Kuppan T. Heat Exchanger Design Handbook
Подождите немного. Документ загружается.

634
Chapter
12
4.5
Bimetal
Concept
The term bimetal is applicable to any combination of two metals or alloys having nearly
matching or widely dissimilar physical and chemical properties and bonded by one of a variety
of processes. The bimetal concept is employed in
(1)
cladding, and
(2)
duplex or bimetallic
tubes.
Cladding
Cladding has been used for many years in pressure vessels, piping systems, and heat ex-
changers (Fig.
34).
Nuclear and petrochemical pressure vessels are weld overlay clad with
300
series stainless steels or high-chromium nickel-base alloys. The severe corrosion problems
in various coal combustion and incineration environments have renewed interest in cladding
technology for both Code-approved and enhanced-strength developmental alloys
[74].
Typical
cladding alloys include ferritic (straight chrome) and austenitic stainless steels, nickel-base
alloys, titanium, zirconium, and tantalum. Clad steels satisfy severe service conditions at less
cost than full-thickness sections of the costly corrosion-resistant material used for the cladding.
Various cladding methods are discussed in detail in a separate section in Chapter
13.
Bimetallic or Duplex Tubing
Two dissimilar corrodents in a heat exchanger can cause havoc, with material having a propen-
sity to corrosion to one fluid and virtually immune to the other fluid, or the problem
of
severe
corrosion of different natures that sometimes attacks both sides of a tube can often be solved
by treating such a condition as two separate corrosion problems. By selecting the specific kind
of metal that is best for each condition, it is possible to combine two such metals into one
tube, known as duplex tubing
or
bimetallic tubing. Duplex tubing is shown schematically in
Fi.g
35.
Specific information on duplex tubing is given in ref.
86.
The tube manufacturing method, mostly hot coextrusion, ensures close mechanical contact
between the two metals without affecting the heat transfer properties. Duplex tubing is made
up of various combinations of ferrous and nonferrous materials. Bimetallic tubes are available
in a variety of stainless steel-carbon steel, Cu, brass, Al, and other combinations. Duplex
tubings have been used in actual service for the past
25
years in oil refining, in production of
synthetic rubber, and in process industries, chemical plants, coke by-products plants, refrigera-
tion systems, and other applications. Typical metal combinations of bimetallic tubes used in
refinery applications, high-temperature boiler corrosion, and condenser applications are dis-
cussed below.
Duplex
Tubing
for
Refinery
Applications.
For oil refining and in the natural gas industry,
duplex tubing with the following combinations is used
[3
I]:
Figure
34
Cladded heat exchanger.

Corrosion
635
/
Inner
tube
Figure
35
Duplex
tubing.
1.
Steel outside to resist various corrosive petroleum vapors and copper or copper alloy inside
toward the fresh water.
2.
Steel outside toward the oil and admiralty, aluminum brass, or cupronickel inside toward
circulating saltwater.
3.
Other applications in these industries call for the combinations of alloy steels with copper
alloys or
of
aluminum with copper or brass either inside or outside.
Duplex Tubing in High-Temperature Boiler Corrosion.
C-Mn or low-alloy steels, clad with
a stainless steel or superalloys, are used in parts of the boiler where loss is excessive due to
high-temperature corrosion
[87].
Duplex Tubing in a Condenser Application.
Ina condenser with stainless steel tubing, hot
corrosive vapors caused little trouble within the tubes. But brackish cooling water on the shell
side caused a problem. This situation was overcome by installing Carpenter bimetallic tubing
consisting of type
304
welded stainless on the inside and deoxidized copper tube on the outside.
Ferrules at the tube ends facilitated expanding of the tubes into type
304
stainless tube sheets.
4.6
Protective
Coatings
Coatings are generally relatively thin films separating the two reactive materials or a metal
from an environment. Applying a protective barrier between a corrosive environment and the
material to be protected is a fundamental method of corrosion control
[88].
It is most widely
used for the protection of steel and other metals. Coatings can be metallic, plastic, paints, or
organic. Typical metallic coatings include nickel coatings, lead coatings, zinc coatings, cad-
mium coatings, tin coatings, and aluminum coatings. Mechanisms by which coatings protect
can be summarized as follows
[89]:
1.
They isolate the metal substrate and the environment, as with nickel electroplating.
2.
They limit contact between the environment and the substrate, as with most organic coat-
ings.
3.
They release substances that are protective or inhibit attack, as with chromate primers.
4.
They produce an electrical current that is protective, as with galvanizing.
Plastic Coatings
Plastic coatings often represent the ideal solution to a cooling water problem. Typical plastic
materials used for coatings are polyethylene, polyvinyl chloride
(PVC),
epoxy resins, and po-
lymides. Under certain conditions, they allow a cheap and corrosion-susceptible metal to be
covered with a high-grade corrosion-resistant plastic coating. To ensure satisfactory protection,
these must be nonporous and sufficient thickness
(0.150-0.250
mm)
[90].

636
Chapter
12
Effectiveness of Coatings
To
be
effective, the coating film must be completely continuous. Any breakdown of the coating
film leads to corrosion. Resistance to water is the most important requirement of the coating,
since all coatings will come in contact with water or moisture in one form or another 188,891,
Surface Treatment
Surface treatment is resorted to on certain metals to improve their corrosion resistance in
specific applications. For example, (1) mild steel or low-alloy steel heat exchanger tubes are
alonized or aluminized (aluminurn vapor diffused) for high-temperature oxidation resistance
and protection from sulfide corrosion in refinery applications [91], and
(2)
titanium tubes are
anodized or thermally oxidized to form an inert surface oxide film for its corrosion resistance,
especially where hydrogen uptake is of concern.
4.7
Electrochemical Protection
(Cathodic and Anodic Protection)
Principle of Cathodic Protection
Cathodic protection is defined as the reduction or elimination of corrosion by making a metal
cathode by means of an impressed current or attachment to a more anodic metal (sacrificial
anode) than the metal in the galvanic couple. These two methods are discussed next, and both
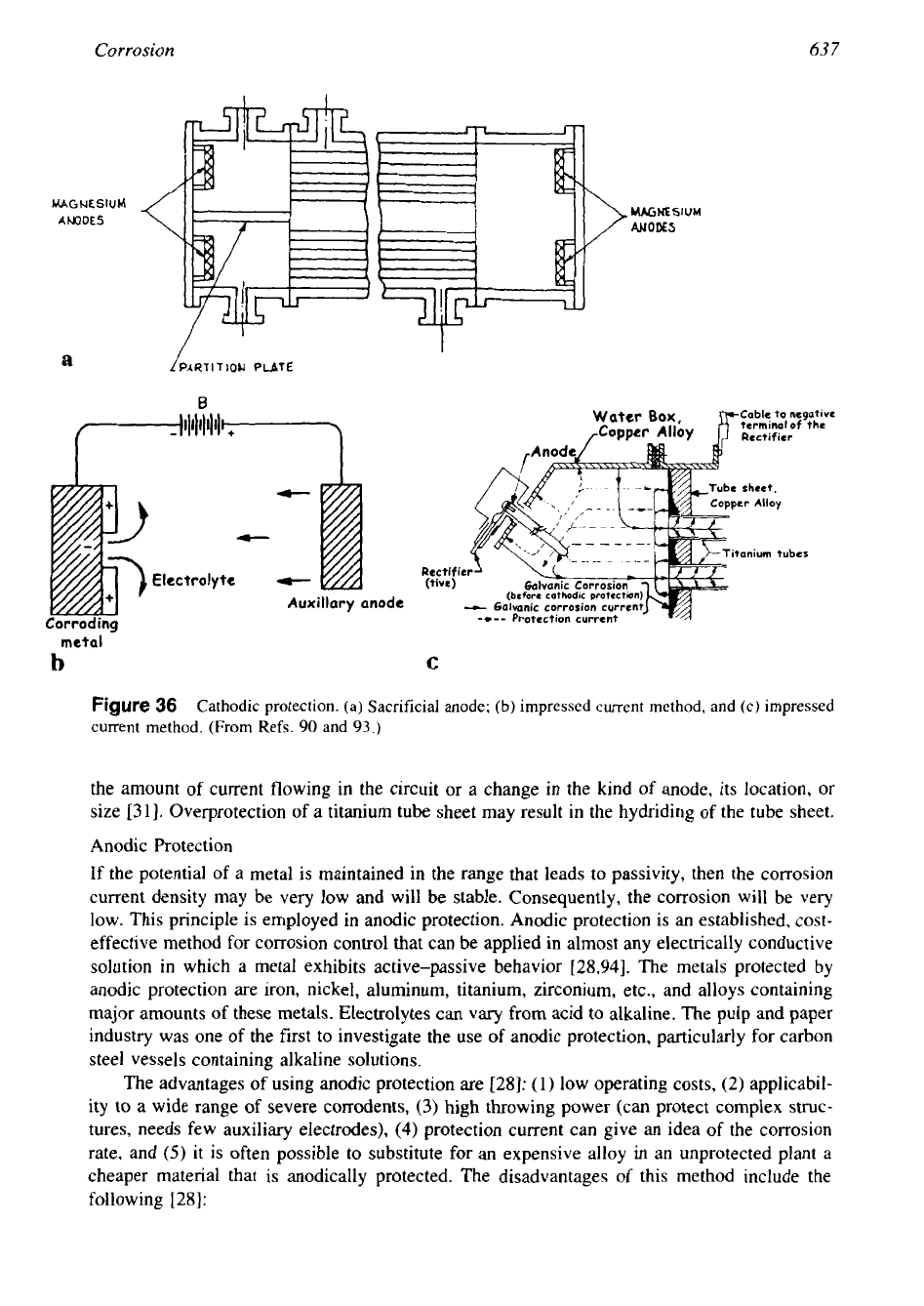
the methods are shown schematically in Fig. 36. Cathodic protection
is
one of the recom-
mended methods of protecting the tube sheet.
Sacrificial Anode.
A cathode is the electrode where practically no corrosion takes place. If
follows, then, that if all anodic areas can be converted to cathodic areas, the entire structure
will become a cathode, and corrosion will be eliminated. Corrosion control by this principle is
known as cathodic protection by sacrificial anode. By coupling the structure with a less noble
metal, it
is
allowed gradually to corrode but is replaced after an interval of time. Zinc is often
coupled with iron for this purpose and is termed a sacrificial anode (Fig. 36a). This method
is
mostly followed in heat exchangers for protecting the end closures.
Galvanic combinations such as zinc or cadmium or magnesium coatings on steel, often
used to avoid corrosion, are known as sacrificial anodic coatings, because they corrode in
preference to steel and protect the latter from rusting. Galvanizing a steel heat exchanger of a
tube bundle did not improve the life expectancy, and galvanizing is not sufficiently effective
to be worth even the slight extra cost involved [92].
Impressed Current Method.
Because the corrosive action is electrochemical, electric current
can be applied to effectively stop the attack of metals exposed to corrosive media. This tech-
nique is called cathodic protection by impressed current. In this method, an impressed current
from a dc source is passed
on
to the metal to be protected and coupled to an insoluble anode
such as platinum, graphite or ferrosilicon alloy or an expendable anode such as zinc, aluminum,
or magnesium, thus forcing
the
structure that has to be protected to have as a large cathode
so
that it will not suffer attack. This method is presently employed in practice to protect buried
pipelines carrying gas, oil, and water from corrosion by the surrounding soil. The protection
of the tube sheet and the end closure when titanium tubes are installed in a exchanger
is
shown
in Fig. 36b [93].
Shortcomings
of
Cathodic Protection.
With a cathodic protection system in seawater and in
certain applications, if condenser and heat exchanger tubes happen to be cathodic, they may
become covered with an undesirable thermal resistance layer. This may require a decrease in
Corrosion
63
7
P
.t-
itanium tubes
Electrolyte
Auxillary anode
Corroding
----
Protection current
metal
b
C
Figure
36
Cathodic protection. (a) Sacrificial anode;
(b)
impressed current method, and (c) impressed
current method. (From Refs.
90
and
93.)
the amount of current flowing in the circuit
or
a change in the kind of anode, its location,
or
size [31]. Overprotection of a titanium tube sheet may result in the hydriding of the tube sheet.
Anodic Protection
If the potential of a metal is maintained in the range that leads to passivity, then the corrosion
current density may be very low and will be stable. Consequently, the corrosion will be very
low. This principle is employed in anodic protection. Anodic protection is an established, cost-
effective method for corrosion control that can be applied in almost any electrically conductive
solution in which a metal exhibits active-passive behavior [28,94]. The metals protected by
anodic protection are iron, nickel, aluminum, titanium, zirconium, etc., and alloys containing
major amounts of these metals. Electrolytes can
vary
from acid to alkaline.
The
pulp and paper
industry was one of the first to investigate the use of anodic protection, particularly for carbon
steel vessels containing alkaline solutions.
The advantages of using anodic protection are [28]:
(1)
low operating costs, (2) applicabil-
ity to a wide range of severe corrodents,
(3)
high throwing power (can protect complex struc-
tures, needs few auxiliary electrodes), (4) protection current can give an idea
of
the corrosion
rate, and
(5)
it is often possible to substitute for an expensive alloy in an unprotected plant a
cheaper material that is anodically protected. The disadvantages
of
this method include the
following [28]:

638
Chapter
12
1.
Anodic protection does not stop corrosion completely, but reduces it to a minimum level.
2.
The failure of the electrical supply may be hazardous because of depassivation.
3.
High installation costs: requires potentiostat, reference and auxiliary electrodes, and high
starting current.
4.
The requirement for electrical current makes this measure unsuitable for protection
in
organic liquid environments, or for components that are not continuously immersed.
4.8
Passivation
"Passivation" is the conditioning of a metal surface to produce a protective surface film that
blocks corrosive ions from reaching the metal, thereby retarding corrosion. To change a metal
from an active to a passive state, the electrode potential must be raised above the passivation
potential. Passivation can be achieved by methods such as the following
[95]:
(
1
)
applying an
external current of sufficient strength or
(2)
using agents of sufficient oxidizing power to give
a
mixed potential above the passivation potential of the metal.
5
CORROSION
MONITORING
Continuous operation, associated with higher operating efficiency, is putting a heavier burden
on heat exchangers operating
in
various process industries such as oil refining, chemical, elec-
tric power plants, food and liquor processing, pulp and paper industries, etc. Changes
in
process
parameters such as temperature, pressure, velocity, and concentration can accelerate corrosion
rates. When the process changes occur, it is imperative to monitor their effects on corrosion
rates to prevent equipment failure caused by corrosion. Corrosion monitoring can be used to
optimize process conditions to achieve the maximum capabilities without sacrificing the integ-
rity of the equipment. Corrosion monitoring has been discussed in detail by Britton et al.
[96]
and in ref.
97.
5.1
Benefits
Effective corrosion monitoring is justified economically for a large, complex plant at which
production continuity is essential. The benefits of a successful corrosion monitoring program
include the following
[96]:
1. Corrosion problems can be predicted.
2.
Plant maintenance and inspection can be scheduled.
3.
Unscheduled shutdowns of plants can be avoided.
4.
improvements in reliability of equipments.
5.
Better
use
of construction materials.
6.
Detection of changes or abnormalities in the process affecting the corrosion rate.
5.2 Approaches to Corrosion Monitoring
in
general there are three approaches for corrosion monitoring
[98]:
(1)
local approach.
(2)
component approach, and
(3)
systems approach. The local approach involves investigations
of
corrosion in terms of local conditions. The component approach involves investigating plant
components and their corrosion phenomena that arise due to the complex environmental and
operational conditions. The systems approach considers the plant system in its totality. This
approach deals with interrelations of phenomena occurring
in
different components of the
system.

Corrosion
639
5.3
Corrosion Monitoring Techniques
Several techniques are used in corrosion monitoring, and the techniques are generally classified
as either online or offline. Various online and offline techniques are described next.
Online Monitoring Techniques
Online corrosion monitoring is conducted to assess the corrosivity of the process stream and
for detecting changes that may occur in operation. Online corrosion data are obtained from
probes or sensors inserted into the system at accessible points that reproduce the particular
area of interest. Various online monitoring techniques include corrosion coupons, electrical
resistance principle, pitting potential, linear polarization principle and Tafel plots, hydrogen
test probe, galvanic measurements, pH measurements, dimensional changes through online
ultrasonic testing, radiography, and acoustic emission technique
[96].
Some of these techniques
are discussed next.
Corrosion Coupons. The most common online monitoring technique is with corrosion cou-
pons. Corrosion coupons can be made in any size or shape, such that they are retrievable from
process equipment without shutting down the unit. Normally, the corrosion coupons are care-
fully weighed before insertion and weighed after retrieval.
Weight Change. When results of other techniques are in question, they are usually verified
with weight loss testing. However, weight
loss
determination would give misleading informa-
tion in the case of localized attack such as pitting.
Hydrogen
Diffusion. Atomic hydrogen can diffuse into steel and form molecules.
If
hydrogen
diffusion is detected, it shows imminent danger due to hydrogen damagehydrogen attack.
Hydrogen diffusion can be measured using either a hydrogen probe (pressure measurement) or
a hydrogen monitoring system (electrochemical).
Electrochenzicnl Techniques. Electrochemical techniques are particularly useful in determin-
ing the corrosion rate that is actually happening in a metal at any given time. The three tech-
niques most often used involve
(
1
)
electrical resistance principles using zero-resistance amme-
ters,
(2)
polarization curves, and
(3)
linear polarization curves. In the electrical resistance
technique, the change in resistance of a thin wire under consideration due to the corrosion
process is measured. Polarization curves may be determined galvanostatically, potentiostati-
cally, or potentiodynamically. Anodic polarization measurements (where the electrode potential
is changed in the positive direction to show current variations over a wide range of oxidizing
potentials) are used primarily to determine critical pitting potentials, or breakdown potentials
for localized corrosion. The principle of linear polarization technique, now referred to as the
polarization technique, is as follows: The amount of externally applied current needed
to
change the corrosion potential of a freely corroding specimen by a few millivolts (approxi-
mately
10
mV) is measured. This current is related to the corrosion rate of the sample; that is,
a linear relationship exists between the applied current and the resulting potential. If the metal
is corroding rapidly, a large external current is needed
to
change its potential, and vice versa.
This is the basis for the precise determination of corrosion rate.
Other methods include Tafel extrapolation (where the linear portion of the anodic or ca-
thodic polarization curve is extrapolated back to the corrosion potential to locate the current
density associated with corrosion rate at that potential), and current measurements at constant
potential (where corrosion rate is monitored for a given oxidizing condition)
[99].
Monitoring
of
Pitting Potential. The potential at which pit initiation occurs is called the
pitting potential. Pitting potential is determined by electrochemical techniques, which consist

640
Chapter
12
of measuring current and potential potentiostatically, either stepwise or by applying a constant-
potential sweep rate in a standard chloride-containing solution.
Online Monitoring
of
Water Purity in Thermal Power Stations.
Corrosion inhibition in
a
cooling-water system involves monitoring calcium hardness, alkalinity, total solids, pH, dis-
solved gases, etc. Automatic analyzers continuously monitor water and steam purities of ther-
mal power stations. These permanent analyzers, whose results appear on a strip chart recorder,
give more information than single manual analyses. Typical parameters monitored by online
instruments include
[
1001:
Conductivity, pH (purity and acidity of the water)
Ammonia, hydrazine phosphate (control of conditioning)
Oxygen, hydrogen (dissolved gases)
Sodium, chloride, silica (harmful impurities in the loop)
Offline Monitoring Techniques
Offline monitoring techniques involve various nondestructive examination methods to deter-
mine the thickness and integrity of the heat exchanger and pressure vessel components. The
various nondestructive testing (NDT) methods employed are:
1.
Visual examination with or without optical aids such as borescopes
2.
Eddy current testing
3.
Magnetic particle examination
4.
Liquid penetrant test
5.
Ultrasonic examination
6. Radiography
Thermograph
y
Corrosion Monitoring of Condensers by Systematic Examination of the State
of
the
Tubes
This procedure involves extracting representative tubes and examining them in the laboratory
with modern analytical equipment
[loll.
In a tube investigation procedure one considers the
three zones (inlet, center, and outlet) separately, because they are exposed to different condi-
tions. On all samples the following four criteria are checked:
1.
Microscopic examination of the condition
of
the tube surface
2.
Residual wall thickness
3.
Weight of the surface layer
4.
Composition of the surface layer
5.4
Limitations of Corrosion Monitoring
It is important to be aware of the limitations of corrosion monitoring data. Some of the impor-
tant limitations of corrosion monitoring are [96]:
1.
The data are only a qualitative guide to the actual behavior of the plant or process.
2.
A confidence factor is established only through experience, and particularly through com-
parison with other sources
of
information, notably that provided by NDT.
5.5
Requirements for Success
of
Corrosion Monitoring Systems
Many factors contribute to the success of a monitoring program. Some of the most important
considerations include the following [96]
:
Corrosion
641
1.
Use of correct technique.
2. Correct location of monitoring probes.
3.
Reliability of the equipment and instrumentation.
4.
Data obtained must be straightforward to interpret.
6
COOLING-WATER
CORROSION
Water, the most commonly used cooling medium, removes unwanted heat from process fluids.
Water is an excellent solvent for numerous substances: It washes the mineral elements out
of
the earth, it absorbs gases
(O?,
COz) from the atmosphere, it entrains suspended matter, and it
promotes growth of microorganisms. If cooling water is used without treatment, it leads to the
twin problems of fouling and corrosion [90]. Corrosion in cooling-water systems is electro-
chemical in nature and the corrosion products may be either soluble or insoluble. The insoluble
material that forms a deposit on the heat transfer surfaces retards heat transfer and promotes
corrosion in long run.
6.1
Corrosion Processes in Water Systems
Corrosion of metals in contact with water is electromechanical in nature. A generalized anodic
oxidation reaction for ferrous metal has already been presented and for copper and aluminum
materials are represented by [69]:
M
-+
M”++ne-
Cu +Cut
+
e-
A1
-+
A13
+
3e-
where
M
represents the metal that has been oxidized to its ionic form having a valence of
nt
and the release of
n
electrons. The reactions at the cathodic site on the metal surface are given
by ref. [69]:
Oz
+
4H’
+
4e-
+
2H,O
(in the presence
of
oxygen)
O2
+
2Hz0
+
4e-
+
40H-
(in the neutral solution)
2H’
+
2e-
-+
H2
(in the absence of oxygen)
6.2
Causes
of
Corrosion in Cooling-Water Systems
Depending on the sources
of
water-river, lake, ocean, or sea-cooling-water corrosion that
attacks heat exchangers is due to one or more of the following impurities [8,69,81,90]:
1.
Dissolved solids and water hardness
2.
Chloride
3.
Sulfate
4.
Silica
5.
Oil
6. Iron and manganese
7.
Suspended mater such
as
turbidity, dirt, clay, silt, sand, etc.
8.
Dry residue
9.
Dissolved gases such as
02,
carbon dioxide, hydrogen sulfide, ammonia
10.
Dissolved organic matter
11.
Microbiological organisms

642
Chapter
I2
Apart from these factors, water temperature, pH, and flow velocity also influence corro-
sion. Effects of these factors on the cooling water corrosion are discussed next.
Dissolved Solids and Water Hardness
All waters containing calcium and/or magnesium salts such as calcium carbonate (CaCO,),
calcium bicarbonate [Ca(HCO+], magnesium carbonate (MgC03), and magnesium bicarbonate
[
Mg(HCO+] in considerable amounts are called “hard.” The following classifications for hard-
ness are often applied to fresh water
[
1021:
Soft:
0-50
ppm
Medium hard: 50-100 ppm
Hard:
>
100
ppm.
Total dissolved solids is the concentration of dissolved solids that cannot be removed by
filtration. Seawater is many times higher in dissolved solids than freshwater.
Total hardness is the amount of calcium and magnesium salts, which may be present
as
bicarbonates (temporary hardness) or as sulfates, chlorides, nitrates, or carbonates (permanent
hardness).
The overall aggressiveness of water is related to its hardness and alkalinity. Soft waters,
which are low in calcium, are more corrosive than hard waters
[69].
Hard water, high in
calcium and magnesium, is less corrosive than soft water because of the tendency
of
the salts
in the hard water to precipitate on the metal surface and form a protective film. However,
increasing the dissolved solid contents of the waters increases its conductivity and hence a
larger corrosion current can flow resulting in higher corrosion rate. The dissolved constituents
can also have a variety of effects including increased scale and deposit formation. Hardness
ions (Ca” and Mg”) and HCOi ions are inhibitive and will suppress corrosion, but chloride
(Cl-) and sulfate
(SOS-)
ions are deleterious and will increase the rate of some forms of corro-
sion
[69].
Scale Deposition.
Water-formed deposits are commonly referred to as scale. This scale in-
creases the resistance for heat transfer and increases pressure drop, and promotes localized hot
corrosion spots. Some hardness in the water may be helpful if the pH and alkalinity can be
controlled to permit formation of a thin protective scale (termed stabilization of the water).
Unfortunately, the buildup of a thick scale on the tube surface seriously interferes with heat
transfer. The normal scale-forming compounds commonly found in cooling-water systems are
calcium carbonate, calcium sulfate, calcium phosphate, and silicates.
Of
these compounds,
calcium carbonate has very low solubility and perhaps is the principal scale-forming material in
cooling waters. Bicarbonate ions react with hydroxyl ions generated at the cathode to produce
carbonate, which precipitates with calcium from the water as calcium carbonate. The equation
for calcium carbonate scaling
is
given by:
Ca(HCO?)?
+
OH-
+
CaC07
+
HCO;
+
H20
(11)
Prevention
of
Calcium Carbonate Scale.
Scale-forming tendencies of cooling water can be
predicted by determining the Langelier saturation index
(LSI),
also known as the calcium
carbonate saturation index, of the water. Some prefer the Ryznar stability index. The Langelier
saturation index is defined as the difference between the actual
pH
value of the water and the
value of pH that the water would have if it was in equilibrium with CaCO?, also known
as
saturation pH. Accordingly, the
LSI
is given by

Corrosion
643
The LSI is not applicable to seawater because of the high salt content
[
1021.
Determination
of
LSI.
The LSI is calculated from
(1)
the alkalinity, (2) the calcium hardness,
(3) the ionic strength (total solids),
(4)
the pH value, and
(5)
the temperature. The index can
also be found out by direct determination of the pH of water as such and after saturating
it
with calcium carbonate. A condition of carbonate equilibrium with the absence of scale can be
accomplished by adjusting any of the variables of the Langelier saturation index. The common
practice is to lower the alkalinity and pH by adding sulfuric acid to the cooling water [70]. A
chart for calculating the saturation index is shown in Fig. 37.
Scaling Control. If the Langelier saturation index is positive, scale will be deposited and will
probably stifle corrosion of the metal. If it is negative, the water is unsaturated with CaCO?,
and it is likely to be corrosive and may dissolve existing hardness salts or may not be scale
forming. Table
5
gives an indication of what may be expected [31]. From Table
5,
the main
choice appears to be between keeping the LSI low and controlling scale formation, or having
a high index and controlling the corrosion. Methods to control scale formation are discussed
in Chapter 9, Fouling.
Chloride
Chlorides are the main salts in seawater, around 19,000 ppm
[
1021. High-chloride waters are
usually corrosive. High chloride ion concentrations easily destroy the protective surface films
including passive films on a large number
of
metals [90], or the chloride forms complex ions
with dissolved iron, thereby preventing or interfering with the formation of protective corrosion
product scales [16]. In the case of austenitic stainless steels, chloride ions will easily destroy
the passive film and result in pitting corrosion or when the metal is subjected to simultaneous
tensile stress can cause chloride SCC. Aluminum is also easily attacked by chloride.
Sulfates
Sulfates are not as corrosive as chlorides [102]. However, concrete cooling water basins are
endangered as soon as the water contains more than
250
ppm of sulfate ions [90].
Silica
Silica will react with magnesium or calcium to form deposits of insoluble magnesium or cal-
cium silicates. Also, it will
form
siliceous glassy scales.
To
avoid such deposits, the silica
concentration must be limited.
Oil
Oil is one of the most undesirable contaminants
of
industrial cooling-water systems. Oils
usu-
ally leak into the system as a result of poor maintenance. They interfere with the action of
corrosion inhibitors; by forming a thin film on metal surfaces, oils retard heat transfer; and
they become nutrients for biological organisms [8].
Iron and Manganese
Iron oxide is produced either by internal corrosion of steel heat exchanger surfaces and trans-
mission lines or from precipitation of soluble iron brought into the cooling system from the
earth by the makeup water. Less than 0.2 ppm of iron and manganese is desirable. With higher
contents, sludge-type hydroxides are precipitated in the presence of oxygen [90]. Iron oxide
scale, which forms as easily dislodged “plate-like” layers, becomes lodged
in
the tubes and
leads to localized impingement attack. Iron oxide scale is prevented by applying cathodic
protection or by protective coatings [33].
