Kuppan T. Heat Exchanger Design Handbook
Подождите немного. Документ загружается.

414
Chapter
9
OEBRIS
our
COOLING
WATER
IN
Figure
6
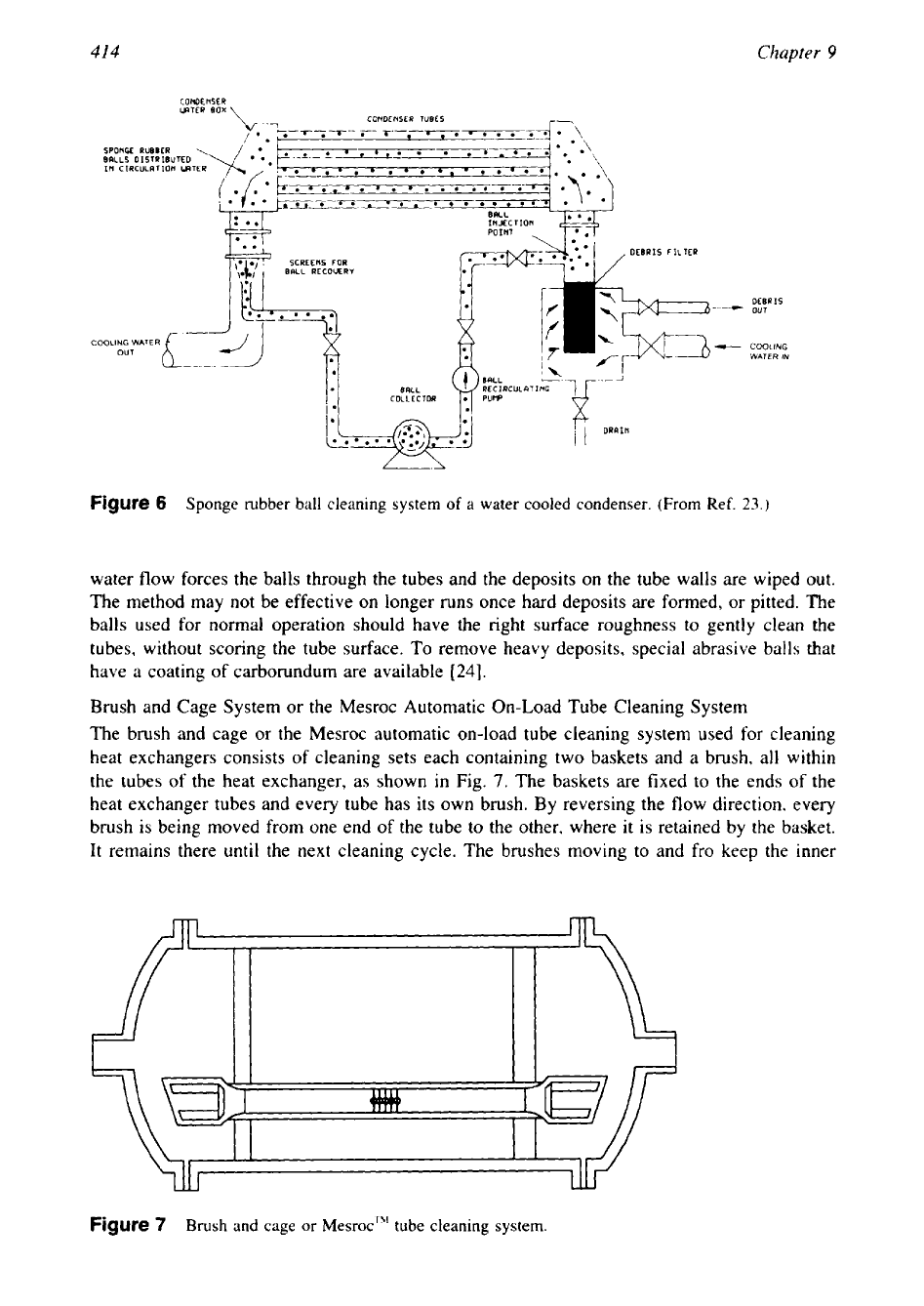
Sponge rubber ball cleaning system
of
a
water cooled condenser. (From
Ref.
23.)
water flow forces the balls through the tubes and the deposits on the tube walls are wiped out.
The method may not be effective on longer runs once hard deposits are formed, or pitted. The
balls used for normal operation should have the right surface roughness to gently clean the
tubes, without scoring the tube surface. To remove heavy deposits, special abrasive balls that
have a coating of carborundum are available
[24].
Brush and Cage System or the Mesroc Automatic On-Load Tube Cleaning System
The brush and cage or the Mesroc automatic on-load tube cleaning system used
for
cleaning
heat exchangers consists of cleaning sets each containing two baskets and a brush, all within
the tubes
of
the heat exchanger, as shown in Fig.
7.
The baskets are fixed to the ends
of
the
heat exchanger tubes and every tube has its own brush. By reversing the flow direction, every
brush is being moved from one end of the tube
to
the other, where it is retained by the basket.
It
remains there
until
the next cleaning cycle. The brushes moving to and fro keep the inner
Figure
7
Brush
and cage
or
MesrocTb' tube cleaning system.
--
-
----
Fou
1
ing
41
5
walls clean. An actuator and a control system initiate the cleaning cycles. A major advantage
of the system is that it does not require a recirculation system as for rubber ball system and
an important disadvantage is the interruption of the flow in the heat exchanger and consequent
disturbance of steady state conditions. Unlike the rubber ball circulation system, the brush and
cage system has not been used to any extent in power plant condensers. However, it has been
applied to single exchangers in process industries
[3].
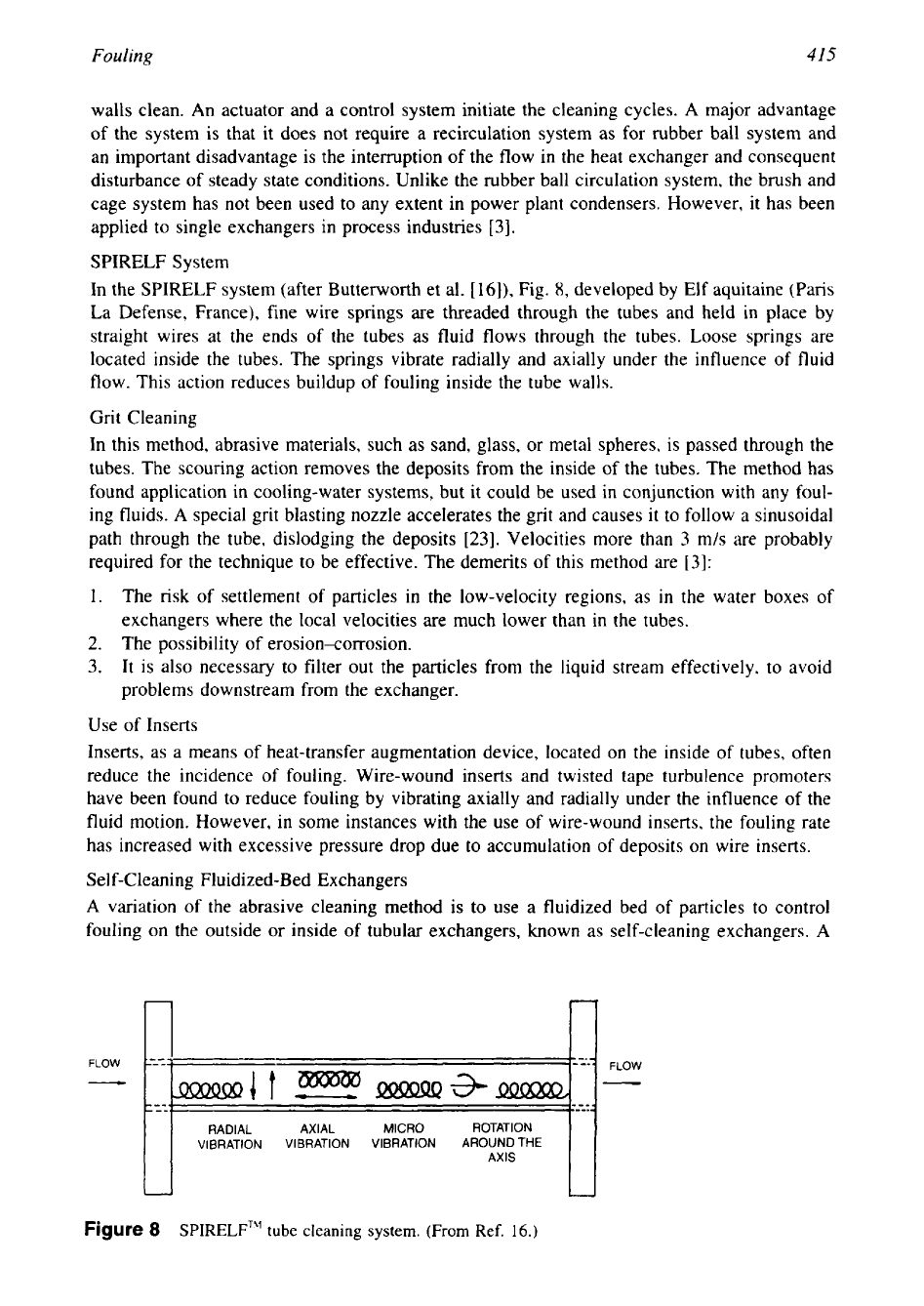
SPIRELF System
In the SPIRELF system (after Butterworth et al.
[16]),
Fig.
8,
developed by Elf aquitaine (Paris
La Defense, France), fine wire springs are threaded through the tubes and held in place by
straight wires at the ends of the tubes
as
fluid flows through the tubes. Loose springs are
located inside the tubes. The springs vibrate radially and axially under the influence of fluid
flow. This action reduces buildup of fouling inside the tube walls.
Grit Cleaning
In this method, abrasive materials, such as sand, glass, or metal spheres, is passed through the
tubes. The scouring action removes the deposits from the inside of the tubes. The method has
found application in cooling-water systems, but it could be used in conjunction with any foul-
ing fluids. A special grit blasting nozzle accelerates the grit and causes it to follow a sinusoidal
path through the tube, dislodging the deposits
[23].
Velocities more than
3
m/s are probably
required for the technique to be effective. The demerits
of
this method are
[3]:
1. The
risk
of settlement of particles in the low-velocity regions, as
in
the water boxes of
exchangers where the local velocities are much lower than in the tubes.
2.
The possibility of erosion-corrosion.
3.
It is also necessary to filter out the particles from the liquid stream effectively, to avoid
problems downstream from the exchanger.
Use of Inserts
Inserts, as a means of heat-transfer augmentation device, located on the inside of tubes, often
reduce the incidence of fouling. Wire-wound inserts and twisted tape turbulence promoters
have been found to reduce fouling by vibrating axially and radially under the influence of the
fluid motion. However, in some instances with the use of wire-wound inserts, the fouling rate
has increased with excessive pressure drop due to accumulation of deposits on wire inserts.
Self-Cleaning Fluidized-Bed Exchangers
A
variation of the abrasive cleaning method is to use a fluidized bed of particles to control
fouling on the outside
or
inside
of
tubular exchangers, known as self-cleaning exchangers.
A
7
-
:==:---
FLOW
-
---.
FLOW
-
---.
-ammmrJt
z!E!bmQ3sMMMIL
~---.-
-
--.
RADIAL AXIAL
MICRO
ROTATION
VIBRATION VIBRATION VIBRATION AROUND THE
AXIS
I
1
Figure
8
SPIRELF"'
tube
cleaning
system.
(From
Ref.
16.)

41
6
Chapter
9
fluidized-bed exchanger consists of a large number of parallel vertical tubes, in which small
solid particles are kept in a fluidized condition by the liquid velocities. The particles have
a
slightly abrasive effect on the tube walls,
so
that they remove the deposits
[25].
A
typical
example for fin-side foulant control is the fluidized-bed waste heat recovery
(FBWHR)
heat
exchanger used to preheat combustion air for industrial furnaces. Fluidized-bed heat ex-
changers consists of horizontal finned heat exchanger tubes with a shallow bed of fine inert
particles, which move upward with gas flow and give up heat to the finned tubes. Similarly
on the tube side, the fine particles scrub the tube surfaces to minimize fouling deposit.
14.1
3
Merits
of
On-Line Cleaning
The merits of on-line cleaning are that it
1. Is more convenient.
2.
Does not require any plant shutdown.
3.
Can save time and labor.
However, the initial cost may be very high in certain cases.
15
FOULANT CONTROL
BY
CHEMICAL ADDITIVES
If fouling cannot be tackled adequately either by process design or equipment design, it can
be further reduced by periodical on-line injection of additives into the process stream
[20].
Chemical additives find wider use in cooling-water systems and to control fouling due
to
crystallization and freezing, chemical reaction or polymerization, precipitation, particulate, and
scaling. The use of additives to prevent chemical reaction or polymerization fouling in liquids
is well known in the petroleum refining industry, where a particular feedstock is a complex
mixture of hydrocarbon and other organic materials
[3].
Various types of additives and their
functions are:
1.
Alkali or acid dosing: By dosing either alkali or acid, pH may be controlled. This
may
be effective to control precipitation fouling. Acid dosing controls the hardness of hard or
brackish water.
2.
Complexing agents: Agents such as chelants complex the metallic ion into a ring structure
that is difficult to ionize.
3.
Chemical reactants: These are used to complex or tie up the active foulants. They can
solubilize or condition the foulants to prevent deposition.
4.
Sequestrant: This additive complexes the metallic ions into a water-soluble structure, thus
preventing its adhesion to the heat exchanger surface. A sequestrator physically surrounds
and isolates particles (e.g., EDTA).
5.
Oxidizing agents: These oxidize the deposits, making them suitable for dissolution (e.g.,
chromic acid, sodium nitrite, potassium permanganate).
6.
Reducing agents: Reduce the compounds in deposits and make them suitable for dissolu-
tion and to prevent the formation of hazardous by-products.
7.
Inhibitors: Control cooling-water corrosion. Corrosion inhibitors such as filming amines
stifle chemical reaction fouling
[3].
8.
Surfactant: Added to chemical cleaning solution to improve the wetting characteristics.
9.
Antiscalants: In aqueous system, these additives chemically combine the scales to form
soluble compounds.
10.
Distortion agents: These interfere with the crystal structure
so
that it becomes more diffi-
cult for coherent crystal structures to form on surfaces.

Fouling
41
7
11.
Dispersants: Dispersants impart an electrical charge to the particles
so
that they are held
in suspension in the bulk of the liquid and the particles pass through the equipment
without deposition on heat transfer surfaces. Dispersants are helpful to control chemical
reaction fouling.
12.
Depressants: Depressants lower the freezing point of the solution such that the potential
forming solids is brought down (e.g., glycols, alcohols).
13.
Flocculating agent: Causes the particles to agglomerate
so
that they may be settled out
of the cooling water or suitably filtered.
14.
Threshold agents that prevent the creation of crystal nuclei around which the larger crys-
tals form. They also arrest the growth of nuclei (e.g., polyphosphates). In cooling-water
systems, threshold agents retard the precipitation of scale-forming salts.
15.
Stabilizers: On reaching the solubility limit, stabilizers are able to retard the nucleation
of individual low-solubility compounds and prevent any existing crystals from forming
adhesive deposits.
16.
Metal coordinators react with the trace metals and prevent them from functioning as
fouling catalysts in the case of chemical reaction or polymerization fouling.
17.
Biocides kills the micro-or macroorganism. Biostats arrest the growth of microorgan-
isms.
16
CONTROL OF FOULING FROM SUSPENDED SOLIDS
Methods of control of fouling from suspended solids include
[3]:
1.
Pretreatment of process fluids by means such as filtration, softening, and desalting to
control precipitation fouling, particulate fouling, and scaling.
2. Chemical treatment using dispersants and flocculating agents.
17
COOLING-WATER MANAGEMENT FOR REDUCED FOULING
Many industries use cooling water for one purpose or another. It is appropriate to include a
section devoted to cooling-water management for fouling control. Traditionally, the treatment
of
cooling water has often been oriented toward corrosion control followed by foulant control.
However, it is impossible to separate these twin problems in any treatment program, since one
can lead to the other, and since both can occur simultaneously
[5].
In addition to some of the
on-line foulant control measures discussed already, specific features
of
cooling-water fouling
control are discussed here. Cooling-water corrosion control measures are discussed in Chapter
12 on corrosion.
17.1
Forms
of
Water-Side
Fouling
Water is by far the most common fluid subject to fouling. The quality of water used in the
cooling system varies depending on its sources, like sea, river, ocean, lake, etc., and on the
three forms of cooling water systems: once through, open recirculating, and closed systems.
Cooling-water quality factors that contribute to fouling include turbidity, salinity, dissolved
solids and hardness, biological organisms, airborne contaminants, etc. The quality of raw water
changes according to weather conditions also. In general, fouling associated with cooling water
can be classified under the following headings
[3]:
Scaling due to crystallization of inverse solubility salts, mainly in cooling-tower water and
sometimes in water drawn from a river, lake, or well.
Biological fouling of water drawn from a river, lake, sea, or the ocean; algae growth in open
recirculating water.

41
8
Chapter
9
Particulate fouling-Deposition of silts, sediments and suspended solids when water is drawn
from a lake, river, or nearby seashore and due
to
airborne objects when water is exposed
to the atmosphere.
Corrosion fouling due
to
cooling-water quality.
17.2 Influence of Surface Temperature on Fouling
The maximum tube surface temperature (the average between the temperature of the inlet fluid
and the exchanger outlet water) on the water side at the tube-water interface is usually the
critical concern. The recommended design surface temperature is
145°F
(63°C)
with 160'F
(71°C) as a practical maximum. Heat exchangers with surface temperature above
160°F
are
prone to localized boiling. Boiling allows concentration of even the most soluble salts, with
the threat of severe deposition and subsequent corrosion. For such a situation, water quality is
to be improved by
full
softening or demineralization. (This material is based on Chenoweth
[41.)
17.3
Foulant Control Versus Type of Cooling-Water System
Fouling problems and their control in each of the three basic types of cooling systems (once-
through, open recirculating, and closed) are often quite different and thus require different
techniques. Cooling-system operation and specific characteristics should be analyzed con-
stantly as a continuing part of the foulant control program.
Once-Through System
In once-through cooling-water systems, usually the major foulants are biological organisms,
mud, silt, debris, or other suspended matter, and pollutants
[5].
This type
of
cooling-water
system generally needs only upstream filtration and a mechanical on-line cleaning method of
passing plugs or sponge rubber balls as in condensers cooled by seawater. Generally, econom-
ics favor the chemical treatment. The chemical additives most generally used are mud fluidiz-
ers, dispersants, and biocides, particularly chlorination. Environmental regulations may restrict
certain chemical additives.
Open Recirculating System
In open recirculating systems with cooling towers or spray ponds, foulants originate
in
the
makeup water, air or process contamination, and from lack of good corrosion control
[5].
Hence, this system is frequently treated for foulant control. Both mechanical and chemical
methods are used singly or jointly to overcome fouling problems. Remove solid particles
through sedimentation ponds and/or continuous filtration. Mud fluidizers, dispersants, floccu-
lating chemicals and foulant solubilizers, biocides, and other chemicals are added to control
fouling.
Closed Recirculating Systems
The source of foulants is usually the corrosion products from heat exchangers and piping
components. Corrosion control is mostly by inhibitors.
On-Line Chemical Control of Cooling-Water Foulants
Foulant control through some of the chemical additives include the following:
Threshold chemicals
Sequestrant and chelating chemicals, such as ethylenediamine tetraacetic acid
(EDTA)
and
derivatives, nitrilotriacetic acid (NTA), organic phosphate esters, and organic phospho-
nates
[5]

Fouling
41
9
Dispersants (e.g., lignins, tannins, alginates, cellulose, starch products, sodium polymethacry-
late, and polyvinyl pyridinium butyl bromide) [5]
Sludge fluidizers
Biocides
17.4
Control of Scale Formation and Fouling Resistances for
Treated Cooling Water
The following values for cooling water assume that corrosion is under control and that biologi-
cal growth does not represent a significant portion of fouling [4]:
1. Since scaling in cooling water system is due to inverse solubility phenomena, which nor-
mally take place about 140°F (60"C), keep the surface temperature below this temperature.
2.
The cooling-water velocity is at least 4 ft/s on the tube side for most nonferrous alloy
tubes and 6 ft/s for carbon steel tubes. Velocities as high as
15
ft/s have been used inside
titanium tubes.
3.
The velocity of cooling water on the shell side is at least
2
ft/s. Higher velocities are
permitted if erosion can be tolerated or flow-induced vibration is not possible.
4.
Under the preceding conditions, a reasonable design value for the cooling-water-side foul-
ing resistance
is
0.001
hr
*
ft2
*
"F/Btu.
5.
The fouling resistance for a clean heat exchanger should be taken as
0.0005
hr
ft'
"F/B tu.
Chemical Means to Control Scaling
Calcium carbonate formation can be controlled by adding acids or specific chemicals, including
sulfuric acid, polymeric inorganic phosphates, phosphonates, and organic polymers like poly-
carboxylates
[
261.
Removal
of
the Hardness Salts. Removal of the scaling chemical species such as magnesium
and calcium from water prior to use in the system, by ion exchange and lime softening proce-
dures, is effective in scaling control. The large volumes of water usually encountered in many
cooling-water systems are likely to make the treatment costs
of
these methods very high. The
modem trend toward very small volumes and high recirculation rates may offer cost restric-
tions [3].
Conversion
of
Hardness Salts to a More Soluble
Form.
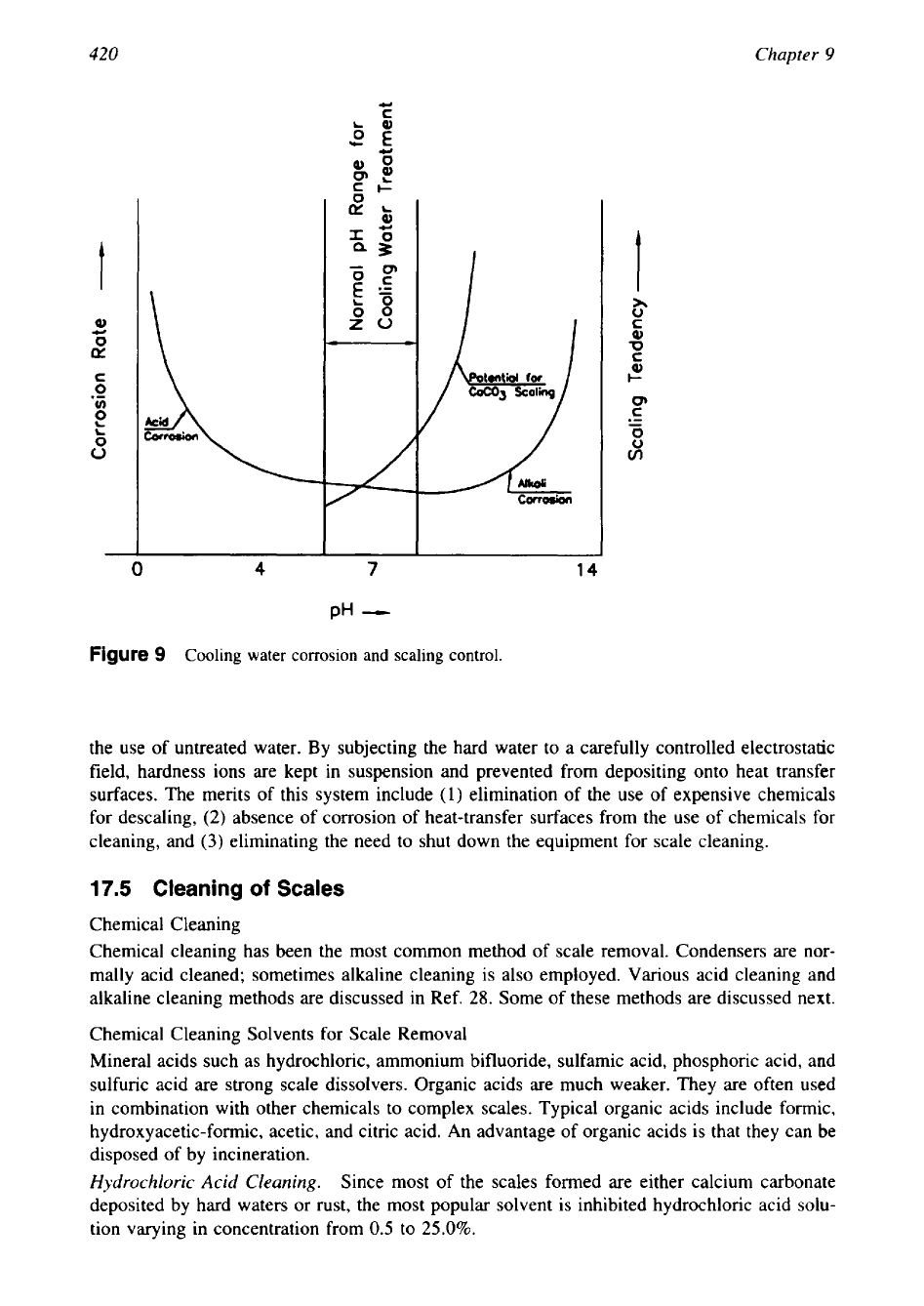
Control of scale formation involves
conversion of hardness salts to a more soluble form. Since the solubility of scale-forming
species in cooling water generally increases with decreasing pH, the addition of acid to main-
tain the pH in the range 6.5-7.5 (with
6.5
as the recommended minimum) may reduce the
scaling. These effects are shown schematically in Fig.
9.
However, corrosion may be involved
if the treatment is not carried out carefully. If large concentrations of sulfate are present, the
use of sulphuric acid may cause sulfate scale to appear. Hydrochloric acid may be preferable
under these circumstances
[3].
Alkaline Cooling Water Operation
for
Scale Control. Alkaline cooling water treatment can
also help in scale control. When the organic phosphorous compounds, including the well-
known phosphates and polyol esters, and low-molecular-weight acrylic-acid-based polymers
and copolymers are used properly, they completely prevent calcium carbonate deposition under
alkaline conditions [27].
Electrostatic Scale Controller and Preventer
This is on-line equipment designed as a one-time fitment that performs the dual function of
scale prevention and scale removal without using chemical additives. The equipment enables
420
Chapter
9
0
4
7
14
Figure
9
Cooling water corrosion
and
scaling control.
the use of untreated water. By subjecting the hard water to a carefully controlled electrostatic
field, hardness ions are kept in suspension and prevented from depositing onto heat transfer
surfaces. The merits of this system include
(1)
elimination of the use
of
expensive chemicals
for descaling,
(2)
absence of corrosion
of
heat-transfer surfaces from the use
of
chemicals for
cleaning, and
(3)
eliminating the need to shut down the equipment for scale cleaning.
17.5
Cleaning
of
Scales
Chemical Cleaning
Chemical cleaning has been the most common method of scale removal. Condensers are nor-
mally acid cleaned; sometimes alkaline cleaning is also employed. Various acid cleaning and
alkaline cleaning methods are discussed in Ref.
28.
Some of these methods are discussed next.
Chemical Cleaning Solvents for Scale Removal
Mineral acids such as hydrochloric, ammonium bifluoride, sulfamic acid, phosphoric acid, and
sulfuric acid are strong scale dissolvers. Organic acids are much weaker. They are often used
in combination with other chemicals to complex scales. Typical organic acids include formic,
hydroxyacetic-formic, acetic, and citric acid. An advantage of organic acids is that they can be
disposed of by incineration.
Hydrochloric Acid Cleaning.
Since most of the scales formed are either calcium carbonate
deposited by hard waters or rust, the most popular solvent is inhibited hydrochloric acid solu-
tion varying in concentration from
0.5
to
25.0%.

Fouling
42
I
Lactic Acid Cleaning.
Lactic acid solutions (20%) are also used for scale removal.
Sulfiric Acid.
This is unsatisfactory for the removal of lime scale due to the formation of
insoluble calcium sulfate. It is generally suitable for removing oxide scales such as rust.
Alkaline Cleaning.
Some waters deposit scales that are very difficult to remove by acid clean-
ing methods. For example, thin scales containing a high percentage of silicate may be removed
in a few hours by hot alkaline solutions such as sodium hydroxide. However, thick silicate
films are difficult to remove.
Mechanical Cleaning.
Calcium sulfate scales are not easily removed by chemical means but
are generally dislodged by mechanical means such as steam shock blasting, rotary cutting, or
sand blasting. Other mechanical methods include the use of bulleting with rubber, nylon, or
twisted wire bristle.
Water Jet Cleaning.
High-pressure water jet cleaning at about 9000 psi has been used in
many situations. It is a time-consuming process and is therefore of little value.
Iron Oxide Removal
Conventional Cleaning Methods.
Two common procedures generally followed in the indus-
tries for iron deposit removal are mechanical cleaning and chemical cleaning [29]:
1.
Mechanical cleaning methods include water hydroblasting, lancing, and passing abrasive
sponges, which remove most of the
soft
deposits but can leave hard, baked-on deposits.
2.
Chemical cleaning with strong mineral acids or high concentrations of chelants up to
10%
is used for temperatures up to 180°F (82°C).
With the chemical cleaning methods, the possibility of corrosion of underlying metal surfaces
as they dissolve away from the iron deposits cannot be ruled out. To overcome this problem,
a new on-line procedure for removing iron-based deposits from cooling water systems has
been developed. This is explained next.
On-Line Removal
of
Iron
Deposits
[29]. The first step of the on-line cleaning process is the
addition of a tannin-based, iron conditioning agent, which penetrates and softens the deposits.
Later, a mild organic acid and dispersants are added and cause sloughing
of
the conditioned
deposits. Postcleaning passivation of all metal surfaces can be accomplished by the normal
corrosion inhibitor or by addition of on-line passivators to prevent flash corrosion. The process
cleans transfer lines as well as heat exchangers and usually can be completed in
3
to
5
days.
NOMENCLATURE
A,,
=
rate constant
A,
=
surface area on the tube inside, m2 (ft2)
A,,,
=tube wall surface at the mid plane, m2 (ft2)
A,
=
surface area on the tube outside, m2 (ft2)
A,
=
chemical reaction fouling rate constant
A,,
=
total wall area for heat conduction, m2 (ft2)
d
=
tube outside diameter, m (ft)
d,
=
tube inside diameter, m (ft)
E
=
activation energy for chemical reaction fouling
h,
=
h,
=
ft'
OF)
ft' OF)
heat-transfer coefficient on the tube inside, W/m2 "C (Btuhr
heat-transfer coefficient on the tube outside, W/m2 "C (Btu/hr
=
tube length, m (ft)
kf
=
thermal conductivity
of
fouling deposit, W/m "C (Btu/hr ft OF)
L

422
Chapter
9
KR
=
constant for crystallization rate
K,
=
chemical reaction fouling (polymerizaton) rate
K,,
=
solubility product of CaCO,, (mol/m')'
k,
=
thermal conductivity
of
the conduction wall material, W/m "C (Btuhr
ft
OF)
h,
=
rate of deposition of fouling mass on the heat-transfer surface, kg/m?
s
(lbm/ft? hr)
rn,
=
net rate of fouling mass deposition per unit area, kg/m'
s
(lbm/ft' hr)
m,*
=
asymptotic value
of
m,
hr
=rate of removal of fouling mass from the heat-transfer surface, kglm'
s
(lbm/ft'
-
hr)
rn,
=
CaC07 scaling rate, kg/m'
s
(lbm/ft'
hr)
R
=
gas constant
RI.
=
thermal resistance due to fouling on both sides of a heat exchanger surface, "C/W
(OF
hr/Btu)
RI
=
thermal resistance of fouling deposit for a unit surface area, m'
/W
(ft' "F hr/Btu)
RI*
=
asymptotic value of thermal resistance due to fouling, "C/W (OF hr/Btu)
R,
=
fouling resistance on tube inside surface,
m2
"C/W (ft'
"F
hr/Btu)
R,,
=
fouling resistance on tube outside surface,
m'
"C/W (ft'
"F
0
hr/Btu)
RT
=
total thermal resistance to heat transfer, "C/W
(hr
"F/Btu)
R,
=
thermal resistance of the separating wall, "C/W (hr "F/Btu)
t
=
time,
s
t,
=
time constant
t,
=
time delay period,
s
T,
=
heat-transfer temperature, "C
(OF)
T,
=conduction wall temperature, "C (OF)
U,,
=
overall heat-transfer coefficient based on tube outside surface, W/m2
"C
(Btu/hr ft'
OF)
U,,,
overall heat-transfer coefficient based on tube outside surface in clean condition,
W/m'
=
"C (Btu/hr
ft2
OF)
I
U,,
=
overall heat-transfer coefficient based on tube outside surface in fouled condition.
W/m'
"C
(Btuhr
ft'
OF)
xI
=
thickness of fouling deposit, m (ft)
p
=
constant
=
l/t,
At,,
=
tube wall thickness, m
(ft)
pl
=
density of the foulant, kg/m' (lbm/ft')
10
Flow-Induced Vibration
of
Shell and
Tube Heat Exchangers
1
PRINCIPLES OF FLOW-INDUCED VIBRATION
Flow-induced vibration
(FIV)
of shell and tube heat exchangers (STHE) has been known for
a long time. Heat exchanger tubes tend to vibrate under the influence of crossflow velocities,
and if the amplitude of vibration becomes large enough, the tubes can be damaged by one or
more of several mechanisms:
(1)
thinning due to repeated mid-span collision,
(2)
impact and
fretting wear at baffle plate and tube interface, and
(3)
fatigue or corrosion fatigue due to high
wear rate. Tube failures are costly because they result in plant shutdown to effect expensive
repairs. These problems can be very serious in nuclear heat exchangers. Therefore, it is impor-
tant to ensure that the modem shell and tube heat exchangers are free from flow-induced
vibration problems at all operating conditions.
In the past heat exchangers were designed conservatively. With the success of the state-
of-the
art
computer programs the trend is to design an efficient and compact heat exchanger.
Higher thermal performance and the desirability of low fouling generally require higher flow
velocities, while fewer baffle plates are desirable to minimize pressure drop. Higher flow
velocities and reduced structural supports can lead
to
severe flow-induced vibration problems.
In addition to these, the incorporation of new materials and processes without adequate consid-
erations of the effects on the structural dynamics contributed to more flow-induced vibration
problems, many of which led to tube failures
[l].
It
is
essential to avoid such costly tube
failures by a detailed flow-induced vibration analysis, preferably at the design stage after ther-
mal design is over.
The flow-induced vibration phenomenon and the mechanism responsible have been studied
extensively over the past
25-30
years.
As
a result, considerable literature has been developed
and there have been efforts to define guidelines for vibration prevention. The subject continues
to receive increasing attention because of its significance in heat exchanger applications, since
as much as
60%
of the heat exchangers in process industries are shell and tube type. References
1-18
brought about a better understanding of the flow-induced vibration phenomenon in shell
and tube heat exchangers. In this chapter, the mechanisms that cause FIV and their evaluation,
acceptance criteria, and design guidelines for vibration prevention are presented. Design guide-
423
