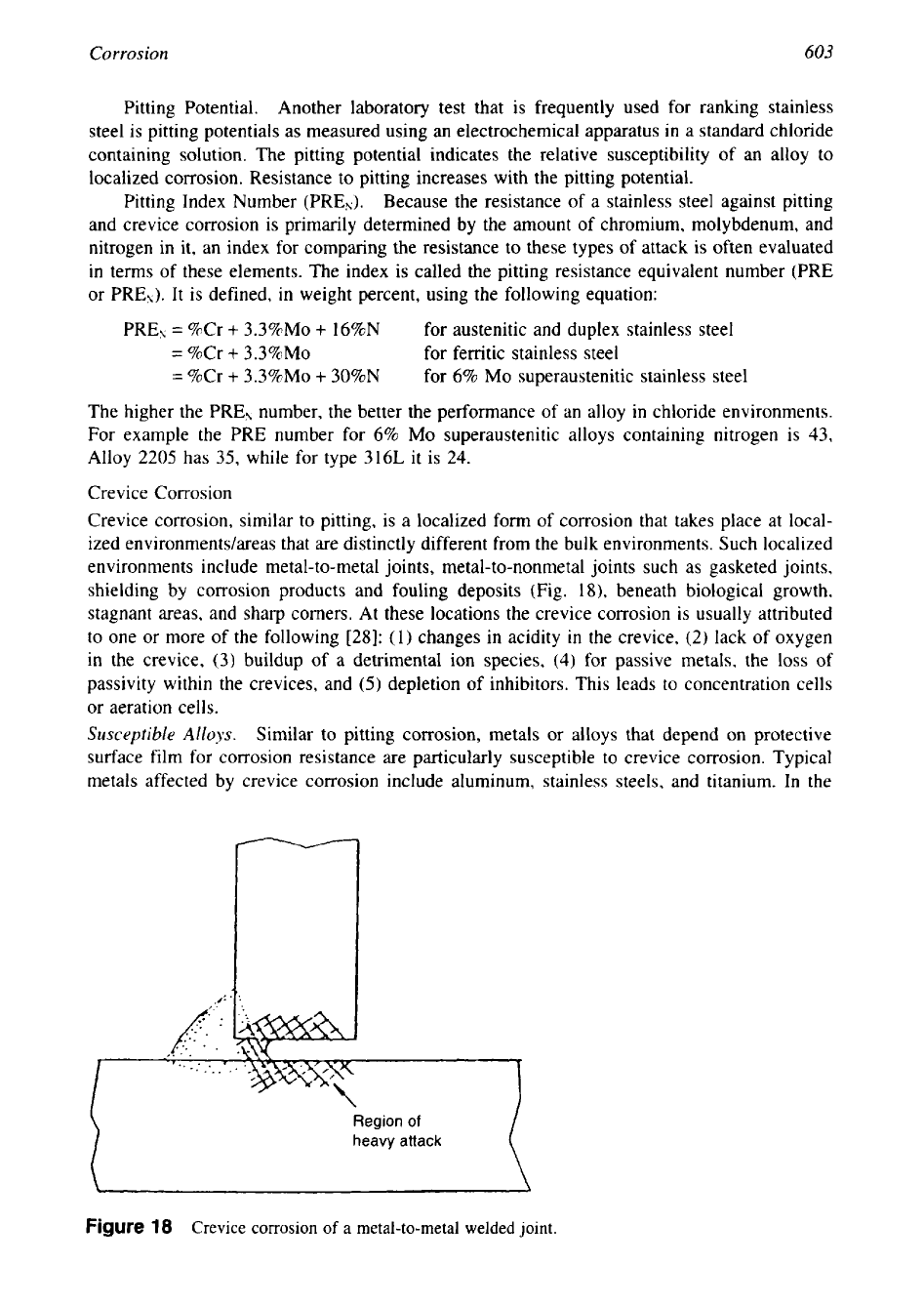
Crevice Corrosion
Crevice corrosion, similar to pitting, is a localized form of corrosion that takes place at local-
ized environmentdareas that are distinctly different from the bulk environments. Such localized
environments include metal-to-metal joints, metal-to-nonmetal joints such as gasketed joints,
shielding by corrosion products and fouling deposits (Fig.
1
8),
beneath biological growth,
stagnant areas, and sharp corners. At these locations the crevice corrosion
is
usually attributed
to one or more of the following
[28]:
(1)
changes in acidity
in
the crevice,
(2)
lack of oxygen
in the crevice, (3) buildup of a detrimental ion species,
(4)
for passive metals, the loss of
passivity within the crevices, and
(5)
depletion of inhibitors. This leads to concentration cells
or aeration cells.
Susceptible
Alloys.
Similar to pitting corrosion, metals or alloys that depend on protective
surface film for corrosion resistance are particularly susceptible to crevice corrosion. Typical
metals affected by crevice corrosion include aluminum, stainless steels, and titanium. In the
Region
of
heavy
attack
\
Figure
18
Crevice corrosion
of
a metal-to-metal welded joint.
