Kuppan T. Heat Exchanger Design Handbook
Подождите немного. Документ загружается.


994
Chapter
1.5
the coupon to the same heat treatment as of the dished end, including the final normalizing.
Problems faced during heat treatment of heads include [49]:
(1)
When the head is quenched,
reasonable escaping of gases and steam should
be
provided to avoid origination of a steam
cushion hindering uniform cooling, and
(2)
the heat treatment is associated with the risk of
distortion.
4
DEFINITION AND GENERAL DESCRIPTION OF BRAZING
One of the more important processes
of
fabrication for complex assemblies is brazing.
Most
of the plate-fin heat exchangers (PFHE), some tube-fin exchangers, especially automobile radi-
ators, and some highly compact metal rotary regenerators are brazed. Brazing joins two similar
or dissimilar metalshonmetals by heating them in the presence of a filler metal having a
liquidus temperature above 840°F (450°C) but below the solidus temperature of the base mate-
rials. Heating may be provided by a variety of processes. The molten filler metal distributes
itself between the closely fitted surfaces
of
the joint by capillary action. Brazing differs from
soldering in that soldering filler metals have a liquidus temperature below 840°F (450°C).
4.1 Brazing Advantages
Brazing has the advantages of greater flexibility and scope. Strong, uniform, leak-proof joints
are made rapidly, inexpensively, and even several joints simultaneously. It is unrivaled for
assembling thin or delicate components or assemblies that are being produced in large quanti-
ties with the facility to produce many joints simultaneously
[50,5
11.
Precise joining
is
compara-
tively easy without application of intense local heat to small areas. There is no heat-affected
zone
in
brazing. Brazing requires little operator skill. Since the base materials do not melt at
the brazing temperature, practically any two materials can be joined together in spite of a
difference
in
composition, melting point, or thermal expansion. This includes similar and dis-
similar metals. However, not all combinations of dissimilar metals can be brazed.
4.2 Disadvantages
of
Brazing
The brazing process is often considered an
art
today
[52].
According to Shah, brazing requires
considerable expenditure and capital cost, as well as development time, before ideal brazed
joints can be manufactured, particularly for complicated assemblies.
If
there is any change in
any one of the brazing process variables, such as flux, filler metals, temperature, brazing atmo-
sphere including vacuum, or equipment and fixture, in general one needs to redefine the braz-
ing process
to
obtain ideal joints.
The objective of this section is to provide comprehensive details on the fundamentals of
brazing, brazing processes, and brazing of important heat exchanger materials including alumi-
num, stainless steel, and nickel. References 50-59 provide general and specific information on
brazing.
5
ELEMENTS OF BRAZING
The proper use of brazing for a given application requires that due consideration be given
to
several factors:
1.
Joint design
2.
Filler metal selection
3.
Precleaning and surface preparation

Heat Exchanger Fabrication
995
4. Fluxing
5.
Fixturing
6.
Heating method
7.
Postbraze treatment and removing flux residues
These factors are discussed in detail next.
5.1
Joint Design
Joint design should consider the following factors:
1.
Joint clearance: Joint clearance determines capillary force. The capillary force draws the
molten filler metal deeply into every joint clearance. The joint clearance must be within
specified limits. Suggested joint clearances as per joint width for various brazing processes
are tabulated in Ref.
50.
2.
Avoid
flux
entrapment: Entrapped flux may make the brazed joints susceptible to in-
service corrosion.
Too
tight joint clearance restricts filler metal flow, whereas too wide
joint clearance allows filler metal flow around flux. Flux trapped
in
the joint may also
falsify leak test indications.
3.
When joining different metals, their differing coefficients of thermal expansion become
vitally important.
4.
When brazing foil is used, the mating surfaces should be held
in
contact. The foil thickness
will then determine the joint clearance.
5.
The brazing symbol on the engineering drawing designates the location, class, and configu-
ration
of
the brazed joint. Such symbols shall be as per ANSVAWS A2.4, Standard Sym-
bols for Welding, Brazing, and Nondestructive Examination.
Joint Types
In common with all forms of brazing, lap joints should be used in preference to butt joints.
Joint types can be butt, lap, and T.
5.2
Brazing Filler Metals
Brazing filler is a nonferrous metal or alloy that melts at a temperature lower than that of the
melting point of the base metal. Most filler metals are alloys,
so
they melt through a range of
temperatures. When molten, the filler metal must wet the base metals, flow, and spread into
joints to form brazed joints. The desired characteristics of molten filler metal are a low contact
angle (Fig. 27), high liquid surface tension, and low viscosity.
Composition of Filler Metals
The American Welding Society lists in AWS 5.8-81 the specifications for brazing filler metal.
Conventional fillers that find use in brazing of heat exchangers are in general based on alumi-
num, copper, nickel, cobalt, silver, and gold. Vacuum-grade fillers are silver-based, gold-pal-
Wetting Dewetting
Figure
27
Molten filler metal contact angle.

996
Chapter
I5
ladium, aluminum-silicon, and copper-based alloys. The selection of filler metals is discussed
by Weymuller [60] and Birchfield [61].
Aluminum Filler Metals
Aluminum filler metals (BAlSi series) are used for brazing aluminum.
To
reduce the possibil-
ity of galvanic corrosion, brazing filler metals are aluminum alloys rather than dissimilar metals
[62]. Most of the brazing alloys are based in the aluminum-silicon eutectic system, containing
between 7 and 12% silicon with a melting point
of
1070°F
(577°C).
Occasionally other ele-
ments are added. They are available as filler metal or as clad brazing sheet. They are used
to
joint the wrought grades such as
1100,
3003,
3004,
3005,
5005, 5050,
5052, 6053, 6061,6062,
6063, 6951, and 7005 [54,60]. Figure 28a shows a comparison
of
brazing temperature ranges
of aluminum alloy base metals and aluminurn filler metals. Figure 28b shows maximum per-
missible brazing temperatures for various aluminum alloys
[50].
1200
1150
1100
1050
-
Approximate
solidus
1000
temperature
Recommrndrd
brazing
range
950
Pure
Aluminkm
ii00.1350
and
3003
5005
3004
5050
sheets
no.
11.12
700L
7005
6061
Figure
28
(a) Brazing temperature ranges for aluminum filler metals
[59].
(b)
Maximum permissible
brazing temperatures for
various
aluminum alloys
[50].

997
Heat Exchanger Fabrication
Copper Fillers
Copper filler (BCu) metal is used to braze ferrous and nickel base alloys.
Copper-phosphorus fillers (BCuP series) apply mostly for joining copper and its alloys
and stainless steel. Avoid using these fillers on ferrous alloys, nickel-based alloys, and copper
alloys containing more than 10% nickel
[54,60].
These fillers are relatively low in cost, since
they lack silver or gold. Copper-based filler alloys are not compatible with sulfur-bearing fuels
[60,63].
Copper-zinc fillers (BCuZn series) are used to braze steels, stainless steels, copper and
its alloys, and nickel-based alloys.
Nickel-Based Filler Metals
Nickel-based filler (BNi series) metals are used primarily to braze heat- and corrosion-resistant
alloys, most commonly nickel- and cobalt-based alloys and the
AISI
300
and
400
series stain-
less steels. These filler metals provide joints that have excellent corrosion resistance and high-
temperature strength [64]. Since they require relatively high melting temperature, their use is
generally restricted to furnace brazing in a controlled atmosphere including vacuum. Also,
nickel fillers tend to be sluggish in the fluid state; therefore, joint design, filler metal selection,
and brazing temperature should be carefully considered [54].
Silver-Based Filler Metals
Silver-based brazing alloys (BAg series) find a multitude
of
uses, because they cover a wide
range of brazing temperatures, 1145°F to 1900°F. They are used to join ferrous and nonferrous
metals and alloys, except low-melting metals such as aluminum, magnesium, and their alloys
[60].
Their advantages are free flow, relatively low brazing temperature, and that they make
ductile and smooth joints [54].
Gold-Based Fillers
Gold-based fillers (BAu series) are preferred for specialized applications such as aerospace
heat exchangers to braze stainless steel, nickel, and cobalt-based alloys that require oxidation
and corrosion resistant at elevated temperatures, and compatibility with sulfur-bearing fuel.
These fillers are primarily used in furnace brazing applications. Because gold interacts little
with base metals, gold-based fillers will not alter properties of the base metal
[60].
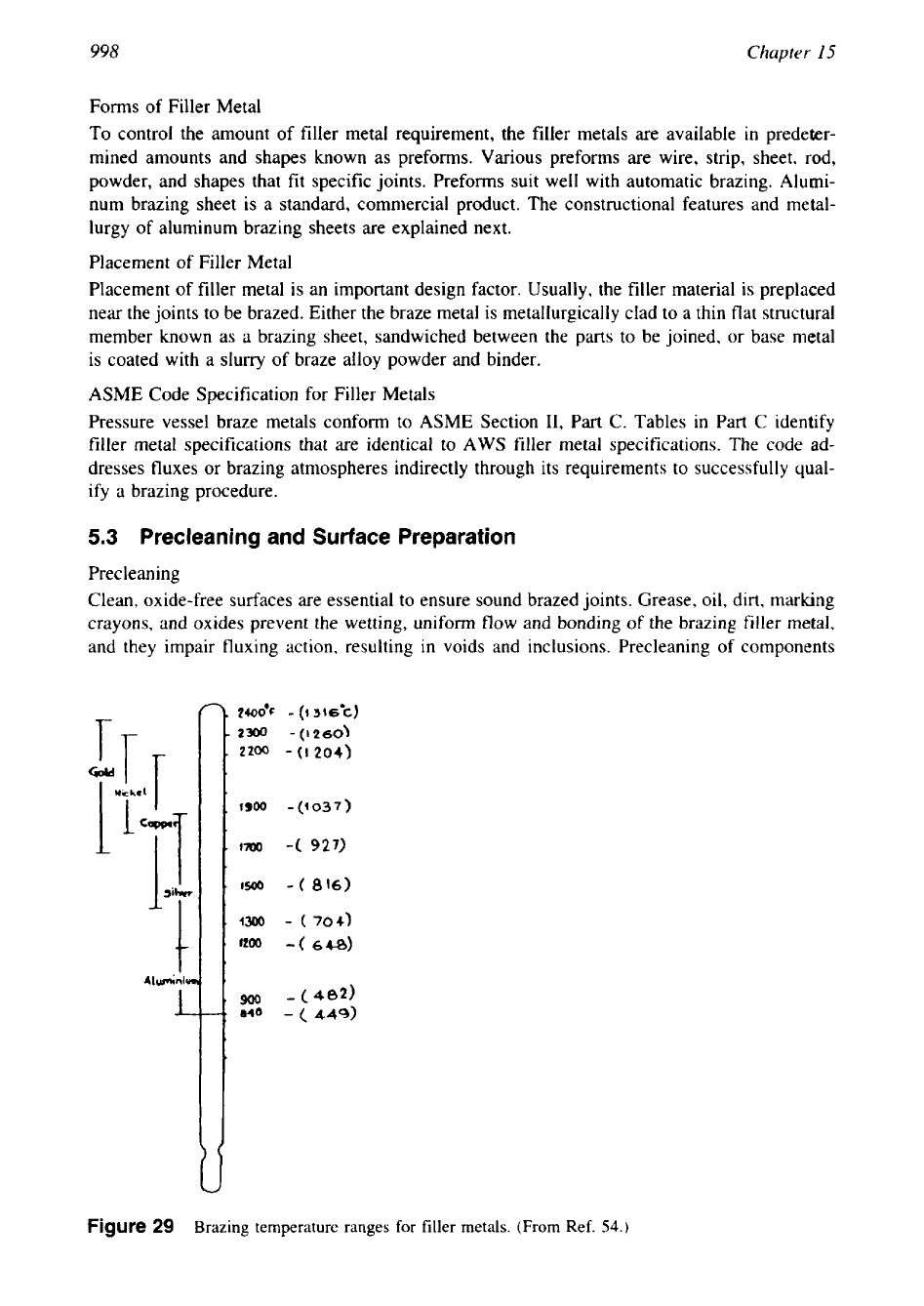
Various
filler metals and their applications are give in Table 1, and brazing temperature ranges for
aluminum, copper, silver, nickel, and gold alloy filler metals are shown in Fig.
29.
Table
1
Typical Filler Metal Applications
Primary metal
in filler
Base materials
A
1
u
mi
n u m
Aluminum-based alloys
Copper
Copper-based alloys, ferrous metals, nickel-based alloys
Copper-phosphorus
Copper-based alloys; avoid using on ferrous alloys, nickel-based alloys, and
copper alloys containing more than
10%
nickel
Copper-zinc
Steels, stainless steels, copper and its alloys, and nickel-based alloys
Nickel
SS
types
300
and
400,
nickel-based and cobalt-based alloys
Silver
Ferrous; nonferrous except aluminum and magnesium and their alloys, which
melt at low temperatures
Gold
Thin sections of
SS,
nickel-based,
or
cobalt-based alloys requiring high corrosion
and oxidation resistance
998
Chapter
I5
Forms of Filler Metal
To
control the amount of filler metal requirement, the filler metals are available in predeter-
mined amounts and shapes known as preforms. Various preforms are wire, strip, sheet, rod,
powder, and shapes that fit specific joints. Preforms suit well with automatic brazing. Alumi-
num brazing sheet is a standard, commercial product. The constructional features and metal-
lurgy of aluminum brazing sheets are explained next.
Placement of Filler Metal
Placement of filler metal is an important design factor. Usually, the filler material is preplaced
near the joints to be brazed. Either the braze metal is metallurgically clad to a thin flat structural
member known as a brazing sheet, sandwiched between the parts
to
be joined,
or
base metal
is coated with a slurry of braze alloy powder and binder.
ASME Code Specification for Filler Metals
Pressure vessel braze metals conform to ASME Section
11,
Part
C.
Tables in Part
C
identify
filler metal specifications that are identical to AWS filler metal specifications.
The
code ad-
dresses fluxes or brazing atmospheres indirectly through its requirements to successfully qual-
ify a brazing procedure.
5.3
Precleaning and Surface Preparation
Precleaning
Clean, oxide-free surfaces are essential to ensure sound brazed joints. Grease, oil, dirt, marking
crayons, and oxides prevent the wetting, uniform flow and bonding
of
the brazing filler metal,
and they impair fluxing action, resulting
in
voids and inclusions. Precleaning of components
Figure
29
Brazing temperature ranges for filler metals. (From Ref.
54.)

Heat Exchanger Fabrication
999
may be accomplished by degreasing with organic solvents or vapors or by a mild chemical
etch, such as dilute caustic soda solution
[50].
Cleaning should always be carried out before
assembly. Otherwise, if an assembled component is immersed in a chemical cleaner, residues
will inevitably be trapped in the joints
[65].
Scale and Oxide Removal
Good performance, which characterizes the soundness (leak tightness, joint strength, and fillet
shape) and integrity of the brazed joint, is possible only when the surface oxide film on the
metal surface is dispersed sufficiently for wetting and flow of filler metal to occur.
Chemical Cleaning.
Scale and oxide removal can be accomplished mechanically or chemi-
cally. Descaling can be done with any of the following solutions
[54]:
1.
Acid cleaning
2.
Acid pickling-sulfuric, nitric, and hydrochloric acid
3.
Salt bath pickling
Prior to descaling, degreasing is recommended.
Mechanical Cleaning.
Mechanical cleaning is usually confined to those metals with heavy
tenacious oxide films. Mechanical methods include abrasive grinding, grit blasting, filing, or
wire brushing using stainless steel bristles. Grit blasting should be done with clean blasting
material such as silica sand or alumina sand. Care must be taken that the mechanical cleaning
materials are not embedded in the metals to be brazed. After mechanical cleaning, air blast-
ing or ultrasonic cleaning should be used to remove all traces of loosened oxides or blasting
medium.
Protection of Precleaned Parts
After cleaning, the cleaned parts should be assembled and brazed without delay to avoid oxida-
tion and build up of contamination. The cleaned surfaces should not be hand touched after
cleaning. They should be stored and transported to the braze preparation areas
in
dry, clean
containers, such as plastic bags.
5.4
Fluxing
Conventional brazing processes performed in air or other oxygen-bearing atmosphere require
fluxing. In other words, vacuum brazing does not require flux, and furnace brazing performed
in inert or strongly reducing atmosphere usually does not require flux. Fluxes are necessary to
displace the oxide layers at the bonding sites and to allow the wetting by the molten filler
metal. Without flux, molten filler forms a ball even though the surface it contacts is clean and
the temperature is high. Additionally, the flux protects the bonding sites against reoxidation of
the cleaned surface during brazing. However, fluxes are not intended to perform the primary
function of removal of oxides, scales, coatings and surface contaminants. Fluxes come as paste,
powder, slurry or liquid.
Selection of a Flux
Flux selection is influenced by these factors
[54,64]:
base material, filler metal, brazing pro-
cess, joint configurations, and flux dispensing methods such as brush, dip, syringe, spray, etc..
which determine
flux
forms and ease of cleaning.
Composition of the Flux
Fluxes contain one or more heavy metal chlorides in addition to active fluoride salts along
with other chemicals. Fluxes are generally mixed with water to form a thick slurry, which is
applied to the precleaned parts to be brazed.

1000
Chapter
15
Demerits of Brazing Using Corrosive Fluxes
Though several advantages can be claimed for the brazing techniques using fluxes [66], brazing
fluxes are chemically active, and their residues are highly corrosive, especially in the presence
of moisture. Therefore, after brazing, flux residuals must be removed by a cleaning process
to
avoid both corrosion and contamination problems. Other problems associated with fluxes in-
clude [62]:
(1)
Postbraze cleaning is costly;
(2)
for complex structures with closed tortuous
passage ways, the elimination of flux residuals can require long cleaning cycles; and
(3)
dis-
posal of spent cleaning solutions can become costly with ever tighter control
of
plant effluent.
5.5
Fixturing
As far as possible, components to be brazed should be self-fixturing. Fixtures, being in contact
with the part, extend the heating time to bring the components up to the brazing temperature.
Slow heating due to attachment of fixturing will change the characteristics of the filler metal
[65]. Examples of self-fixturing construction include aluminum screws, rivets, resistance welds,
argon arc tack welds, piercing, and tagging. For external fixtures, austenitic stainless steels or
heat-resisting nickel alloys is recommended. Mild steel can also be used for low-volume pro-
duction. Use clean and
dry
fixtures to avoid pumpdown delays.
5.6
Brazing Methods
Brazing processes are classified according to the sources or methods of heating. There are
numerous brazing methods, including:
1. Torch brazing
2.
Dip brazing
3.
Furnace brazing
4.
Vacuum and controlled-atmosphere brazing
5.
Induction brazing
6. Resistance brazing
7.
Infrared brazing
All these brazing methods include the same brazing procedures. The prime difference lies in
the way the parts are heated and the way flux and filler metals are applied. Except for vacuum
and controlled-atmosphere brazing, all methods require flux. In this section, the first four braz-
ing methods are discussed.
To
braze, surfaces must be cleaned, freed
of
excess oxide, coated with flux, and spaced a
few thousandths of an inch apart. Brazing filler is placed in or near the joint to be formed,
assembled, and fixtured. When heat is applied, the flux displaces oxide and shields the metal
from air. As the filler flows, it displaces flux and wets the base metal, adapting to submicm-
scopic irregularities and dissolving the small high points it encounters.
Torch Brazing
Any joint that can be reached by a torch and brought to brazing temperature can be readily
brazed by this technique. Torch brazing can be either hand-held or automatic. Hand-held man-
ual torch brazing is most frequently used for repairs, one-of-a-kind brazing jobs, short produc-
tion runs, and as an alternative to gas or arc welding. For example, repairs or low-production
copper-to-copper joints in return bends of an evaporator or a condenser of a airconditioner are
preformed by torch brazing. Most commercial torch brazing is carried out by use of oxyacety-
lene flame in which the torch is adjusted to produce slightly reducing flame for most applica-

1001
Heat Exchanger Fabrication
tion. In automatic torch brazing, the assembly is moved automatically in relation to the torch,
or vice versa. It is used for mass production.
Dip Brazing
In the dip brazing process, often referred to as salt-bath brazing, the part or assembly being
joined is held together and immersed in a bath of molten salt, which flows into the joints when
the parts reach a temperature approaching that of the bath. The molten
flux,
at a temperature
slightly above the liquidus of the filler metal, serves as a heat source and partially supports the
submerged metal parts. In addition to providing the heat for brazing, many of the salts have
fluxing properties. Dip brazing has been extensively used in industry for daily production of
aluminum and other alloy parts. It has the unique advantages that
[52]
(1)
the time required
for heating is about one-fourth that required for furnace brazing,
(2)
distortion due to self
weight is less due to buoyancy, and
(3)
the parts reach the brazing temperature uniformly.
Choice
of
Flux.
Commercial dip-brazing fluxes are generally similar to fluxes used with other
brazing methods. Their main ingredients include the combinations of sodium chloride, potas-
sium chloride, aluminum fluoride, and lithium chloride. Dip brazing requires water-free flux
to avoid spattering of moisture from the bath.
Dip Bath Heating Means.
Heating may be by gas for lower temperature systems or by one
of the two forms of electrical heating: either resistance elements mounted around the pot, or
electrodes immersed in the molten salt bath.
Flux Pot.
Flux pots comprise steel-reinforced vessels lined with high-alumina, acid-proof
fire brick. Pot covers are well insulated. Large covers may be mounted on rollers and power
operated.
Preheating the Assemblies.
Dip brazing generally requires preheating. This helps in a flux
preplaced joint, dries the flux, and vaporizes moisture from the assembly before being im-
mersed into the salt bath and thereby prevents accidents from steam explosions. It also can
reduce brazing time, minimize the salt residue that forms on the part, and reduce distortion, as
well as reduce bath size
[54].
Preheating is achieved either by the flue gases or by electrical
heating in a suitable furnace to a temperature
50°F
to
100°F
(30°C
to
60°C)
below the solidus
temperature of the brazing filler metal until the entire assembly has attained this temperature.
For aluminum brazing, the assembly is preheated to approximately
1000°F
(538°C).
Dip Brazing Procedure.
The dip brazing steps (along with furnace-brazing steps) are given
in Table
2.
Immersion Time.
The time needed to form the joints depends on the mass of the assembly
and its temperature at the moment it enters the molten salt bath. Dip time varies from as little
as a few seconds to as long as
10
or
20
min. There is
no
formula to arrive at the immersion
time, but it is arrived by trial-and-error practice
[50].
Maintaining the Flux Bath.
A
properly maintained flux bath will produce a work piece that
is bright and shiny, with fillets well formed and complete
[54].
To achieve these properties,
the bath should be relatively free of sludge and surface film, slightly acidic with a pH between
6.4
and
7.0
(for aluminum a pH between
5.3
and
6.9),
and relatively constant chemical compo-
sition. The bath is periodically checked for moisture, acidity, chemical composition, tempera-
ture, and other physical characteristics by the laboratory and corrective action made as required.
Scum and Sludge Removal.
Contaminants in and on top of the hot flux interfere with quality
of brazing. Contaminants that float to the surface are called scum, and they are readily removed
by skimming the bath’s surface with a sheet of aluminum. There are a number of commercial
preparations that, when added to the bath, cause the particles to coagulate, making their re-

1002
Chapter
15
Table
2
Dip and Furnace Brazing Steps
Dip brazing Furnace brazing
Precleaning and surface preparation
Precleaning and surface preparation
Preparation of flux bath
Flux preparation and application"
Assembly and fixturing
Assembly and fixturing
Preheating
Brazing in flux bath
Brazing in furnace
Cooling or quenching
Cooling
or
quenching
Hot water washing
Hot water washing"
Flux removal
Flux removal"
Finishing and inspection
Finishing and inspection
"Not
applicable
for
vacuum brazing.
moval easier
[50].
Contaminants that sink to the bottom of the pot are called sludge. The
sludge is removed periodically by ladling with a perforated tool.
Ventilation. Salt-bath dip brazing can emit toxic or noxious gas, fumes, and dust. Ventilation
to remove air emissions from the above the bath is a must.
Limitations of Dip Brazing.
Some of the limitations of dip brazing are:
1.
Dip brazing generally requires preheating to prevent accidents from steam explosions.
2.
The shape of the part must be designed to avoid trapping air or salt and to be drained
completely.
3.
Maintenance problem due to power shutdown for internally electric heated salt bath.
4.
A
different salt will probably be required for the next application if either the parent metals
or the brazing alloy has been changed; frequent changing is not economical.
Furnace Brazing
The termfurnace brazing can be applied to any brazing process where a furnace is used as the
heat source to raise the parts to be joined to their brazing temperature. Furnace brazing
is
extensively used in industry for brazing heat exchangers and joining complex parts. It offers
uniform heating, and hence accurate temperature control is possible. The finished product has
excellent quality. The process is economically attractive for brazing heavy assemblies
in
which
multiple brazed joints are to be formed simultaneously or many assemblies are to be joined
simultaneously.
Furnace Heating. The furnaces may be heated by gas, oil, or electricity, and provided with
temperature controls. Fluxes or specially controlled atmosphere that perform fluxing functions
must be provided. Filler metal must be preplaced in the form
of
sheet, wire, rings, powder, or
other suitable forms.
Atmospheres. Furnace brazing takes place in a vacuum or in a controlled atmosphere of high-
purity inert or reducing gas. Both reduce the amount of flux needed or in some cases eliminate
the need for
flux
completely, and prevent oxidation and scaling during heating. There
are,
however, examples of this type of furnace being used with an atmosphere of normal air or
ultradry air in association with flux. These aspects are discussed later. The brazing atmosphere,
whether gaseous or vacuum, should be free from harmful constituents such as sulfur, oxygen,
and water vapor. When brazing in a gaseous atmosphere, it is a common practice to monitor
the water vapor content of the atmosphere as a function of dewpoint.
In
general, a furnace
atmosphere having a dewpoint less than about -60°C (-75°F) produces better quality.

1003
Heat Exchanger Fabrication
Brazing Furnaces.
In general, brazing furnaces are classified as (1) batch furnace,
(2)
semi-
continuous or locked furnace, or
(3)
continuous furnace. Single-chambered, batch-type units
are used for low-volume production, while multichambered semicontinuous or continuous fur-
naces are employed for high-volume production applications. Selection of a particular type
production furnace depends on the size and geometry of the parts to be brazed, as well as
production rates required.
Classification
of
Batch Furnaces.
Batch furnaces are classified as follows [54,67]:
1.
Direct-combustion furnaces
2. Muffle furnaces or hot-wall furnaces
3.
Retort-bell type combustion furnaces
4. Vacuum brazing furnaces: single pumped retort furnaces, double pumped retort furnace,
batch-type vacuum furnace (hot or cold wall design)
These furnace types are briefly discussed next. For more details see Refs. 54 and 67.
Direct Combustion Furnace.
The simplest and least expensive furnaces are the direct-
combustion type, in which combustion products pass through the brazing zone and hence come
into contact with the workpiece.
As
moisture is always a by-product of combustion and as
moisture is a hindrance to good brazing, the direct-combustion furnace will not offer optimum
quality joints.
Muffle Furnace or Hot-Wall Furnaces.
A
gas-fired muffle furnace will normally incorpo-
rate a refractory chamber
so
that the flames pass around the outside of the chamber, which
ensures that the products of combustion do not come into contact with the workpiece. Because
of this, this furnace offers optimum quality joints. For still better quality, employ electric-
resistance or radiant-tube furnaces, or furnaces that combine resistance and combustion heating.
Retort-Bell Type Combustion Furnaces. The retort-bell type combustion furnaces have
been developed for high-temperature brazing applications. For brazing in a purified hydrogen
atmosphere, there is an inner container
of
a heat-resistant alloy sealed from outside air and the
products of combustion. The workpiece is placed into this container; the container
is
purged
with dry hydrogen and then lowered into a pit-type furnace. Hydrogen flows through the
container during preheating, brazing, and cooling.
Vacuum Brazing Furnaces. Vacuum brazing can be of these types: (1) single pumped
retort, (2) double pumped retort,
(3)
batch-type furnace, and
(4)
continuous furnace.
The
single pumped retort
is loaded with the brazement, evacuated and heated externally
by a resistance or by gas or by oil combustion.
The
double pumped retort
is for higher temperature brazing. Manufacturers can employ a
double-wall retort; the work load sits in a high vacuum
(lO-’
torr or lower) container that is
placed in a rough vacuum
(1
to
10
torr) chamber.
The
batch-type vacuum furnace
consists of a chamber with doors at one or both ends and
is typically equipped with mechanical roughing and oil vapor diffusion pumps. Batch-type
vacuum furnaces can be hot- or cold-walled design; cold-wall furnaces are more popular. The
term
cold wall
design refers to the fact that the chamber walls are water cooled and that
conventional refractory insulation is not present between the hot zone and chamber walls.
A
typical batch vacuum furnace is shown schematically in Fig.
30.
Depending on the geometry
of the workpiece and the desired production rate, the hot zone size can be in the range of
1-600 ft’ (0.028-17 m3). The hot zone can be rectangular or cylindrical in shape. Batch furnace
cycle times can vary few minutes to longer than a day. Large components such as cryogenic
heat exchanger cores have cycle times of many hours.
