Kuppan T. Heat Exchanger Design Handbook
Подождите немного. Документ загружается.


624
Chapter
I2
steels, and high-nickel alloys (Monel
400
and Alloy B-2) have exhibited susceptibility to MIC
Wl.
Sources
of
Microorganisms.
Microorganisms are widely distributed in nature, being present
in streams, rivers, lakes, seawater, and coastal estuaries. Such microorganisms include bacteria,
fungi, algae, and yeasts, depending on the sources of water.
Classification
of
Microbiological Organisms.
Microbiological organisms, or microbes,
known to cause corrosion of metals may be classified in four general flora groups [60]:
(1)
bacteria, (2) fungi,
(3)
algae, and
(4)
yeasts. According to their metabolic activities, corrosive
microbes are classified into six general metabolic groups [60]:
(1)
acid producers,
(2)
mokl
growers, (3) slime formers, (4) sulfate reducers,
(5)
hydrocarbon feeders, and (6) metal ion
concentrators/oxidizers.
The three major types of microbes commonly associated with MIC are
(1)
sulfate-reducing bacteria
(SRB),
(2)
iron and manganese bacteria, and
(3)
sulfur oxidizing
bacteria
[
1 31.
Sulfate-Reducing Bacteria (SRB).
The sulfate-reducing bacteria seem to be involved in
at least some form of MIC of most of the susceptible alloys like iron and mild steels, aluminum
alloys, and copper alloys [61]. They reduce sulfates to sulfides and depolarize cathodic sites
on metal surfaces by consuming hydrogen.
Iron and Manganese Bacteria.
Iron-and manganese-oxidizing bacteria, known as metal
ion
concentrators/oxidizers,
are the most often associated with the corrosion of stainless steels
[
131. Such bacteria produce iron and manganese metabolites that form deposits that in turn
create concentration cells or harbor other corrosive microbes.
Sulfur-Oxidizing Bacteria or Acid Producer.
Some microbes can oxidize sulfur com-
pounds to sulfuric acid; a pH as low as
2
has been recorded where sulfur-oxidizing microbes
are active.
Attachment, Growth, and Influence
of
Microorganisms on Metal Suqaces.
On attachment
to
metal surfaces, microorganisms begin colonizing and produce a biofilm, known as slime or
mat. Certain microbes can metabolize nutrients and other chemical compounds (e.g., sulfur
and iron) to create corrosive environments or they can directly participate in electrochemical
reactions. The metabolic processes of the microorganisms can influence the corrosion behavior
of materials by [60,62,63]:
(1)
destroying protective surface films,
(2)
producing a localized
acid environment, (3) creating corrosive deposits,
(4)
creating corrosion cells, notably differen-
tial aeration and ion concentration cells, and
(5)
altering anodic and cathodic reactions, depend-
ing on the environment and organisms involved. These processes may occur either alone or in
combinations. The microorganisms tolerate elevated pressures and a wide temperature range,
and oxygen content from
0
to almost
100%.
Temperatures greater than 40°F (4°C) but less
than
140°F
(60°C) tend to promote MIC.
Categories
of
MIC.
MIC can be divided into two categories: aerobic and anaerobic. Aerobic
microbes exist in the presence of air. Anaerobic microbes can exist and multiply in the absence
of air. Sulfate-reducing bacteria are the most important microorganisms in the anaerobic cate-
gory, whereas slime-forming bacteria, sulfur bacteria, and iron bacteria are important microor-
ganisms
in
the aerobic category.
MIC
of
Industrial Alloys.
Mild Steel.
Due to MIC, carbon steels have experienced random pitting, general
corro-
sion, and formation of tubercles on surfaces. MIC of carbon steels can be prevented by chemi-
cals, coatings, or cathodic protection [61].

Corrosion
625
Austenitic Stainless Steel and Weldments.
Pitting failures of austenitic stainless steel
components and weldments by MIC commonly results when residual natural water is left in
stainless steel equipments after hydrotesting. MIC failures of austenitic stainless steel welds
have been reviewed by Pope et al.
[61]
and in refs.
63
and
64.
The conditionslmechanisms
responsible for the MIC of austenitic stainless steels include
[@I:
1. Austenitic stainless steels form small tubercles from microbial action under which severe
pitting occurs.
2.
Oxygen concentration cells produce carbonic acid, which is corrosive to stainless steels.
3.
Crevices are ideal sites for MIC to occur; perhaps the consumption of oxygen by the
biofilm prevents the oxygen in the external environment from reaching the interior of the
crevice.
4.
Depassivation: Microbes, by forming a slime layer, produce a crevice in which regions of
the normally passive film damaged by mechanical means or through halide attack go
unrepaired.
5.
Weldment surface conditions that are commonly associated with poor corrosion resistance,
such as heat tint and conditions that are associated with residual stresses, such as gouges,
may produce conditions that are susceptible to MIC.
Measures to overcome MIC in stainless steel include, solution annealing and pickling, and a
temporary measure, spraying the heat exchanger tube interior by a quick set epoxy to a distance
of
5-6
ft instead of plugging
[65].
Copper Alloys. Copper and copper alloys are more resistant to the attachment of biofou-
ling organisms than steel and most of the other common materials of construction. This is due
to the inherent characteristic of copper alloys and appears to be associated with copper ion
formation within the corrosion product film. Therefore, coupling of steel or less noble materials
or cathodic protection, which suppresses copper ion formation, allows biological fouling to
occur
[66].
However, the belief that copper ions and salts formed
by
copper alloy corrosion are
lethal to most of the microorganisms is now known to be erroneous. For example,
Thiobacillus
thiooxiduns
can withstand copper concentrations as high as 2%
[67].
Copper alloys are particu-
larly sensitive to sulfate-reducing bacteria and ammonia-producing bacteria and have exhibited
failures by pitting, plug type dealloying, stress corrosion cracking, and erosion<orrosion
[6
I].
Titanium’s Resistance to MIC. Titanium and its alloys exhibit excellent resistance to
MIC under both anaerobic and aerobic conditions. More than
30
years of extensive titanium
alloy use in biologically active process and raw cooling waters, especially seawater, appears
to substantiate titanium’s resistance to MIC
[68].
Characteristic features of titanium alloys
resistance to MIC include the following
[68]:
1. Titanium alloys are fully resistant to the reduced chemical species associated with anaero-
bic activity over the total range
of
concentrations and temperature as high as 212°F
(
100°C).
2.
Titanium alloys are exceptionally resistant to the oxidizing and acidic conditions and com-
pounds associated with aerobe activity.
3.
The surface oxide film remains intact under fully deoxygenated, reducing conditions down
to a pH of
2
at
100°C.
4.
The surface oxide film exhibits excellent resistance to atomic and diatomic hydrogen ab-
sorption, the hydrogen being produced by the metabolism
of
sulfate-reducing bacteria.
5.
Both algae and fungi cannot produce conditions that affect titanium alloy passivity.

626
Chapter
I2
Nevertheless, titanium alloys are not biotoxic and permit growth or attachment of any
micro or macro biofilm or organism on metal surfaces. However,
in
no case has localized
corrosion ever been observed beneath these biofilms. Periodical cleaning to avoid the buildup
of the biofouling is necessary or the water velocity should be sufficiently high.
Control
of
MIC.
Prevention of MIC in most of the metals involves trying to prevent the
occurrence, growth, and metabolic activities of MIC causing microbes
in
the vicinity of the
metals. The important means of microbial control are:
1. Untake filtration
2.
Systematic cleaning and elimination of stagnant areas
3.
Proper use of a biocide
4. Thermal shock treatments.
Uptake Filtration. Intake screens are now available with mesh sizes down to
0.5
mm,
small enough to filter out stringy debris and all but the smallest organisms [26].
Systematic Cleaning and Elimination of Stagnant Areas. Since the presence of scales,
shellfish, corrosion products, etc. stimulates pitting and, in particular, crevice corrosion in the
tubes, keep the tubes clean either continuously by on-line cleaning systems, or intermittently
by off-line cleaning methods [26].
Biocides.
The most practical and efficient method of controlling microbiological activity
in cooling waters is through chemicals known as biocides. These biocides kill the organism or
inhibit their growth and reproductive cycles. Biocides used in cooling water system include
chlorides, chlorine dioxide, bromine, organo-bromide, methylene bisthiocynate, isothiazoli-
none, quaternary ammonium salts, organo-Wquaternary ammonium salts [69], copper salts,
chlorine donors (e.g., phenates), thiocynates, and acrolein
[70].
Limitations of Biocides.
1.
Even though these chemicals can do a good job of killing organisms
in
the bulk water
phase, they are much less effective in penetrating biofilms (slime) and killing the organ-
isms therein.
2.
While selecting biocides, consideration should be given for toxicity to plant and animal
life exposed
to
the discharged water.
3.
Hard-shelled organisms such as barnacles, mussels, and others possess the ability to tightly
close their shells when first sensing a toxic substance such as chlorine
in
the water,
to
remain closed until it passes, and then to reopen and resume feeding [66].
Thermal Shock. The thermal shock treatment involves recirculating the cooling water to
allow the temperature
to
increase
to
120°F
(49"C), a condition that ensures the death of
most
organisms
.
Microbiologically Influenced Corrosion Failure Analyses.
MIC can be diagnosed by tech-
niques such as in situ bacterial sampling of residual water, bacterial analysis of corrosion
products using analytical chemistry, culture growth, and scanning electron microscopy, as well
as nondestructive examination using ultrasonics and radiographic techniques. Metallographic
examination can reveal MIC characteristics such as dendritic corrosion attack in weld metal
[71].
If
special techniques are not followed, they can be misdiagnosed as attack caused by
conventional chloride crevice/pitting corrosion attack.
Corrosion of Weldments
Weld Metal Versus Parent Metal.
Corrosion of weldments occurs in spite of proper selection
of base metal and filler metal, codes and standards followed, and postweld heat treatment
(PWHT) carried
out.
In welded joints, the weld metal is usually
of
matching composition to
the parent material or overmatched, with its choice normally being dictated by the need to

-
----
Co rrosion
62
7
obtain a sound weld of mechanical properties comparable with those of the parent material and
resistance to galvanic corrosion [72]. A survey of weld failures, reported at Corrosion
82.
a
forum on corrosion sponsored by the National Association of Corrosion Engineers, showed
that poor welding practices such as poor fitups, misalignments, and incompletely fused root
beads have caused many weld failures in process vessels. Incomplete fusion, particularly
in
root passes, is a common source of notches and crevices
[6].
The factors that control the
corrosion resistance of the weldments and how to optimize the weld quality are discussed
in
this section.
Causes
of
Corrosion
of
Weldments.
In addition to the material composition, the following
welding desigdprocess-related features contribute to weld material corrosion
[73]:
1.
Weldment design
2.
Fabrication technique
3.
Heat input and welding practice
4.
Protection of welding environment; carbon and nitrogen pickup during welding
[6].
5.
Formation of oxide film and scale
6.
Weld slag and spatter
7.
Welding defects like, incomplete weld penetration or fusion, porosity, cracks, crevices,
HAZ, and high residual stresses
8.
Metallurgical factors like microsegregation, precipitation of intermetallic phases, forma-
tion of unmixed zones, recrystallization and grain growth
in
the weld HAZ, volatization
of
alloying element(s) from the molten weld pool
9.
Postweld heat treatment (PWHT)
10.
Postweld cleaning
The thermal cycle due to the welding process affects the microstructure and surface com-
position of welds and adjacent base metal. Often both crevice corrosion (Fig.
31)
and pitting
corrosion are associated with the weld defects. The presence of secondary phases having differ-
ent oxidation potential and the attack on the phases of highest potential can be quite common.
Weld deposits containing nonmetallic inclusions and porosity can accelerate the corrosion rate.
Sometimes the weld metal may be deficient
in
corrosion resistance compared to the parent
metal due to microsegregation of an important alloying elements of the parent metal. This is
especially true for welding
6-MO
superaustenitic stainless steel. This situation is overcome by
overalloying the filler metal. Weld deposits containing entrapped slag particles or residues
from fluxes act as sites for cathodic reaction and can lead to increased weld metal corrosion.
Measures to Improve the Corrosion Resistance
of
Weld Metal.
Corrosion resistance of the
weld metal can be improved by:
Crevice
corrosion
starts
in
small
gaps
Figure
31
Crevice corrosion due to weld defect. (From Ref.
6.)

628
Chapter
12
1.
Shielding the molten and hot metal surfaces from reactive gases
in
the weld environment,
including protection of underside of the root
2.
Controlling grain growth in the HAZ
3.
Controlling the precipitation of the intermetallic phases during welding/postweld heat treat-
ment.
4.
Proper postweld heat treatment
5.
Postweld cleaning and passivating the surfaces in the case of stainless steels
6.
Subjecting the weldments for thorough nondestructive testing (NDT) for weld defects
4
CORROSION
PREVENTION
AND CONTROL
The majority of the corrosion control techniques employed center around the ideas of either
isolating the corroding metal from the environment, or modification of the environment
so
that
either the anodic or the cathodic reaction is brought under control
[27].
Alternately, use corro-
sion-resistant metals in equipment construction. The last 25 years have seen the development
of many specialized methods for improving the corrosion resistance
of
structural components
in
corrosive environments. These include coatings and cladding of alloy steels and minor alloy
additions to the
300
series stainless steels, chromizing
of
internal surfaces of 2.25Cr-1Mo
[74],
and alonizing steel tubes. Corrosion control measures have been mentioned already briefly
while discussing various forms of corrosion. However, some generalized procedures are
ex-
plained in this section.
4.1
Principles
of
Corrosion Control
Corrosion control can be divided into two principal approaches: (1) corrosion prevention, and
(2) corrosion protection. Corrosion prevention is based on the idea of designing equipment
so
that corrosion cannot occur, whereas corrosion protection aims at minimizing the corrosion
attack. Since corrosion of a material takes place due to electrochemical reaction with its envi-
ronment,
in
most practical situations this attack cannot be prevented; it can only be controlled
so
that a useful life is obtained from the equipment. Various techniques for corrosion control
are as follows:
1.
Use of proper design
2.
Changing the characteristics of the corrosive environment
3.
Use of corrosion-resistant materials
4.
Bimetal concept involving cladding and bimetallic tubes
5.
Application of barrier coats, surface treatment
6.
Providing electrochemical protection by cathodic or anodic protection
7. Passivation
There are advantages, disadvantages, and areas of the
most
economical use for each
of
these methods.
No
single method is a universal cure for all corrosion problems [l]. Each
problem must be individually studied before implementing the corrosion control measure. The
technical solution most suitable to these problems should be decided through cost-benefit anal-
ysis by the plant user.
4.2
Corrosion Control
by
Proper Engineering Design
Design is an important aspect in the prevention of corrosion. In fact, in many engineering
structures the “weakest spot” is the lack of consideration given to corrosion control during the
design stage.
Corrosion
629
Design Details
The design details of pressure vessels and heat exchangers can have a significant effect on
corrosion. Design details to minimize corrosion are discussed in ref.
15.
During the design
stage, give consideration to crevices, galvanic couples, drainage, and ventilation. Vessels
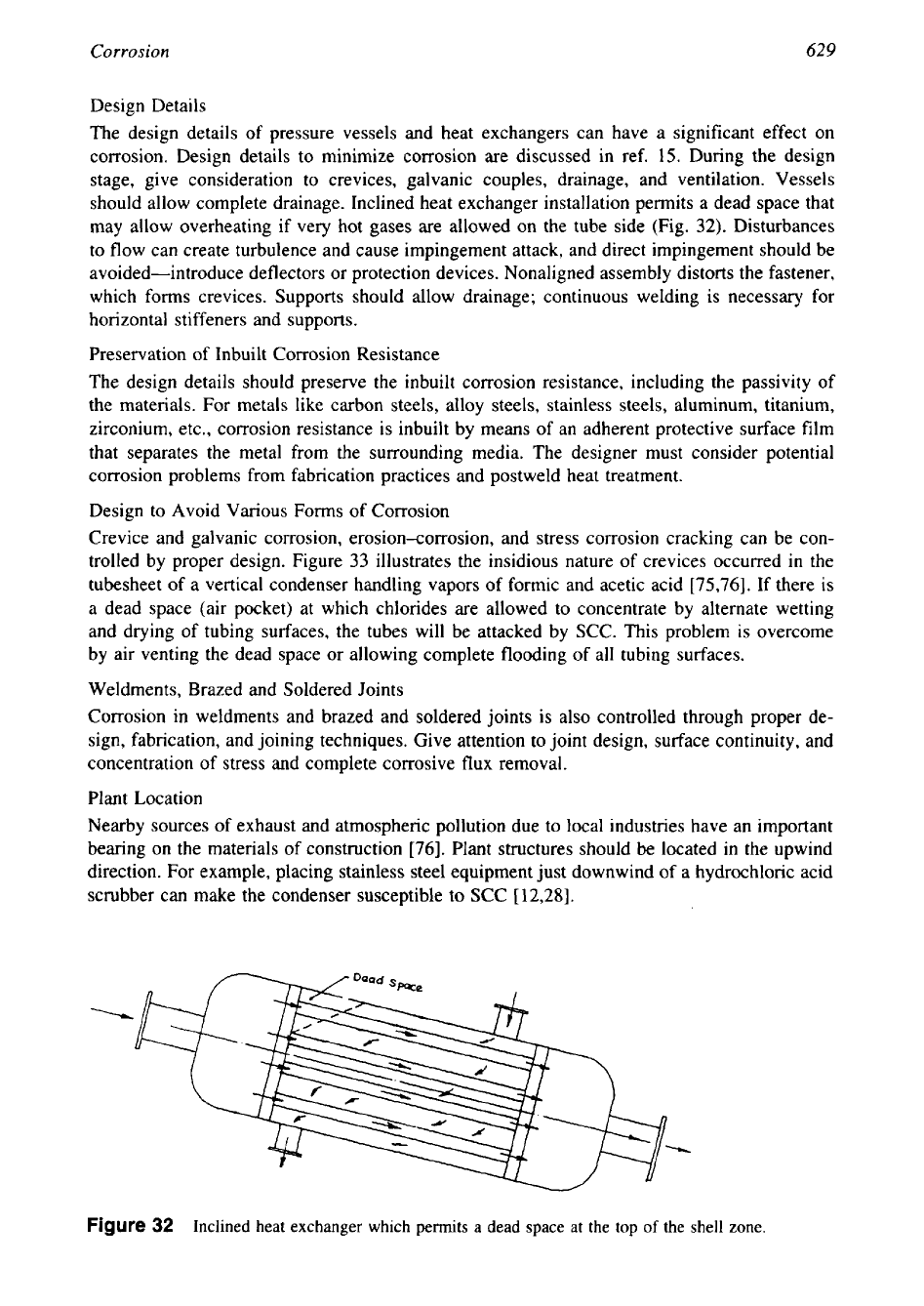
should allow complete drainage. Inclined heat exchanger installation permits a dead space that
may allow overheating if very hot gases are allowed on the tube side (Fig.
32).
Disturbances
to flow can create turbulence and cause impingement attack, and direct impingement should be
avoided-introduce deflectors or protection devices. Nonaligned assembly distorts the fastener,
which forms crevices. Supports should allow drainage; continuous welding is necessary for
horizontal stiffeners and supports.
Preservation of Inbuilt Corrosion Resistance
The design details should preserve the inbuilt corrosion resistance, including the passivity of
the materials. For metals like carbon steels, alloy steels, stainless steels, aluminum, titanium,
zirconium, etc., corrosion resistance is inbuilt by means of an adherent protective surface film
that separates the metal from the surrounding media. The designer must consider potential
corrosion problems from fabrication practices and postweld heat treatment.
Design to Avoid Various
Forms
of
Corrosion
Crevice and galvanic corrosion, erosion-corrosion, and stress corrosion cracking can be con-
trolled by proper design. Figure
33
illustrates the insidious nature of crevices occurred in the
tubesheet of a vertical condenser handling vapors of formic and acetic acid
[75,76].
If there is
a dead space (air pocket) at which chlorides are allowed to concentrate by alternate wetting
and drying of tubing surfaces, the tubes will be attacked by
SCC.
This problem is overcome
by air venting the dead space or allowing complete flooding of all tubing surfaces.
Weldments, Brazed and Soldered Joints
Corrosion in weldments and brazed and soldered joints is also controlled through proper de-
sign, fabrication, and joining techniques. Give attention to joint design, surface continuity, and
concentration of stress and complete corrosive flux removal.
Plant Location
Nearby sources of exhaust and atmospheric pollution due to local industries have an important
bearing on the materials of construction
[76].
Plant structures should be located in the upwind
direction. For example, placing stainless steel equipment just downwind of a hydrochloric acid
scrubber can make the condenser susceptible to
SCC
[
12,281.
Figure
32
Inclined heat exchanger which permits a dead space at the top
of
the shell zone.

630
Chapter
12
TUBESHEET
I
APPED
APOR
UBESHEE
T
1
f
VENT
ING
VERT
I
CAL
(b)
HEAT EXCHANGER
Figure
33
Crevice corrosion due to concentration
of
vapor phase and remedial measure.
(a)
Poor
design, and
(b)
correct design.
Startup and Shutdown Problems
The construction materials should withstand not only the requirements at the design conditions
but also the process upsets, startups, shutdowns, and standby. During these conditions, corro-
sion conditions can change significantly. Many corrosion problems do not originate when a
plant is onstream. Rather, they can be traced to irregularities during startup or shutdown.
Startup problems are often related to high temperature, difference in corrodent concentration,
inadequate distribution of inhibitors, or incomplete oxygen removal
[28].
Downtime problems
are usually caused by residual process fluids or dry residues that result from prolonged shut-
down periods or decaying of organic matter during shutdown periods. Residual fluids can
promote localized corrosion such as pitting or crevice corrosion of stainless steel. Typical cases
for shutdown problems are polythionic acid corrosion of stainless steel vessels
in
refinery
applications, and
SCC
and sulfide attack
of
copper alloys due to ammonia liberated from
putrefying organisms during shutdown periods.
Overdesign
In many instances, when the corrosive effect of a system is known, additional thickness to
various components will be provided for in the design. This is known as a corrosion allowance.
Because this thickness is in addition to that required for the design conditions, an extra cost is
involved. Highly corrosive fluids may require the use
of
expensive corrosion-resistant metals
such as stainless steel, titanium, nickel base alloys, zirconium, tantalum, and nonmetals such
as ceramics, graphite, glass, Teflon, etc. However, with the use of the special materials nor-
mally the corrosion allowance is neglected or minimized.
Efiect
of
the Service Environment: Code and Regulatory Requirements.
The codes recognize
that corrosion can occur and provide rules for including corrosion allowances to be specified
by the designer
in
the calculation of required thickness.

Corrosion
63
1
4.3
Corrosion Control by Modification of the Environment
(Use of Inhibitors)
Since corrosion is the reaction between a metal and its environment, any modification to the
environment that makes it less aggressive will be beneficial in limiting the corrosion attack
upon the metal. The corrosivity of a corroding medium can be changed by using various
methods such as the following
[77]:
1. Use a chemical additive, known as an inhibitor, that will have an effect on the electrochem-
ical reaction to stifle corrosion.
2.
Change the corrosivity of the environment by removing the active corrosive constituents.
Some examples are removal of oxygen and carbon dioxide from boiler feed water, soften-
ing, demineralization
of
cooling waters, removal of chloride ions from a solution, and
deaeration of acid solutions in contact with copper and nickel alloys.
Inhibitors
An inhibitor can be generally defined as a material that, when added to a corrodent (liquid),
interferes with or retards the electrochemical reaction. To control corrosion, inhibitors are
commonly added in small amounts to acids, cooling waters, steam, and other environments,
periodically or continuously
[78].
Inhibitors can be roughly classified according to the way
in
which they retard the electrochemical reaction to stifle corrosion. On this basis, if they inhibit
the anodic reaction, they are called anodic inhibitors; those that inhibit the cathodic reaction
are cathodic inhibitors; and if they inhibit both the anodic and cathodic reactions, they are
called mixed inhibitors. The effect of an inhibitor in most cases is to form a barrier between
the metal surface and the environment. Inorganic compounds such as chromates and nitrites
interfere with the anodic reaction while the polyphosphates suppress the cathodic reaction.
Another classification
of
inhibitors is single-component inhibitors and multicomponent inhibi-
tors. The underlying principles of anodic and cathodic inhibitors are discussed next.
Anodic
Inhibitors.
During electrochemical reaction, a metal is dissolved at anodic areas to
give metal ions, and electrons that flow to cathodic areas. This may be represented as follows
for a divalent metal:
M-+M"+2e
(7)
A
chemical that prevents or restricts action (in Eq.
7)
is called an anodic inhibitor and
usually functions by combining with the
M"
ions emerging from the corresponding area to
form an insoluble compound on the metal surface. Typical anodic inhibitors include chromates,
nitrites, phosphates, molybates, orthophospates, silicates, and some organic materials.
There are of three general types of anodic inhibitors:
1.
Passivators
2.
Oxidizing inhibitors
3.
Nonoxidizing inhibitors such as orthophosphate
The passivators function by converting an anodic area to cathodic area, usually by means of
impermeable oxide films. Oxidizing inhibitors function by increasing the oxidation potential
at the anodic surfaces to increase the rate of formation of gamma-iron oxide
[79]
or are effec-
tive in repairing discontinuity in the passive film by quickly oxidizing iron wherever the sur-
face is exposed. Chromate is the best known oxidizing anodic inhibitor in cooling-water sys-
tems. Its merits and demerits are discussed next.

632
Chapter
I2
Chromate. Chromate is probably the most effective corrosion inhibitor for water systems.
Protection is afforded by a film consisting of alpha-ferric oxide and chromic oxide
[8].
Chro-
mates are the least expensive for use in water systems and are widely used in the recirculating
cooling-water systems of internal combustion engines. However, chromates and heavy metals
have recently become ecologically unacceptable in many places. To avoid toxic chemicals and
meet regulations on effluent discharge, a large number of substitutes were developed, including
zinc, poly-and orthophosphates, phosphonates, and a variety of polymers.
Cathodic Inhibitors.
Cathodic inhibitors work by increasing the degree
of
cathodic polariza-
tion, thereby reducing the overall corrosion rate and current density, or they reduce corrosion
by interfering with any of the steps
of
the oxygen reduction reaction. The cathodic reactions
that complement the anodic process (Eq.
6)
are Eqs.
2
and
3,
and these reactions are necessary
to absorb the electrons released by the dissolution of the metal at the anode.
A
compound that
restricts reactions by Eq.
2
or
3
is called a cathodic inhibitor. Reaction by Eq.
2
will prevail
in acid solutions. Reaction by Eq.
3
will prevail over reaction by Eq.
2
when there is an ample
supply of dissolved oxygen and a low concentration of hydrogen ions as in alkaline solutions.
By preventing absorption
of
the electrons released by the anodic reaction, corrosion must stop.
Zinc is a well-known cathodic inhibitor
[SO].
Typical cathodic inhibitors are calcium bicarbon-
ate, polyphosphates, phosphonates, metal cations, and organics. Cathodic inhibitors are often
termed “safe” because they do not usually cause localized pitting attack
[
161.
Cathodic inhibi-
tors are somewhat less effective than anodic inhibitors
[Sl].
Adsorption Inhibitors
or
Organic (Nonchromate) Corrosion Control Polymers.
Some organic
compounds are effective as inhibitors because of adsorption mechanisms. Oil inhibitors are
effective because of the physical adsorption process
[82].
Organic inhibitors used in automotive
diesel engine cooling-water systems include amines, benzoates, organic phosphates, mercap-
tants, triazoles, and polar type oils. Organic inhibitors such as starch quinoline and its deriva-
tives and thiourea and its derivatives are commonly used for inhibition in acid media.
Multicomponent Systems.
Single-component inhibitors include chromates, sodium nitrite, sili-
cates, sodium molybdate, and sodium phosphate. Multicomponent systems with many combi-
nations have pronounced synergism in controlling steel corrosion in recirculating cooling-water
systems compared with the individual components. Multicomponent systems can be either
heavy metal treatments or non-heavy metal treatments (no zinc or chromate). Typical heavy
metal multicomponent treatments include zinc chromate, zinc chromate/phosphonate, zinc pol-
yphosphate, and zinc phosphonates. Non-heavy metal multicomponent systems (no zinc or
chromium) in current use are
[69,81]
(1)
combination of the phosphates (AMP/HEDP),
(2)
polyphosphate-phosphonate
mixtures, and
(3)
the
polyphosphate-orthophosphate.
Non-heavy metal treatment programs are receiving increased attention because of environ-
mental regulations against discharge.
Passivation Inhibitors.
Passivation inhibitors, also known as film formers, work by deposit-
ing protective films over the entire surface, which provides a barrier to the dissolution of the
metal
in
the corrosive environment. Typical passivation inhibitors include
(
1
)
chemical oxidiz-
ing substances, such as chromate and nitrate, and
(2)
organic substances such as tannin, gela-
tine, saponin, and beta-diketones, used in alkaline solutions.
Precipitation Inhibitors.
Precipitation inhibitors produce insoluble films on the cathode under
conditions of locally high pH and isolate the cathode from the environment
[69].
Sodium
polyphosphate and zinc salts such as zinc sulfate and zinc chloride are examples of precipita-
tion inhibitors.
Copper Inhibitors.
Though the previously mentioned ferrous inhibitors exert some control
over corrosion of copper base alloys, three specific inhibitors are extensively used on to protect

Corrosion
633
copper base alloys
[69]: (1)
mercaptobenzothinzole (MBT), (2) benzotriazole, and
(3)
polytria-
zone.
Requirements
for
EfSectiveness
of
Inhibitors.
The key to the success of an inhibitor is the
maintenance of clean metal surfaces, which are essential for effective inhibitor film formation
[83].
The inhibitors used should be compatible with the process fluids being used. Consider-
ation should be given to avoiding adverse effects such as foaming, decrease in catalytic activ-
ity, degradation of another material, and loss of heat transfer
[78].
Inhibitor
Evaluation.
To determine the effectiveness
of
an inhibitor for use in a specific
application, a comparison is made by using any of the corrosion testing techniques to determine
the corrosion rate of the medium without inhibitor and the test is repeated with each inhibitor
present in the medium. The effectiveness of each inhibitor can be calculated from the following
equation:
where
q,
is the inhibitor efficiency,
rn,
the corrosion rate without inhibitor, and
rn,
the corrosion
rate with inhibitor.
Electrochemical techniques such as the galvanostatic pitting potential test can be used to
determine the effectiveness of various corrosion inhibitors in the automobile cooling-water
system. This method correlates the effectiveness of inhibitors in the prevention of pitting corro-
sion by measuring the inhibitor’s effect on the pitting potential
(Ep).
4.4
Corrosion-Resistant
Alloys
Corrosion of certain metals is due to impurities in them. By controlling the impurities, the
corrosion resistance can be improved markedly. Before the advent
of
the advanced refining
techniques, ferritic stainless steels had relatively high levels of interstitials like carbon and
nitrogen, and as a result had serious limitations with respect to fabricability, toughness, and
corrosion resistance. The newer ferritics, especially those with high chromium content and
very low carbon content, have become possible through argon oxygen decarburization (AOD),
vacuum induction melting (VIM), and vacuum oxygen decarburization (VOD)
[84].
On the
other hand, a small addition of alloying element may have a profound effect on an alloy’s
resistance to certain types of corrosion. Addition of small amounts of copper to steel increases
its atmospheric corrosion reistance; small amounts of phosphorus, antimony, and arsenic added
to brasses inhibit dezincification; and small amounts of arsenic added
to
copper, and iron added
to copper-nickel, increase the resistance to impingement attack and erosion-corrosion.
Besides changes in composition, metallurgical variations and mechanical effects may have
an effect on reducing corrosion damage. Introduction of compressive stresses on the surface
by shot peening will reduce corrosion fatigue or
SCC.
Annealing to reduce the internal stresses
and solution annealing to remove the grain boundary segregation help in eliminating SCC and
intergranular attack, respectively.
If corrosion of a metal in an environment is inevitable, use an alternate material, such as
borosilicate glass, impervious graphite, zirconium, tantalum, Teflon, etc., that shows chemical
inertness to most of the chemicals.
A
recent focus to tackle severe corrosion is on three alloy families
[85]:
(1)
duplex stain-
less steels, which exhibit good corrosion resistance to both pitting and SCC, and have about
twice the yield strength of the typical austenitic stainless steel,
(2)
6-MO
superaustenitic stain-
less steels, and
(3)
high-nickel alloys such as
UNS
NO
6625,
UNS
NO
4400,
and
UNS
NO
7716.
