Kuppan T. Heat Exchanger Design Handbook
Подождите немного. Документ загружается.

394
Chapter
9
TIME
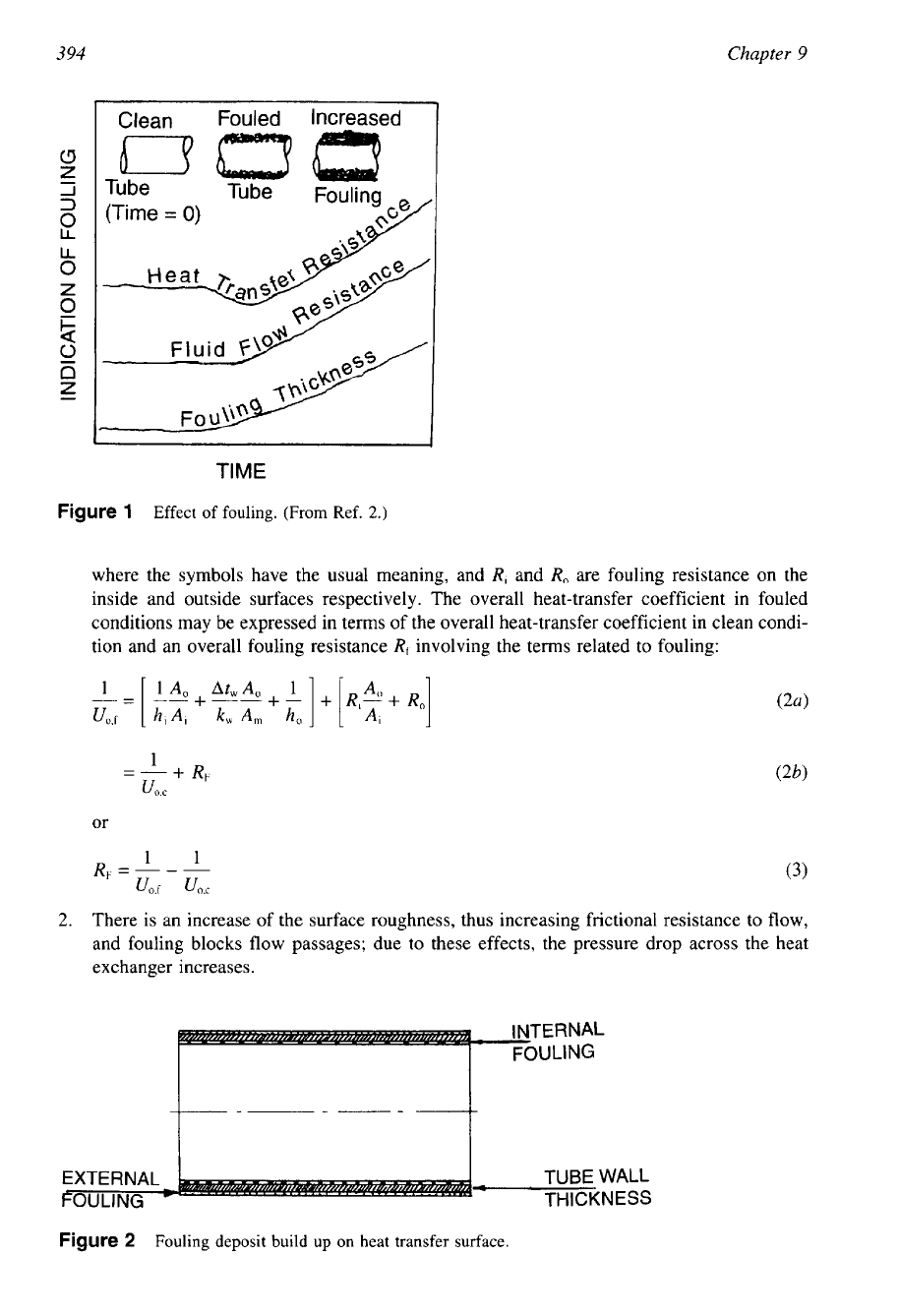
Figure
1
Effect of fouling. (From Ref.
2.)
where the symbols have the usual meaning, and
Ri
and
R,
are fouling resistance on the
inside and outside surfaces respectively. The overall heat-transfer coefficient in fouled
conditions may be expressed in terms of the overall heat-transfer coefficient in clean condi-
tion
and
an
overall fouling resistance
Rf
involving the terms related to fouling:
or
1
R
F-
1
(3)
U,,f
u0,c
2.
There is an increase of the surface roughness, thus increasing frictional resistance to flow,
and fouling blocks flow passages; due to these effects, the pressure drop across the heat
exchanger increases.
1-----
I
EXTERNAL
TUBE
WALL
FOULING
=-
THICKNESS
Figure
2
Fouling deposit build up on heat transfer surface.

Fouling
395
3.
Fouling may create a localized environment where corrosion is promoted.
4.
Fouling will reduce the thermal effectiveness of heat exchangers, which in turn affect the
subsequent processes or will increase the thermal load on the system.
5.
An additional goal become prevention
of
contamination
of
a process fluid or product.
2
COSTS OF HEAT EXCHANGER FOULING
Fouling of heat exchangers has been estimated to represent an annual expense
in
the United
States
of
somewhere between
$4.2
and
$10
billion
[4].
Fouling is something that is unwanted
and counterproductive. The presence of fouling on heat exchange surfaces causes additional
costs due to the following reasons:
1.
Increased capital expenditure due to oversizing.
2.
Energy losses associated with poor performance of the equipment.
3.
Treatment cost to lessen corrosion and fouling.
4.
Lost production due to maintenance schedules.
Economic considerations should be among the most influential parameters for determining
appropriate allowances for fouling. It is important to determine a strategy as to whether first
cost, operating and maintenance cost, or total cost over a period of years is the objective.
2.1
Oversizing
While sizing
a
heat exchanger it is a normal practice to oversize the heat-transfer surface area
to account for fouling, and the oversizing is normally of the order of
2040%.
2.2
Additional Energy Costs
Since fouling reduces the heat-transfer rates, additional energy is expended to increase the
heat-transfer rate; fouling also increases the pressure drop across the core and hence more
pumping power is required to meet the heat load.
2.3
Treatment Cost to Lessen Corrosion and Fouling
The formation of fouling deposits on heat-transfer surfaces necessitates periodical cleaning,
which costs money for cleaning materials and process, personnel required, and recently envi-
ronmental problems to discharge the effluents.
2.4
Lost Production Due to Maintenance Schedules and
Down Time
for
Maintenance
Periodical cleaning requires plant shutdown and hence the unavailability of system for produc-
tive purposes. Critical industries that cannot afford for downtime and loss in production will
maintain standby units, which again raises additional capital costs for spares.
3
PARAMETERS THAT INFLUENCE FOULING RESISTANCES
Many operational and design variables have been identified as having most pronounced and
well-defined effects on fouling. These variables are reviewed here
in
principle to clarify the
fouling problems and because the designer has an influence on their modification. Those pa-
rameters include the following:

396
Chapter
9
1.
Properties of fluids and their propensity for fouling
2.
Surface temperature
3.
Velocity and hydrodynamic effects
4.
Tube material
5.
Fluid purity and freedom from contamination
6.
Surface roughness
7.
Suspended solids
8.
Placing the more fouling fluid on the tube side
9.
Shell-side flow
10.
Type of heat exchanger
11.
Heat exchanger geometry and orientation
12.
Equipment design
13.
Seasonal temperate changes
14.
Heat-transfer processes like sensible heating, cooling, condensation, vaporization, etc.
3.1
Properties of Fluids and Usual Propensity for Fouling
The most important consideration is the fluid and the conditions conducive for fouling. At
times a process modification can result in conditions that are less likely to cause fouling.
3.2
Temperature
A good practical rule to follow is to expect more fouling as the temperature rises. This is due
to a “baking on” effect, scaling tendencies, increased corrosion rate, faster reactions, crystal
formation and polymerization, and loss in activity by some antifoulants [5]. Lower tempera-
tures produce slower fouling buildup, and usually deposits that are easily removable [4]. How-
ever, for some process fluids, low surface temperature promotes crystallization and solidifica-
tion fouling. For those applications, it is better to use an optimum surface temperature to
overcome these problems. For cooling water with a potential to scaling, the desired maximum
surface temperature is about 140°F (60°C). Biological fouling is a strong function of tempera-
ture. At higher temperatures, chemical and enzyme reactions proceed at a higher rate with a
consequent increase in cell growth rate [6]. According to Mukherjee [6], for any biological
organism, there is a temperature below which reproduction and growth rate are arrested and a
temperature above which the organism becomes damaged or killed. If, however, the tempera-
ture rises to an even higher level, some heat-sensitive cells may die.
3.3
Velocity and Hydrodynamic Effects
Hydrodynamic effects such as flow velocity and shear stress at the surface influence fouling.
Within the pressure drop considerations, the higher the velocity, higher will be the thermal
performance of the exchanger and less will be the fouling. Uniform and constant flow of
process fluids past the heat exchanger favors less fouling. Foulants suspended in the process
fluids will deposit in low-velocity regions, particularly where the velocity changes quickly, as
in heat exchanger water boxes and on the shell side
[5].
Higher shear stress promotes dislodg-
ing of deposits from surfaces. Maintain relatively uniform velocities across the heat exchanger
to reduce the incidence of sedimentation and accumulation of deposits.
3.4
Tube Material
The selection of tube material is significant to deal with corrosion fouling.
Carbon steel is corrosive but least expensive.
Copper exhibits biocidal effects in water. However, its use is limited in certain applications:
(
1
)
Copper is attacked by biological organisms including sulfate-reducing bacteria; this

Fouling
397
increases fouling.
(2)
Copper alloys are prohibited in high-pressure steam power plant heat
exchangers, since the corrosion deposits of copper alloys are transported and deposited in
high-pressure steam generators and subsequently block the turbine blades.
(3)
Environ-
mental protection limits the use of copper in river, lake, and ocean waters, since copper is
poisonous to aquatic life.
Noncorrosive materials such as titanium and nickel will prevent corrosion, but they are expen-
sive and have no biocidal effects.
Glass, graphite, and Teflon tubes often resist fouling and/or improve cleaning.
Although the construction material is more important to resist fouling, surface treatment by
plastics, vitreous enamel, glass, and some polymers will minimize the accumulation of
deposits
[3].
3.5
Impurities
Seldom are fluids pure. Intrusion of minute amounts of impurities can initiate or substantially
increase fouling. They can either deposit as a fouling layer or acts as catalysts to the fouling
processes
[4].
For example, chemical reaction fouling or polymerization of refinery hydrocar-
bon streams is due to oxygen ingress and/or trace elements such as Va and
MO.
In crystalliza-
tion fouling, the presence of small particles of impurities may initiate the deposition process
by seeding. The properties of the impurities form the basis of many antifoulant chemicals.
Sometimes impurities such as sand or other suspended particles in a cooling water may have
a scouring action, which will reduce or remove deposits
[3].
3.6
Surface Roughness
The surface roughness is supposed to have the following effects
[3]:
1.
The provision of “nucleation sites” that encourage the laying down of the initial deposits.
2.
The creation of turbulence effects within the flowing fluid and, probably, instabilities in
the viscous sublayer.
Better surface finish has been shown to influence the delay
of
fouling and ease cleaning.
Similarly, nonwetting surfaces delay fouling. Rough surfaces encourage particulate deposition.
After the initiation of fouling, the persistence of the roughness effects will be more a function
of the deposit itself
[3].
Even smooth tubes may become rough in due course due to scale
formation, formation of corrosion products, or erosion.
3.7
Suspended
Solids
Suspended solids promote particulate fouling by sedimentation or settling under gravitation
onto the heat-transfer surfaces. Since particulate fouling
is
velocity dependent, prevention is
achieved if stagnant areas are avoided. For water, high velocities (above
1
m/s)
help prevent
particulate fouling. Often it is economical to install an upstream filtration.
3.8
Placing the
More
Fouling Fluid
on
the Tube Side
As a general guideline, the fouling fluid is preferably placed on the tube side for ease of
cleaning. Also, there is less probability for low-velocity or stagnant regions on the tube side.
3.9
Shell-Side
Flow
Velocities are generally lower on the shell side than on the tube side, less uniform throughout
the bundle, and limited by flow-induced vibration. Zero-or low-velocity regions on the shell
398
Chapter
9
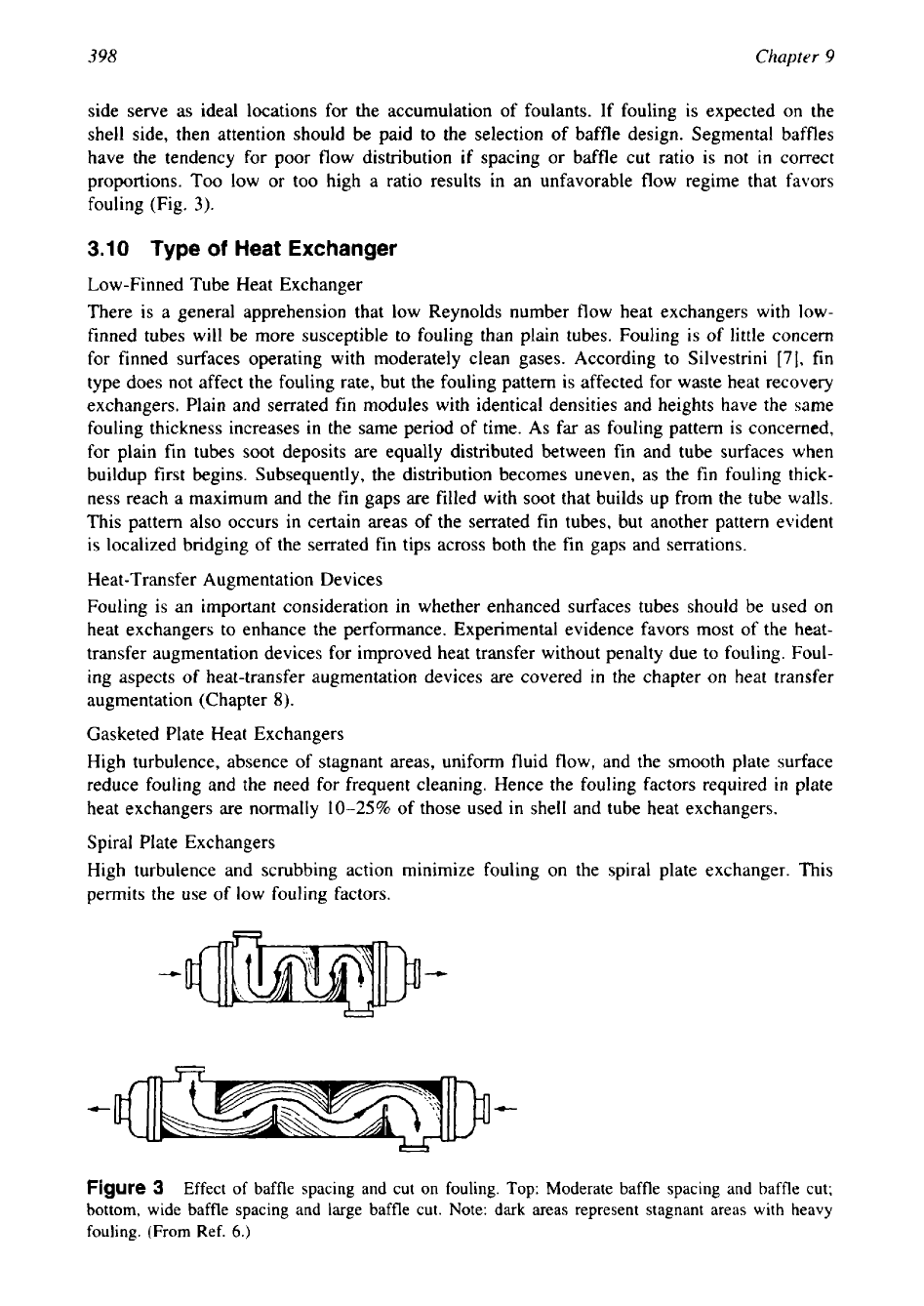
side serve as ideal locations for the accumulation of foulants. If fouling is expected on the
shell side, then attention should be paid to the selection of baffle design. Segmental baffles
have the tendency for poor flow distribution if spacing or baffle cut ratio
is
not
in
correct
proportions. Too low or too high a ratio results in an unfavorable flow regime that favors
fouling (Fig.
3).
3.10
Type
of
Heat
Exchanger
Low-Finned Tube Heat Exchanger
There
is
a general apprehension that low Reynolds number flow heat exchangers with
low-
finned tubes will be more susceptible to fouling than plain tubes. Fouling is of little concern
for finned surfaces operating with moderately clean gases. According to Silvestrini [7], fin
type does not affect the fouling rate, but the fouling pattern is affected for waste heat recovery
exchangers. Plain and serrated fin modules with identical densities and heights have the same
fouling thickness increases in the same period of time.
As
far as fouling pattern is concerned,
for plain fin tubes soot deposits are equally distributed between fin and tube surfaces when
buildup first begins. Subsequently, the distribution becomes uneven, as the
fin
fouling thick-
ness reach a maximum and the fin gaps are filled with soot that builds up from the tube walls.
This pattern also occurs in certain areas of the serrated fin tubes, but another pattern evident
is localized bridging of the serrated fin tips across both the fin gaps and serrations.
Heat-Transfer Augmentation Devices
Fouling is an important consideration in whether enhanced surfaces tubes should be
used
on
heat exchangers to enhance the performance. Experimental evidence favors most of the heat-
transfer augmentation devices for improved heat transfer without penalty due to fouling. Foul-
ing aspects of heat-transfer augmentation devices are covered
in
the chapter on heat transfer
augmentation (Chapter
8).
Gasketed Plate Heat Exchangers
High turbulence, absence of stagnant areas, uniform fluid flow, and the smooth plate surface
reduce fouling and the need for frequent cleaning. Hence the fouling factors required
in
plate
heat exchangers are normally
10-25%
of
those used in shell and tube heat exchangers.
Spiral Plate Exchangers
High turbulence and scrubbing action minimize fouling on the spiral plate exchanger. This
permits the use of low fouling factors.
Figure
3
Effect
of
baffle spacing and cut on fouling. Top: Moderate baffle spacing and baffle cut;
bottom, wide baffle spacing and large baffle cut. Note: dark areas represent stagnant areas with heavy
fouling. (From Ref.
6.)

Fou
1
ing
399
3.11 Seasonal Temperature Changes
When cooling tower water is used as coolant, considerations are to be given for winter condi-
tions where the ambient temperature may be near zero or below zero on the Celsius scale. The
increased temperature driving force during the cold season contributes to more substantial
overdesign and hence overperformance problems, unless a control mechanism has been insti-
tuted to vary the water/air flow rate as per the ambient temperature.
3.12
Equipment Design
Equipment design can contribute to increased fouling. Heat exchanger tubes that extend beyond
tube sheet, for example, can cause rapid fouling.
3.13 Heat Exchanger Geometry and Orientation
Heat exchanger geometry influences the uniformity of flows on the shell side and tube side.
Orientation of heat exchangers eases cleaning [4]. Finned tube heat exchanger geometry and
surface characteristics like tube layout, tube pitch, secondary surface density, etc. influence
fouling. The influences of surface modification and
fin
density on fouling were discussed
in
Chapter 4.
3.1
4
Heat-Transfer Processes Like Sensible Heating, Cooling,
Condensation, and Vaporization
The fouling resistances for the same fluid can be considerably different depending upon
whether heat is being transferred through sensible heating or cooling, boiling,
or
condensing [4].
4
FOULING CURVES/MODES
OF
FOULING
The amount of material deposited per unit area,
mf
is related to the fouling resistance
R,
and
the density of the foulant
pf,
thermal conductivity
kf,
and thickness of the deposit
xI
by the
following equation
[I]:
mf
=
PfXl
=
PfkfR,
(4)
The incidence of fouling with reference to time is normally defined by the following three
modes [I]:
1.
Linear mode-Increase of
rnf
(or
Rf)
with time
t.
2.
Falling rate mode-The rate of deposition decreases with increasing time.
3.
Asymptotic mode-The value of
mf
(or
Rf)
is not time variant after initial fouling.
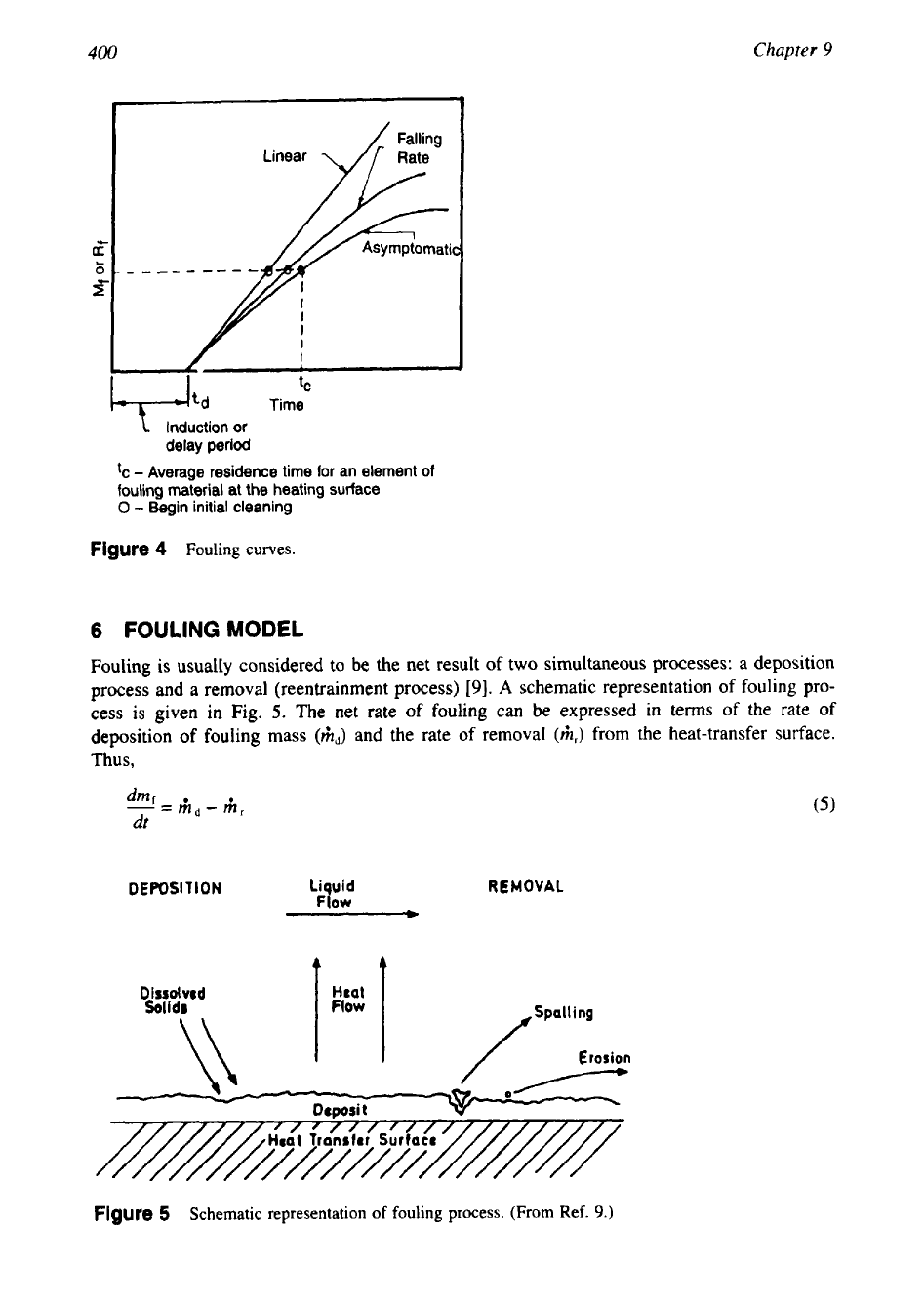
The representation of various modes of fouling with reference to time is known as a
fouling curve. Typical fouling curves are shown in Fig. 4. The linear modes and falling rate
modes may be the incidence of fouling in the early stages of asymptotic behavior. The asymp-
totic mode is of particular interest to heat exchangers, since the incidence of fouling raises the
possibility of continued operation of the equipments without additional fouling. The time delay
period,
td,
is
time required for the formation of initial fouling substrata. Smooth and nonwetting
surfaces like glass and Teflon will extend the delay period.
5
STAGES
OF
FOULING
For all the categories of fouling, the successive events that commonly occur
in
most situations
are up to five in number. They are initiation of fouling, transport to surface, attachment to
surfaces, removal from surfaces, and aging of deposit [S].
400
Chapter
9
t
tC
Time
Induction or
delay
period
tc
-
Average residence time for an element of
fouling material at the heating surface
0
-
Begin initial cleaning
Figure
4
Fouling
curves.
6
FOULING
MODEL
Fouling is usually considered to be the net result
of
two simultaneous processes: a deposition
process
and
a removal (reentrainment process)
[9].
A
schematic representation of fouling
pro-
cess
is
given in
Fig.
5.
The
net rate of fouling
can
be expressed in terms of the rate
of
deposition
of
fouling mass
(hd)
and the rate of removal
(h,)
from the heat-transfer surface.
Thus,
dmf
-
=
md
-
riz,
dt
DEPOSITION
Liquid
REMOVAL
Flow
L
Figure
5
Schematic representation
of
fouling process.
(From
Ref.
9.)

Fouling
401
One of the earliest models of fouling was that by Kern and Seaton
[
101.
In this model, it was
assumed that
h,
remained constant with time
t
but that
h,
was proportional to
rnf
and therefore
increased with time to approach
hd
asymptotically. Thus
hd
=
pmf;
then integration of
Eq.
5
from the initial condition
mf
=
0
at
t
=
0
gives
rnf
=
mr*(
1
-
e-P')
(6)
where
mf*
is the asymptotic value of
mf
and
p
=
MC.
The time constant
t,
represents the average
residence time for an element of fouling material at the heating surface. Equation
6
can be
expressed in terms of fouling resistance
Rf
at time
t
in terms of the asymptotic value
Rr*
by
Rf
=
Rf*(
1
-
e-P'>
(7)
Modeling efforts in the past
25
years have centered on evaluating the functions
md
and
m,
for
specific fouling situations.
7
MECHANISMS OF FOULING
It is of great importance to understand the fouling mechanisms in principle, as they will indi-
cate the causes and conditions of fouling and hence give clues how fouling can be minimized.
Based on the mechanism of fouling, Epstein
[8]
classifies fouling into six types:
1.
Particulate fouling
2.
Reaction fouling
3.
Corrosion fouling
4.
Precipitation fouling
5.
Biological fouling
6.
Solidification fouling
7.1
Particulate
Fouling
Particulate fouling may be defined as
the
accumulation
of
particles suspended in the process
streams onto the heat-transfer surfaces. This type
of
fouling includes sedimentation of settling
under gravitation as well as deposition of colloidal particles by other deposition mechanisms
on to the heat transfer surfaces.
Various
forms
of
particulate fouling are:
1.
Fouling that occurs in once-through cooling-water systems using sea, river, and lake water
containing mud, silt, and sediments. They are capable of depositing in low-flow areas,
forming a physical barrier, and preventing oxygen from reaching the metal/solution inter-
face. The deposit buildup will promote localized corrosion.
2.
Gas-side fouling: Gas-side fouling is mostly by particulate fouling by dirty gas streams,
airborne contaminants, and the leakage and mixing of one process stream with the other,
in addition to its own contaminantsrarbon soot in the case of combustion gases and
exhaust gases. In some cases, gas-side fouling may also be accompanied by corrosion,
particularly when condensation of corrosive acids take place from combustion gases. Level
of
gas-side fouling depends on features such as
Tube layout pattern-in-line or staggered
Tube pitch
Fin density
Fin surface characteristic such as plain fin, surface-modified fin

402
Chapter
9
3.
Another important area of particulate fouling is due to the aluminum transport phenome-
non. The problem consists of corrosion of heat-rejecting aluminum surfaces (engine block)
followed by deposition of insoluble aluminum salts in the radiator. The corrosion process
can be prevented by the inclusion of silicate inhibitors in the coolant formulation.
7.2
Chemical Reaction Fouling (Polymerization)
Deposits formed by chemical reactions at the heat-transfer surface in which the surface material
itself is not a reactant are known as chemical reaction fouling. Polymerization, cracking, and
coking of hydrocarbons are prime examples of reaction fouling. The factors likely to affect
reaction fouling include the following [3,11]:
Temperature is the most sensitive variable. It is usual that below a certain surface temperature
polymerization does not initiate, but increases rapidly above that. The reaction rate is
related to the temperature by the Arrhenius law:
where
E
is the activation energy,
R
the gas constant,
A,,
the rate constant, and
T,
the heat
transfer surface temperature.
The presence of most sulfur compounds, nitrogen compounds, and the presence of trace ele-
ments (metallic impurities) such as
MO
and Va in hydrocarbon streams significantly in-
creases the fouling rates.
Composition of the process stream, including contaminants and, especially, oxygen ingress
will affect reaction fouling.
Sound prevention measures for chemical reaction fouling should include the following
[3,11]:
1.
Avoidance of feed contact with air or oxygen by nitrogen blanketing.
2.
Elimination or reduction of unsaturates, which are particularly high in cracked stocks.
3.
Caustic scrubbing to remove sulfur compounds.
4. Desalting, which reduces trace metal contamination.
5.
The use of antioxidation additives that inhibit the polymerization reaction, along with steps
taken to minimize oxygen ingress.
6.
Use of additives known
as
metal coordinators, which react with the trace elements and
prevent them from functioning as fouling catalysts. Other additives recommended are cor-
rosion inhibitors and dispersion agents [3].
7.3
Corrosion Fouling
Corrosion fouling is due to the deposition of corrosion products on heat-transfer surfaces. In
this category of fouling process, the heat-transfer surface material itself reacts to produce corro-
sion products, which foul the heat-transfer surface. The most common forms of this type of
fouling are material
loss
due to general thinning, iron oxide on carbon steel tubes in cooling-
water systems, and fouling of soldered radiator tube ends on the water side by solder bloom
corrosion. Corrosion fouling is highly dependent upon the choice of material of construction
and the environment. Hence,
it
is
possible to overcome corrosion fouling if the right choice of
material has been made to resist the environment. Measures such as the use of inhibitors,
cathodic protection, and surface treatment such as passivation of stainless steel will minimize
corrosion and hence corrosion fouling.

Fouling
403
7.4
Crystallization
or
Precipitation Fouling
This type of fouling mostly takes place in cooling-water systems, when water-soluble salts,
predominantly calcium carbonates, become supersaturated and crystallize on the tube wall to
form scaling. Such scaling occurs because many of the dissolved salts in water exhibit inverse
solubility effects, a condition that reverses the normal solubility (increasing with temperature)
into one that decreases with temperature. Thus an inverse solubility solution will crystallize
when heated (e.g., cooling water), while normal solubility salts will crystallize when cooled.
Chemical additives can be helpful to reduce fouling problems due to crystallization and freez-
ing in a number of ways. Broadly there are four groups of chemicals to control crystallization
[3]:
distortion agents, dispersants, sequestering agents, and threshold chemicals.
Modeling for Scaling
According to Hasson
[
121, scaling is due to diffusion of calcium and carbonate ions from the
bulk of the fluid, followed by crystallization of CaC03 on the hot wall surfaces. Their model
for predicting CaCO? scaling rates is given by
rn,
=
KR[(Ca2+),(Co,
-
K,,]
(9)
where
rn,
is the scale deposition rate (kg/m'
s),
KR
the constant for crystallization rate, and
the solubility product of CaC03 (mol/m3)'.
The principle of fouling and the factors promoting scaling are discussed
in
the section on
cooling water corrosion are discussed at the end of this chapter.
7.5
Biological Fouling
The attachment of microorganisms (bacteria, algae, and fungi) and macroorganisms (barnacles,
sponges, fishes, seaweed, etc.) on heat-transfer surfaces where the cooling water is used in as-
drawn condition from river, lake, sea and coastal water, etc., is commonly referred to as biolog-
ical fouling. On contact with heat-transfer surfaces, these organisms can attach and breed,
sometimes completely clogging the fluid passages, as well as entrapping silt or other suspended
solids and giving rise to deposit corrosion. Concentration of microorganisms in cooling-water
systems may be relatively low before problems
of
biofouling are initiated. For open recirculat-
ing systems, bacteria concentrations
of
the order of 1
x
10'
cells/ml and fungi of
1
x
103 cells/
ml may be regarded as limiting values
[3].
Corrosion due to biological attachment to heat-
transfer surfaces is known as microbiologically influenced corrosion (MIC). MIC is discussed
in detail in Chapter
12
on corrosion. The techniques that can be effective in controlling biologi-
cal fouling include the following:
1.
Select materials that posses good biocidal properties.
2.
Mechanical cleaning techniques like upstream filtration, air bumping, back flushing, pass-
ing brushes, sponge rubber balls, grit coated rubber balls, and scrapers [4].
3.
Chemical cleaning techniques that employ biocides such as chlorine, chlorine dioxide,
bromine, ozone, surfactants, pH changes, and/or salt additions.
4.
Thermal shock treatment by application of heat, or deslugging with steam
or
hot water.
5.
Some less well-known techniques like ultraviolet radiation.
7.6
Solidification Fouling
or
Freezing Fouling
The freezing of a liquid or
of
higher-melting constituents of a multicomponent solution on a
subcooled heat-transfer surface is known as solidification fouling. Notable examples include
frosting of moisture in the air, freezing of cooling water in low-temperature processes, and
