Kuppan T. Heat Exchanger Design Handbook
Подождите немного. Документ загружается.

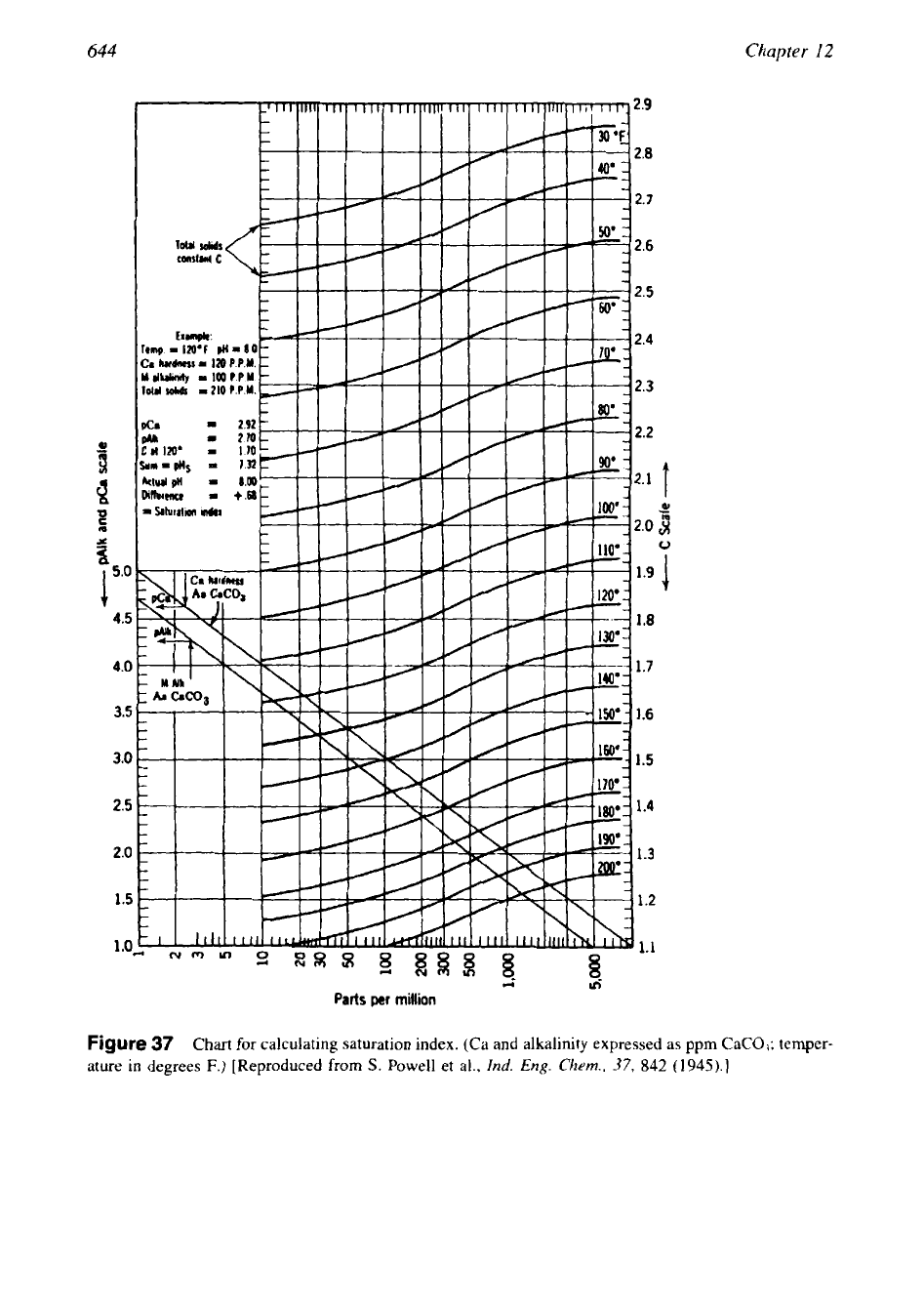
644
Chapter
12
4.!
3.1
2.
2.1
1.
I!
Figure
37
Chart
for
calculating saturation index. (Ca and alkalinity expressed as ppm
CaCO,;
temper-
ature in degrees
F.)
[Reproduced from
S.
Powell
et
al.,
lnd.
Eng.
Chem.,
37,
842
(1945).]
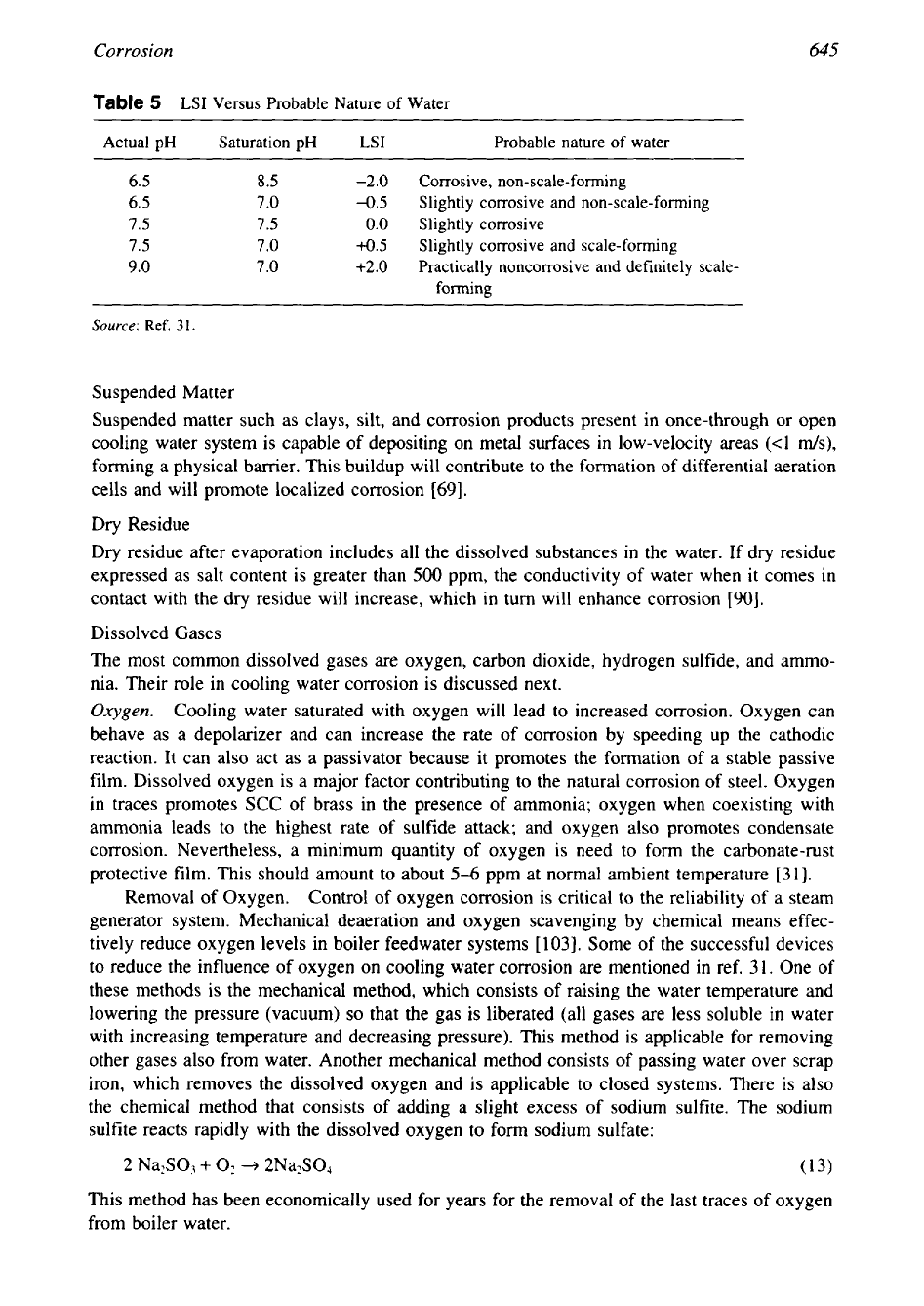
Corrosion
645
Table
5
LSI
Versus Probable Nature
of
Water
Actual
pH
Saturation
pH
LSI
Probable nature
of
water
~~~ ~~ ~~ ~~~~~~~
6.5
8.5
-2.0
Corrosive, non-scale-forming
6.5 7.0
-0.5
Slightly corrosive and non-scale-forming
7.5
7.5
0.0
Slightly corrosive
7.5
7.0
4.5
Slightly corrosive and scale-forming
9.0
7
.O
+2.0
Practically noncorrosive and definitely scale-
forming
Source:
Ref.
31
Suspended Matter
Suspended matter such as clays, silt, and corrosion products present in once-through or open
cooling water system is capable of depositing on metal surfaces in low-velocity areas (<1
ds),
forming a physical barrier. This buildup will contribute to the formation of differential aeration
cells and will promote localized corrosion [69].
Dry Residue
Dry residue after evaporation includes all the dissolved substances in the water. If dry residue
expressed as salt content is greater than
500
ppm, the conductivity of water when it comes in
contact with the dry residue will increase, which in turn will enhance corrosion [90].
Dissolved Gases
The most common dissolved gases are oxygen, carbon dioxide, hydrogen sulfide, and ammo-
nia. Their role in cooling water corrosion is discussed next.
Oxygen.
Cooling water saturated with oxygen will lead to increased corrosion. Oxygen can
behave as a depolarizer and can increase the rate of corrosion by speeding up the cathodic
reaction. It can also act as a passivator because it promotes the formation of
a
stable passive
film. Dissolved oxygen is a major factor contributing to the natural corrosion
of
steel. Oxygen
in traces promotes SCC
of
brass in the presence of ammonia; oxygen when coexisting with
ammonia leads to the highest rate of sulfide attack; and oxygen also promotes condensate
corrosion. Nevertheless, a minimum quantity of oxygen is need to form the carbonate-rust
protective film. This should amount to about
5-6
ppm at normal ambient temperature [31].
Removal of Oxygen.
Control
of
oxygen corrosion is critical to the reliability
of
a steam
generator system. Mechanical deaeration and oxygen scavenging by chemical means effec-
tively reduce oxygen levels in boiler feedwater systems
[
1031. Some
of
the successful devices
to reduce the influence of oxygen on cooling water corrosion are mentioned in ref. 31. One of
these methods is the mechanical method, which consists
of
raising the water temperature and
lowering the pressure (vacuum)
so
that the gas is liberated (all gases are less soluble in water
with increasing temperature and decreasing pressure). This method is applicable for removing
other gases also from water. Another mechanical method consists of passing water over scrap
iron, which removes the dissolved oxygen and is applicable to closed systems. There is also
the chemical method that consists of adding a slight excess of sodium sulfite. The sodium
sulfite reacts rapidly with the dissolved oxygen
to
form sodium sulfate:
2 Na2S03
+
0:
-+
2Na2S04
(13)
This method has been economically used for years for the removal of the last traces
of
oxygen
from boiler water.

646
Chapter
12
Carbon Dioxide.
Natural cooling water always contains free carbon dioxide, in larger or
smaller quantities. Dissolved free carbon dioxide makes water acidic. The free carbon dioxide
is defined as the quantity that is required to retain the alkaline earth carbonate in accordance
with the equation:
CaC03
+
CO2
+
H20
*
Cat*
+
2HCOj
(14)
Cooling waters without excess free carbon dioxide lead to the formation of a protective
carbonate-rust scale, which represents one of the most important protective surface layers
[90].
However, excessive carbon dioxide will cause corrosion. Excess carbon dioxide is removed
from cooling water by spraying water over a mass of coke or other inert material exposed to
the air. Sometimes by this process the carbon dioxide content can be reduced to
5-10
ppm.
The second method involves treating the water with lime
[31].
Hydrogen
SulJide.
Hydrogen sulfide in cooling waters generally results from the action of
bacteria on organic matter, particularly sewage. The presence of even few parts per million
may change the corrosivity of water drastically. The presence of hydrogen sulfide
in
a water
has the tendency to reduce inlet-end or impingement corrosion, but increases the probability
of pitting corrosion occurring along the entire length
of
a tube
[102].
Additionally, the salts
created by this attack (copper sulfide, iron sulfide, etc.) are insoluble and precipitate on the
metal surface as loose, noninhibitive deposits that promote galvanic couple and thus promote
corrosion
[
81.
Ammonia.
Ammonia is originally derived from the thermal degradation of various nitrogen-
containing compounds added to the boiler feedwater to reduce corrosion. Ammonia does not
cause excess corrosion on ferrous metals. However, traces of ammonia will cause both rapid
general thinning and stress corrosion cracking in brasses. Pure copper and copper-nickel alloys
are not susceptible to ammonia SCC, but a higher rate of uniform corrosion will take place
with higher ammonia contents
[90].
Dissolved Organic Matter.
Dissolved organic matter in excess quantity tends to produce
sludge-type deposits and fouling. Dissolved organic matter is detected indirectly by detennin-
ing the potassium permanganate (KMnOJ consumption. If the KMnOl consumption exceeds
about
25
mgL water, then the water is *‘dirty,” which tends to produce sludge-type deposits
and fouling
[90].
Microbiolugical Organisms.
Corrosion due to microbiological organisms has been discussed
in
the section on microbiologically influenced corrosion.
pH
Value.
The pH of the water can have several specific effects. High pH levels make the
formation of heavy calcium carbonate and calcium phosphates scales likely. From Eq.
6,
it is
evident that where a greater number of hydrogen ions (low pH) is available, the corrosion
reaction should occur more rapidly. Natural waters produce more or less neutral solution (pH
value about 7). Clean seawater has a pH of about
8.
In general the neutral and weakly alkaline
waters usually cause no or only very slight corrosion on metallic materials, but weakly to
strongly acidic water is always corrosive. Type of water also influences corrosion. The corro-
siveness of a natural freshwater with
a
pH of
7-8
usually is much less than that of seawater
with a similar pH. The difference is due to the presence of more mineral salts in seawater.
Nevertheless, the pH
is
not the only determining factor. Water hardness, particularly the car-
bonate hardness, is of decided importance
[90].
An additional factor that decides the water
corrosion is the dissolved mineral salts.
Temperature.
In general an increasing temperature will increase corrosion rates. Temperature
plays a dual role with respect
to
oxygen corrosion. For an open system, beyond
175°F
(80°C)
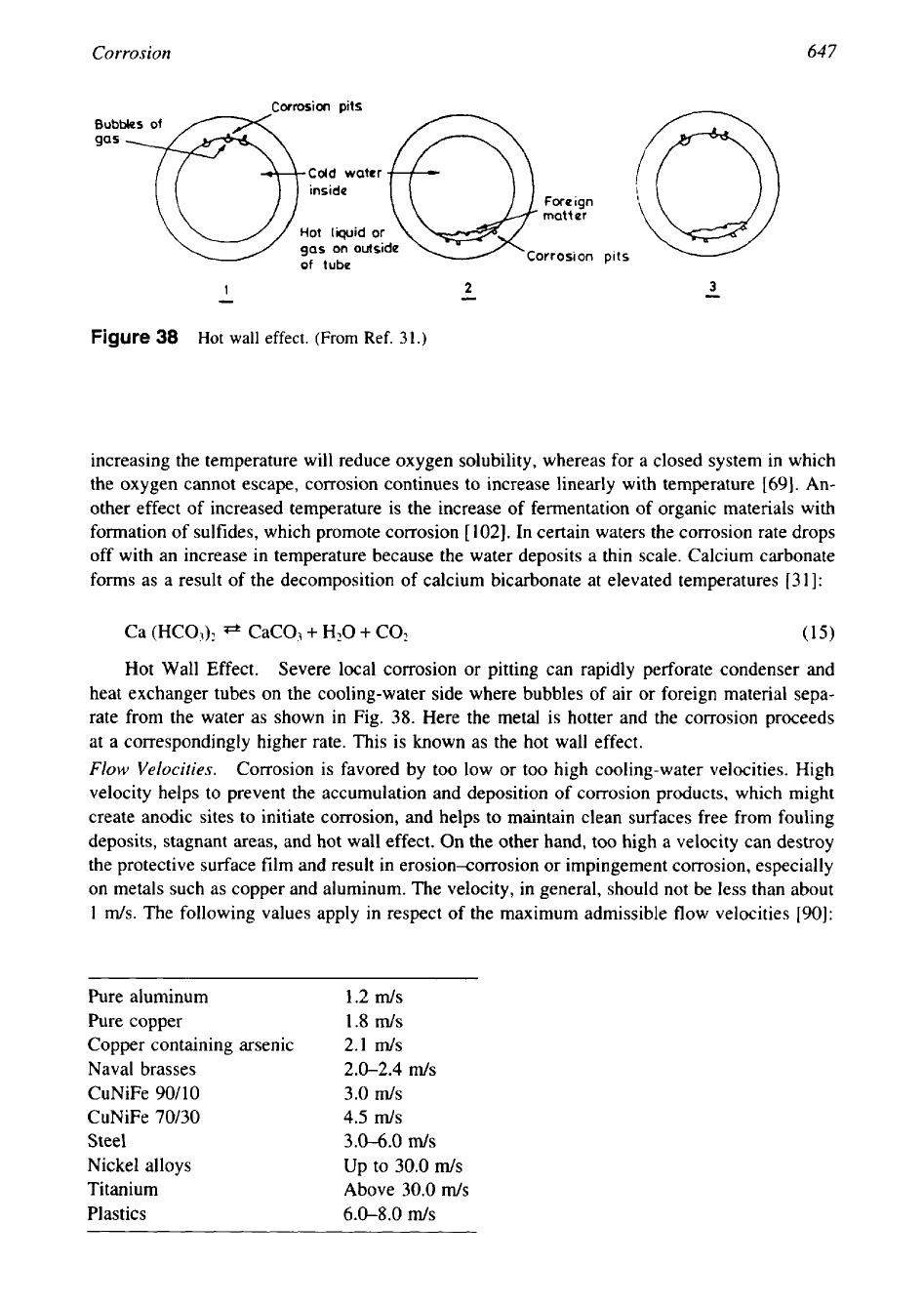
-
-
Corrosion
647
r
ore
ign
matt
er
Corrosion
pits
3
-
1
2
Figure
38
Hot
wall
effect.
(From
Ref.
31.)
increasing the temperature will reduce oxygen solubility, whereas for a closed system in which
the oxygen cannot escape, corrosion continues to increase linearly with temperature
[69].
An-
other effect of increased temperature is the increase
of
fermentation of organic materials with
formation
of
sulfides, which promote corrosion
[
1021. In certain waters the corrosion rate drops
off with an increase in temperature because the water deposits a thin scale. Calcium carbonate
forms as a result of the decomposition of calcium bicarbonate at elevated temperatures
[31]:
Ca (HCO& CaC03
+
H20
+
CO2 (15)
Hot Wall Effect. Severe local corrosion or pitting can rapidly perforate condenser and
heat exchanger tubes on the cooling-water side where bubbles of air or foreign material sepa-
rate from the water as shown in Fig.
38.
Here the metal is hotter and the corrosion proceeds
at a correspondingly higher rate. This is known as the hot wall effect.
Flow
Velocities.
Corrosion is favored by too low or too high cooling-water velocities. High
velocity helps to prevent the accumulation and deposition
of
corrosion products, which might
create anodic sites to initiate corrosion, and helps to maintain clean surfaces free from fouling
deposits, stagnant areas, and hot wall effect. On the other hand, too high a velocity can destroy
the protective surface film and result in erosionxorrosion or impingement corrosion, especially
on metals such as copper and aluminum. The velocity, in general, should not be less than about
1
m/s.
The following values apply in respect of the maximum admissible flow velocities
[90]:
Pure aluminum
1.2
m/s
Pure copper
1.8
m/s
Copper containing arsenic
2.1
m/s
Naval brasses
2.0-2.4
m/~
CuNiFe
90/10
3.0
m/s
CuNiFe
70/30
4.5
m/s
Steel
3.0-6.0
m/s
Nickel alloys
Up to
30.0
m/s
Titanium
Above
30.0
m/s
Plastics
6.0-8.0
m/s
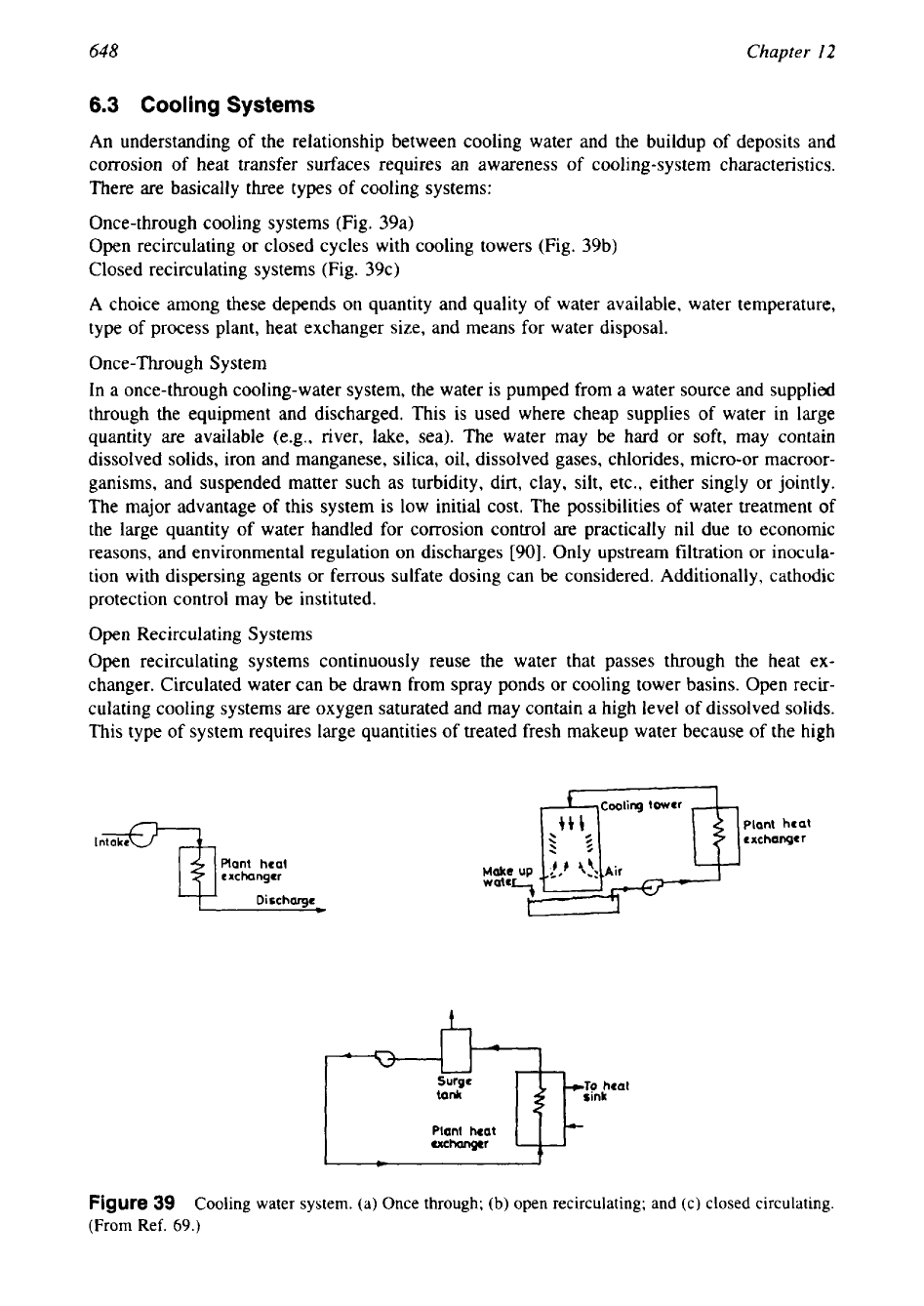
648
Chapter
12
6.3
Cooling
Systems
An understanding of the relationship between cooling water and the buildup of deposits and
corrosion of heat transfer surfaces requires an awareness of cooling-system characteristics.
There are basically three types of cooling systems:
Once-through cooling systems (Fig. 39a)
Open recirculating or closed cycles with cooling towers (Fig. 39b)
Closed recirculating systems (Fig. 39c)
A
choice among these depends on quantity and quality of water available, water temperature,
type of process plant, heat exchanger size, and means for water disposal.
Once-Through System
In a once-through cooling-water system, the water is pumped from a water source and supplied
through the equipment and discharged.
This
is
used where cheap supplies of water in large
quantity are available (e.g., river, lake, sea). The water may be hard or soft, may contain
dissolved solids, iron and manganese, silica, oil, dissolved gases, chlorides, micro-or macroor-
ganisms, and suspended matter such as turbidity, dirt, clay, silt, etc., either singly or jointly.
The major advantage
of
this system is low initial cost. The possibilities of water treatment of
the large quantity
of
water handled for corrosion control are practically nil due to economic
reasons, and environmental regulation on discharges [90]. Only upstream filtration or inocula-
tion with dispersing agents or ferrous sulfate dosing can
be
considered. Additionally, cathodic
protection control may be instituted.
Open Recirculating Systems
Open recirculating systems continuously reuse the water that passes through the heat ex-
changer. Circulated water can be drawn from spray ponds or cooling tower basins. Open recir-
culating cooling systems are oxygen saturated and may contain a high level of dissolved solids.
This type of system requires large quantities of treated fresh makeup water because of the high
cooling
tower
Plant heat
c
xchanger
Plant
heat
c
xchangcr
Discharge
/
A
-
Surge
tank
+TO
heat
sink
Plant
heat
c
exchanger
.
Figure
39
Cooling water system. (a) Once through;
(b)
open recirculating; and
(c)
closed circulating.
(From Ref.
69.)

Corrosion
649
evaporation losses. Open recirculating systems are susceptible to corrosion and deposits. The
intensive contact between the cooling water and air, as well as the solar radiation, stimulates
the growth of algae and microorganisms. The treatment of cooling water of this system calls
for the following measures
[90]:
Monitoring and control of pH
Adding a corrosion inhibitor
Adding an algae-destroying agent
An additive (stabilizer) to prevent calcium carbonate precipitations
Possible makeup water treatment
Partial filtration
Closed Recirculating Systems
Closed recirculating systems continuously recirculate the same water. This system has little
water loss and is free from algal growth. Because there are no evaporation losses, the require-
ment for makeup water is minimal, and the mineral content remains essentially constant. How-
ever, corrosion by-products can fould the heat exchanger. Hence, fouling deposits should be
removed periodically by suitable offline cleaning methods. The completely closed cycle repre-
sents the ideal possibility for a clean and effective water treatment. Corrosion control by inhibi-
tors is the most important control measure.
6.4
Corrosion Control Methods for Cooling-Water Systems
In principle, damage to cooling systems can be checked in many ways:
1.
Proper material selection
2.
Cooling water system design
3.
Continuous water treatment system
4.
Use of inhibitors
5.
Ferrous
sulfate dosing
6.
Protective coatings
7.
Cathodic protection
8.
Passivation
9.
Biological control
10.
Scale control
11.
Systematic cleaning
Corrosion control measures such as protective coating and cathodic protection, biological con-
trol, and various scale control measures have been discussed earlier in this chapter. Additional
measures to control scaling as a foulant were discussed in Chapter
9,
Fouling.
Material Selection
Corrosion control by means of cooling water design should take into account two possibilities:
(1)
the water treatment program, and
(2)
selecting corrosion-resistant material. Proper material
selection involves selecting a material that can be exposed to the cooling water without the
danger of corrosion. The question of whether corrosion in cooling systems can be checked by
means of suitable material selection at the design stage or by instituting a permanent water
treatment program when the unit
starts
functioning must be considered on the basis of corrosion
damage and economic considerations
[90].
On the material side, cupronickel and titanium are
costly compared to carbon steel, aluminum and copper, and certain copper alloys such as
brasses. One the water treatment side, continuous online treatment including dosing of chemi-
cals and additives involves money, especially when the quantity of water handled is large, as

650
Chapter
I2
in once-through cooling water system. The objectives of such a strategy are to ensure that the
operating costs are minimized throughout the life of the plant. This can be established by
studying the following technical and economic considerations
[33]:
Estimated life of candidate tube materials from trials, manufacturer’s literature, and experience
Predicted number of outages and associated costs
Number
of
retubes required
Capital costs of materials and retubing costs
Heat transfer efficiency and thermal performance effects
Compatibility with other cooling- water sys tem materials
Cooling-Water System Design
In planning a heat exchanger cooled by natural water, the first step is to obtain the following
information about the water
[32]:
Analysis of water
Water temperature upon entry
Solids content
Content of organic matter including
H2S
Certain guiding principles to be considered at the design stage are as follows
[90,92]:
Empty the cooling system completely when in the standstill condition. Many more cases of
corrosion have taken place when the cooling system was at a standstill than in operation.
When welding on the cooling-water side, care should be taken to avoid protruding weldments
or crevices as a result of unsatisfactory weld penetration.
Design sealing in such a way that seals cannot lift on the water side.
Narrow gaps should be avoided in the design stage. If this is not possible, the gap width should
preferably be large
(>OS
mm).
The flow velocities should be neither too low nor too high. Except for coppers and brasses,
maintain water velocities as high
as
practical. Velocities of
3-8
ft/s are the minimum
desired.
Beware of galvanic coupling.
Always maintain vents on top tube sheets.
Locate water inlet and outlet nozzles on the shell side as near the tube sheets as possible to
reduce “dead pockets.”
Place water on the tube side, and the process liquid on the shell side.
Additional guidelines were given by Forchhammer
[32]:
In the construction phase, check the integrity of system components for tightness with low-
chloride water free from solids.
To
form the
first
oxide layer
in
the tubes, the unit should run several weeks without interruption.
Water Treatment
By water treatment, we either remove the aggressive components to a great extent
or
add
specific chemicals to the water. In this way, both corrosion and fouling are avoided. Water
treatments can be generally subdivided into three main groups:
Chlorination/settling/filtration to remove the turbidity and microorganisms
Water softening to remove water hardness
Partial or full demineralization for the removal of hardness and all dissolved salts
Corrosion
651
Removal of hardness is effected by (1) the cold lime process, (2) the sodium cation exchange
process, (3) demineralization, and (4) the acid process.
Corrosion Inhibitors
The use
of
corrosion inhibitors
is
one of the foremost methods
of
controlling corrosion
in
a
cooling-water system. The main effect that corrosion inhibitors have in aqueous ferrous sys-
tems is to reduce the initial corrosion rate sufficiently to allow the gamma-iron oxide passive
film to form and,
in
some cases, to take part directly
in
film formation. Corrosion control by
inhibitors has been discussed
in
the section on corrosion prevention and control.
Ferrous Sulfate Dosing
It is difficult to form protective films on copper alloys such as aluminum brass while subjected
to
brackish water and seawater. The addition of ferrous sulfate offers a possibility of building
up a protective film and diminishing the danger of erosion-corrosion [104]. Experience has
shown that the best results are obtained under the following conditions [32,93]:
Ferrous sulfate dosing, tube cleaning
by
sponge balls or brushes, and deaeration must start
working from the very beginning, as startup after the first corrosion attack has occurred
cannot stop corrosion.
Cleaniness of tube surfaces is especially important while commissioning.
A water temperature above
8-
10°C.
Association with continuous tube cleaning.
Solution injected at water box inlet.
Chlorination stopped during injection, since investigation shows that a synergistic effect be-
tween iron and chlorine can lead to rapid attack and early failure.
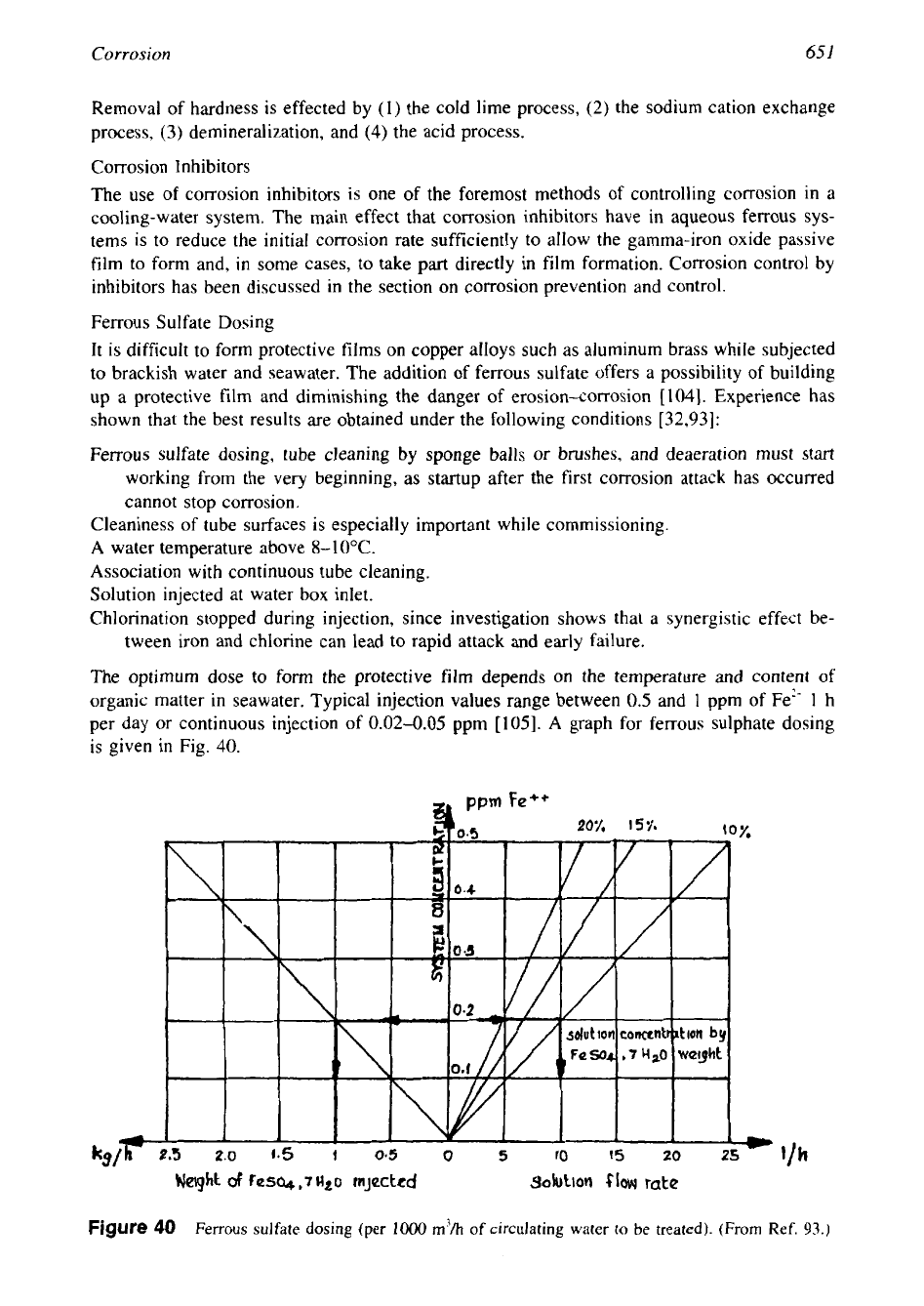
The optimum dose to form the protective film depends on the temperature and content
of
organic matter in seawater. Typical injection values range between
0.5
and
1
ppm of Fe?'
1
h
per day or continuous injection
of
0.02-0.05
ppm [105].
A
graph for ferrous sulphate dosing
is given
in
Fig. 40.
,Y
Weight
of
~QSCL+
rnjectcd
30!UhOtl
$IOW
rate
Figure
40
Ferrous sulfate dosing (per
10oO
m'/h
of circulating water to
be
treated). (From Ref.
93.)

652
Chapter
12
Passivation
Donohue et al. [95] discussed passivation of clean metal surfaces in cooling-water system
through pretreatment of surfaces by application of a
polyphosphates-surfactant
combination
and prefilming with chromate or nonchromate zinc polyphosphate or polymer polyphosphates.
Chromate, nitrite, and orthophosphate (in the presence of oxygen) can promote passivity
on
clean iron surfaces.
6.5 Influence of Cooling-Water Types on Corrosion
Cooling waters may
vary
from the purest of distilled or demineralized waters to full-strength
seawater. High-conductivity chloride-bearing water, saturated with oxygen, leads to concentra-
tion cell corrosion or deposit attack on almost all alloys, ferrous or nonferrous, regardless of
their inherent resistance to water.
Fresh Water
In general, if fresh water is involved, no serious corrosion problems are likely to arise. Fresh
waters are normally handled with tubes of arsenical copper, inhibited admiralty brass, inhibited
aluminum brass, aluminum bronze, red brass, or copper-nickels
[
1021.
Seawater Corrosion
Some of the key findings of
20
years of corrosion research on seawater are as follows
[
1061:
1.
The presence of dissolved oxygen in seawater and desalting brine is the single most impor-
tant factor in determining the performance of mild steel, low-alloy steels, copper alloys,
and, to some extent, stainless steels.
2.
For optimum performance of mild steel, low-alloy steel, copper alloys, and stainless steels
in
seawater and brine, the pH should be as high as possible without promoting calcium
carbonate scale or precipitation of magnesium hydroxide.
3.
Low-alloy steels can perform well, as much as
30%
better than mild steels in typical
desalination environments.
In seawater applications, arsenical copper and red brass
85:
15
would not be used for tubes
in power plant condensers cooled with seawater. Inhibited aluminum brass tubes are most
widely used. Aluminum bronze
(5%)
would be suitable for acid-polluted seawater. Copper-
nickels will give better service than other tubes in polluted waters [102].
Brackish Waters
Brackish waters are a mixture of seawater and fresh water. They are generally less corrosive
than seawater except where contaminated with industrial wastes and sewage. In general, when
contaminants are present, aluminum brass and aluminum bronze prove more satisfactory than
other alloys. In the absence
of
contaminants, arsenical admiralty and the cupronickel alloys are
satisfactory. The choice between these two depends upon water velocity [31]. Among ferrous
alloys, the ferritic stainless steels, such as type
410
(12% chrome) and type
430
(18%
chrome),
are unsatisfactory in brackish cooling water due to insufficient alloy content to repair the
passive films that are broken down by the deposit attack. Hence, the use of straight chrome
stainless steel
is
to be avoided
in
brackish cooling water [92].
Boiler Feedwaters
Generally, boiler feedwaters are only slightly corrosive toward copper and its alloys due to
deaeration and water treatment.
The
selection of the proper alloy is generally based on the
temperature and pressure requirements. Copper and arsenical admiralty brass are the most
commonly used for the low-pressure and low-temperature feedwater heaters, whereas cupron-

Corrosion
653
ickels are used for the higher pressure and higher temperature heaters because of their higher
creep strength
[3
11.
6.6
Corrosion
of
Individual Metals in Cooling-Water Systems
Aluminum
Waters containing small amounts of the heavy metal ions such as iron, copper, chlorides, and
mercury will cause pitting corrosion. Waters that are essentially neutral offer no problem.
Pitting of aluminum may be experienced beneath deposits. Use of the Alclad products often
makes aluminum usable
in
cooling-water services like automobile radiators.
Copper and Its Alloys
Copper and its alloys are very important heat exchanger materials because of their generally
high resistance to corrosion in waters. However, these alloys too can suffer corrosion under
deposits, erosion-corrosion, dezincification of the brasses, and sulfide attack.
Steel
The corrosion of steel in cooling water
is
governed mainly by the oxygen availability and
water composition [92]. Corrosion attack is overcome by addition of inhibitors and cathodic
protect ion.
Austenitic Stainless Steel
The corrosion resistance of chromium-nickel
(1
8-8)
stainless steels depends upon a passive
surface film. This film is readily penetrated by the chloride ion. For this reason, the stainless
steels are subject to pitting and stress corrosion cracking from the chlorides. The pitting rate
of the
18-8
alloys is usually quite low in closed-cycle cooling waters and in inhibited water
associated with periodic tube cleaning.
Superferritics and Superaustenitics
These steels have been primarily developed for seawater applications.
Titanium
Titanium is an ideal material for seawater applications in the absence of fouling deposits.
6.7
Forms
of
Corrosion in Cooling
Water
A wide spectrum of materials is used on
the
cooling-water side. These materials are affected
by most forms of corrosion discussed earlier. The corrosion prevention and control measures
discussed therein are equally applicable to combat cooling-water corrosion also. Various forms
of corrosion, susceptible alloys or susceptible components, and remedial measures are men-
tioned very briefly in the following paragraphs.
Uniform Corrosion
All alloys, especially copper and its alloys, suffer uniform corrosion, which is controlled by
pH adjustment, inhibition, and proper material selection.
Galvanic Corrosion
Less noble metals experience galvanic corrosion. Susceptible components are tube sheets, wa-
ter boxes, bolts, and flanges. Alloys close to one another in the galvanic series should be used.
Use insulation to separate the active metal from the noble metal.
