Kuppan T. Heat Exchanger Design Handbook
Подождите немного. Документ загружается.

1014
Chapter
I5
4
Lowers
sotidus
T
1nCreaSeS
liquidus
T
Decreases
fludit
y
4
"9
-
Increases
liquidus
1
Decreases
fluidity
Mg
b
Decreases
liquidus
T
lncrearer
fluidity
AI
c
Contributes
to
D
i
rrdJion
4
Bi
Minor
cf
f
cc
t
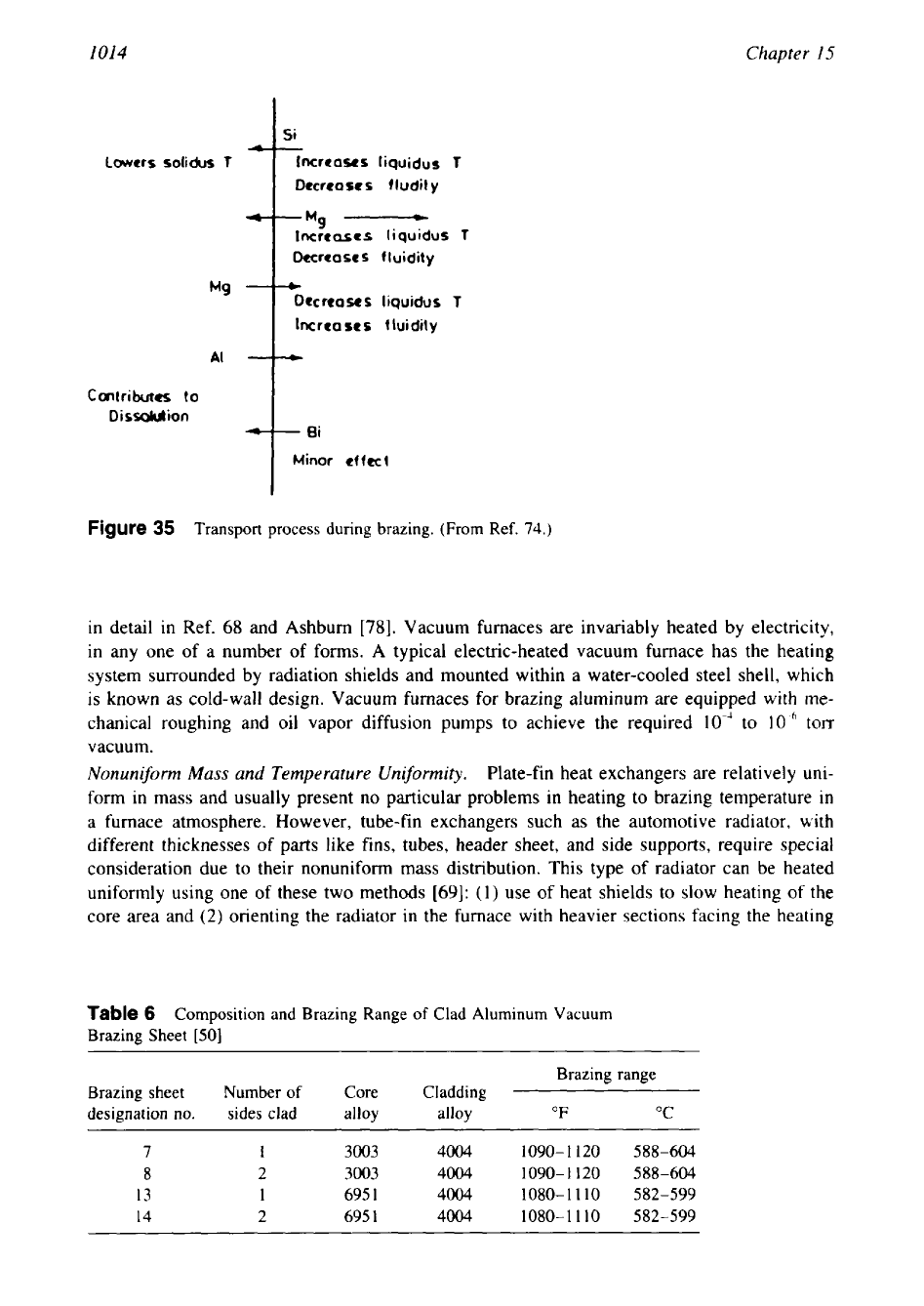
Figure
35
Transport process during brazing. (From Ref.
74.)
in detail in Ref.
68
and Ashburn
[78].
Vacuum furnaces are invariably heated by electricity,
in any one of a number of forms. A typical electric-heated vacuum furnace has the heating
system surrounded by radiation shields and mounted within a water-cooled steel shell, which
is known as cold-wall design. Vacuum furnaces for brazing aluminum are equipped with me-
chanical roughing and oil vapor diffusion pumps to achieve the required
10-'
to
10-h
tom
vacuum.
Nonuniform
Mass
and
Temperature Uniformity.
Plate-fin heat exchangers are relatively uni-
form in mass and usually present no particular problems in heating to brazing temperature
in
a furnace atmosphere. However, tube-fin exchangers such as the automotive radiator, with
different thicknesses of
parts
like fins, tubes, header sheet, and side supports, require special
consideration due to their nonuniform mass distribution. This type of radiator can be heated
uniformly using one of these two methods
[69]:
(1) use of heat shields to slow heating of the
core area and
(2)
orienting the radiator in the furnace with heavier sections facing the heating
Table
6
Composition and Brazing Range of Clad Aluminum Vacuum
Brazing Sheet
[50]
Brazing range
Brazing sheet Number of Core Cladding
designation no. sides clad alloy alloy "F
"C
7
1
3003
4004
1090-1
120
588-604
8
2
3003
4004 1090-1120
588-604
13
1
695
1
4004 1080-1110
582-599
14
2
695
1
4004 1080-1110
582-599

Heat Exchanger Fabrication
1015
elements. While both methods provide
f5"F
temperature uniformity, the latter method is more
amenable to production use.
Postbraze Cleaning and Finishing.
The brazed aluminum alloys should be free from residual
flux to avoid corrosion during service. The postbraze cleaning methods include ultrasonic
means or using chemical solutions, or using one of the chemical brighteners.
Cleaning of a Chloride Type Flux.
1.
The most rapid and efficient method of removing the chloride type
flux
is by hot water
cleaning, i.e., by the immersion of the still hot part into boiling water.
A
chloride
flux
is
highly soluble, and boiling water will quickly remove most of it.
2.
Ultrasonic cleaning with proprietary cleaning solutions.
3.
Chemical cleaning.
Cleaning of a Fluoride Type Flux.
Even though residual fluoride
flux
is noncorrosive,
for aesthetic reasons and for surface coating, the fluoride flux is cleaned using a caustic etch
or chemical brighteners, since fluoride fluxes are not water washable [50].
Finishing.
When a brazing fillet has been properly formed, no finishing
is
required ex-
cept flux removal by postbraze cleaning. If necessary, the fillet, like the brazed metal, may be
ground, filed, or polished. Caution: The brazed assembly should not be cleaned in caustic
solution.
8
BRAZING
OF
HEAT-RESISTANT ALLOYS
AND STAINLESS STEEL
Heat-resistant superalloys are either nickel-based or cobalt-based alloys, with the alloying ele-
ments chromium, iron, tungsten, and molybdenum. These alloys exhibit strength, oxidation
resistance, and resistance to corrosion at elevated temperatures
(1
200-2200°F).
Some of the
nickel-based wrought alloys used as heat exchanger materials are Hastelloy
X,
Inconel
625,
and Inconel 718, and cobalt-based alloy Haynes
188
[52].
Elements of brazing of nickel-based
and cobalt-based alloys are discussed in this section.
8.1
Brazing
of
Nickel-Based Alloys
Requirements for Brazing of Nickel-Based Alloys
The success of brazing of nickel-based alloys depends on the careful consideration of the
following parameters [79]:
1.
Physical metallurgy of the alloys
2.
Surface preparation
3.
Thermal cycles
4.
Brazing atmosphere
Physical Metallurgy
of
the Alloys.
The surfaces of precipitation-hardenable alloys containing
more than 1% A1 and Ti are covered with hard, tenacious oxide films. These surface films
hinder the wetting of molten filler metal, and the films are
in
general impossible to reduce,
either in a controlled atmosphere or in a vacuum brazing atmosphere. However, these difficul-
ties are not normally encountered with solid-solution-strengthened alloys.
Su$ace Preparation.
Successful brazing requires removal
of
surface oxide films. To improve
the wettability by filler metals, additional surface treatment followed by precleaning may be

1016
Chapter
I5
required. The surface treatment consists of electroplating of nickel on the surfaces, known
as
nickel flashing.
Thermal Cycles.
The effect of high-temperature thermal cycles are [79]: (1) Brazing tempera-
ture above their hardening temperatures of 1100
to
1500°F may alter the alloy properties;
(2)
at the high brazing temperature of 1
850"F,
the
precipitation-hardenable
alloys may be subjected
to grain growth and decrease in stress rupture properties, which cannot be recovered by subse-
quent heat treatment; and
(3)
the liquid metal is subjected
to
embrittlement when the molten
metal is under the influence of tensile stress. Hence, the residual or applied tensile stress should
be relieved before brazing.
Brazing
Atmospheres.
For successful brazing of nickel-based alloys, either a
dry,
oxygen-
free reducing atmosphere or a vacuum
is
preferred. The effects of various controlled atmo-
spheres have been covered earlier. Vacuum brazing in the range of 104 torr has proved ade-
quate for brazing most of the nickel-based alloys. The brazing atmosphere, whether gaseous
or vacuum, should be free from harmful constituents such as sulfur, oxygen, and water vapor.
In general, a gaseous atmosphere having a dewpoint less than about
-60°C
(-75°F) will pro-
duce better quality.
Brazing Filler Metals
Generally, nickel-based alloys are brazed with nickel-based alloys containing boron andor
silicon, which serve as melting-point depressants. Phosphorous is another effective melting-
point depressant, and it also aids good flow in applications of low stress, there temperatures
do not exceed 1400°F (760°C). Sometimes chromium is present to provide oxidation and
corrosion resistance. Other filler metals include copper and silver brazing alloys for low-tem-
perature applications, and proprietary filler metals for service temperature above
1
800°F
(982°C).
8.2
Brazing of Cobalt-Based
Alloys
Brazing of cobalt-based alloys can be accomplished by adopting the same techniques used for
brazing of nickel-based alloys but with less stringent brazing requirements [79]. This is due
to
the absence of appreciable amounts of aluminum and titanium in the base metals. The filler
metals are usually cobalt based, nickel based, or gold-palladium compositions. Most cobalt-
based filler metals contain boron and/or silicon as melting-point depressants and Cr, Ni, and
W
for improved strength, corrosion, and oxidation resistance.
8.3
Brazing
of
Stainless Steel
In recent years, there has been a demand, particularly from the aircraft and nuclear energy
industries, for complex heat exchangers that operate under arduous conditions
of
temperature
and pressure and adverse environments.
To
meet these requirements, heat exchangers have
been fabricated from stainless steel, usually the 18
:
8
type stabilized with titanium. Other
wrought stainless steels such as of AISI types 316, 316L, 347, and 430 are also extensively
used as heat exchanger materials for high-temperature applications. Many aircraft heat ex-
changers are of modular construction [80]. Typical brazed forms of stainless steel heat ex-
changers include plate-fin heat exchangers, shell and tube heat exchangers, and regenerators.
To
save weight in aircraft and for greater efficiency in heat transfer, the heat exchangers have
been made from thin sheet and thin-wall tubing; typical sizes are sheet
0.005
in thick and
tubing of 0.006 in wall thickness. One of the problems in fabricating structures from these thin
materials has been to make completely sound joints without any distortion in the heat ex-
changer matrix.

Heat Exchanger Fabrication
101
7
Brazeability of Stainless Steel
Brazing of stainless steels is carried out at high temperatures (see Table 4). Among the various
factors, the quality of stainless steel brazing depends on the following
[63]:
1.
Ability to create a proper brazing atmosphere to eliminate oxidation, scaling, and surface
reaction that forms sulfides and nitrides on its surface. Brazing either in a controlled
atmosphere or in vacuum eliminates the oxidation of the base metal, is effective in reduc-
ing the oxides, and promotes the flow of filler metal.
2.
Ability to arrest the sensitization of austenitic stainless steel during brazing, or proper
solution treatment of sensitized steels to restore their corrosion resistance.
Brazing Process.
Stainless steel can be brazed by all conventional brazing processes includ-
ing torch, furnace, dip, and induction brazing. Stainless steel heat exchangers are mostly fur-
nace brazed, either in a controlled reducing atmosphere such as dry hydrogen, dissociated
ammonia, or argon, or in vacuum.
Furnace Brazing in Dry Hydrogen.
Dry hydrogen is the most preferred for brazing of
stainless steel since it eliminates the oxidation of the base metal and is effective in reducing
the oxides. The principal disadvantages are high cost; difficulty in getting hydrogen in dry
condition, free from oxygen and other oxidants; and handling and storage problems
[65].
Furnace Brazing in Dry Dissociated Ammonia.
Stainless steels are brazed in a dry disso-
ciated ammonia, i.e., with the composition of H2 and N2. Even small traces of NH3 will nitride
stainless steel and dissolve in water if traces of moisture are present.
Vacuum Brazing.
For high-temperature service applications, vacuum brazing can offer
excellent heat and corrosion resistance. A vacuum of
10-*
torr of mercury is sufficient to braze
most stainless steels. Many structures that could not be brazed in hydrogen have been brazed
successfully in vacuum furnaces
[65].
Filler Metals.
In addition
to
brazeability, for most applications filler metals are selected for
mechanical properties, corrosion resistance, service temperature, and compatibility with pro-
cess fluids. Filler metals for brazing stainless steels include copper alloys, silver alloys, gold
and gold-palladium alloys, and nickel alloys. Nickel-based filler metals alloy with stainless
steel and form secondary phases with two undesirable characteristics
[79]:
(1)
low ductility
and hence susceptibility to hot cracking, and
(2)
higher melting point than the base metal and
hence likely to freeze and retard the filler metal
flow.
To achieve flow in deep joints, diametral
clearances of as much as
0.004
to
0.008
in (0.1 to
0.2
mm)
are necessary. Selection of brazing
filler metals for brazing stainless steels is
also
discussed by Amato et al. [81]. For guidance
on selection of brazing filler metals for stainless steels brazing, Welding Engineer Data Sheet
No.
493
[82]
is useful.
Flues.
Brazing of stainless steel in a controlled atmosphere or in vacuum does not require
flux. In some furnace brazing applications, flux is necessary. But flux is required for torch
brazing, dip brazing, or for any other conventional brazing process carried out in an uncon-
trolled atmosphere.
9
QUALITY CONTROL, INSPECTION, AND NDT
OF
BRAZEDHEATEXCHANGERS
The principles of quality control, quality assurance, inspection and NDT techniques have been
dealt with in Chapter 14. Even though the focus in that chapter was shell and tube heat ex-
changers, the underlying principle is relevant for any type of heat exchanger and for any manufac-
turing process. With this in mind, quality control and inspection pertaining to brazing are dis-
cussed here. Quality assurance for aluminum brazing is discussed in ANSVAWS
C3.7-93,

1018
Chapter
I5
Specification for Aluminum Brazing
[7
I]. Salient features of quality systems for brazing are:
Data cards. An essential part of the quality system is the use of data cards for each job to
record brazing parameters. The data cards contain such information as materials used, joint
configuration, brazing alloy, thermal cycle, furnace load configuration, and inspection and
NDT requirements.
Responsibility for inspection. Unless otherwise specified in the contract or purchase order,
the manufacturer is responsible for the performance of all inspections during various stages
of brazing operation.
Sequence of inspection and manufacturing operations. Brazed joints may be inspected at
the assembly or subassembly stage, and after postbraze heat treatment. Flux residue testing
shall be performed after completion of all operations of the brazed joint.
Temperature measuring devices. All furnaces and molten salt baths used
in
the brazing
shall have automatic temperature controlling and recording devices in proper working
order. Periodically calibrate temperature measuring devices, furnace atmosphere control-
ling instruments, and vacuum measuring devices.
Integrity of vacuum furnaces: A critical step for vacuum furnace is to ensure a sound
furnace atmosphere through regular, scheduled leak testing.
Elements of quality control for dip brazing: Quality control aspects should cover mainte-
nance of
flux
composition, temperature, pH, removal of scum and sludge, preheating the
assembly, etc., as discussed earlier in the section on the dip brazing process.
Flux removal test: All components brazed using corrosive flux shall be tested for the
presence of residual flux after postbrazing cleaning. The silver nitrate test is popular for
determining the presence of flux either in the wash water or remaining on the workpiece
after it has been washed and dried. Evidence of any precipitate or cloudy appearance
indicates incomplete cleaning, and the piece should be cleaned further or may be rejected.
Other test methods may be used as alternates with the written approval of the buyer.
9.1
Quality
of
the
Brazed Joints
The quality of brazed joint shall be such that the brazed assemblies are suitable for the intended
purpose and that surfaces are free of excess braze filler material. Quality problems of brazed
joints
of
aluminum heat exchangers are discussed by Shah
[52].
Some of these problems may
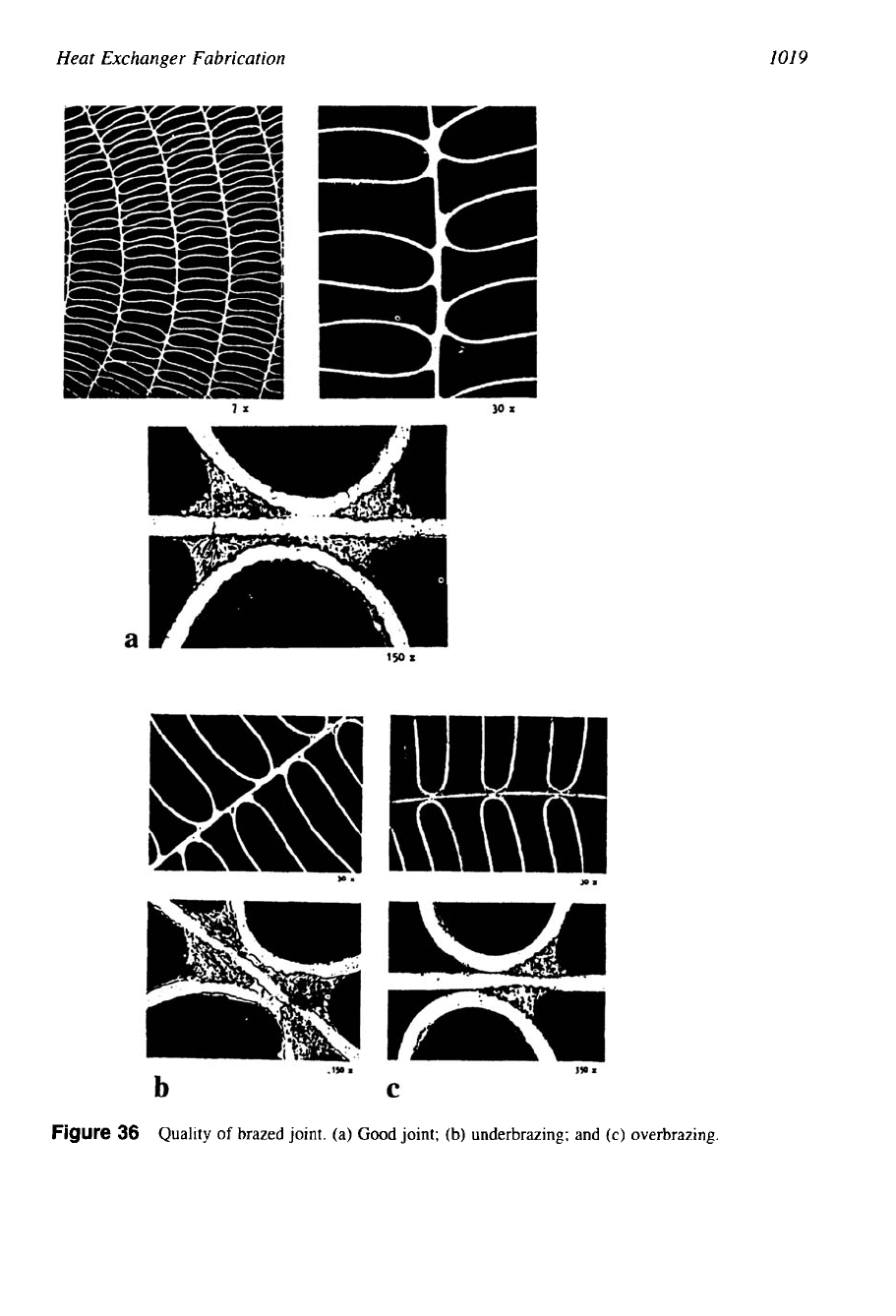
also be common to other metals. Quality of a good brazed joint is shown schematically in
Fig.
36. A good brazed joint, as shown in Fig. 36a, has large smooth fillets, absence of discontinu-
ities and voids, and the width of the joint is sufficient. Imperfect brazed joints include the
following
[52]:
1. Poor fillets: a braze joint with narrow-width fillets, due to insufficient time at the braze
temperature.
2.
Partial joint (underbrazing): due to low brazing temperature, low amount of melting-point
temperature depressant elements in filler metal, or improper heating rate (Fig. 36b).
3. Overbrazing: a braze joint that is a result of too high brazing temperature or the brazing
time; filler metal may dissolve the base metal and reduce its strength (Fig. 36c).
Discontinuities
Discontinuities result in all brazing processes. Some may be process specific. Discontinuities
may be associated with structural discontinuities or associated with the braze metal or the
brazed joint. Typical discontinuities include
[50,53,54]:
(1) lack
of
fill,
(2)
flux entrapment,
(3) intermittent bond,
(4)
noncontinuous fillets,
(5)
voids, (6) porosity, (7) cracks,
(8)
base
metal erosion,
(9)
unsatisfactory surface appearance, (10) discoloration, and
(
1 1)
distortion.
1019
Heat Exchanger Fabrication
7.
30
I
Figure
36
Quality
of
brazed joint. (a)
Good
joint;
(b)
underbrazing; and
(c)
overbrazing.
1020
Chapter
15
All discontinuities reduce joint strength. Acceptance limits for discontinuities must identify
shape, orientation, location in the brazement, and relationship to other discontinuities.
9.2
Inspection
Inspection of brazed joints may be conducted on test specimens or by tests of the finished
brazed assembly. The tests may
be
nondestructive or destructive methods, such as peel tests,
tension
or
shear tests, fatigue test, impact tests, torsion tests, and metallographic examination
or proof testing. Various NDT methods include
PT,
RT, and UT. The scheme of NDT tech-
niques is shown in Fig.
37.
Visual Examination
Visual examination is the most commonly used means for rapidly assessing brazed joint qual-
ity, dimensions, and distortion, if any. If the fillet is fully formed and smoothly curvilinear,
the joint may be accepted as being reasonably sound pending further, more detailed examina-
tion
[so].
Visual inspection
is
unsuitable for the detection of flux entrapment.
Leak Testing
To
make certain no leaks exist, heat exchangers are leak tested. The choice of pressure medium
will depend to some extent upon the maximum acceptable leak aperture. Thin
oil
such
as
kerosene, heated oil, water, air, or Freon may be used. For critical services, where it is neces-
sary
to identify the presence
of
very minute leaks, helium mass spectrometer leak detection
technique may be employed.
Heat
exchanger
Figure
37
Scheme of
NDT
techniques for brazing.
(From
Ref.
58.)

Heat Exchanger Fabrication
1021
9.3
Brazing Codes and Standards
AWS Brazing and Soldering Documents
BRH
Brazing Hand Book
SM
Soldering Manual
A 2.4
Standard symbols for welding, brazing, and nondestructive testing
A
5.8
Specification for filler metals for brazing, A5.01, filler metal procurement guide-
lines
B
2.2
Standard for brazing procedures and performance qualification
C
3.2
Standard method for evaluating the strength of brazed joints in shear
c
3.3
Recommended practice for design manufacture and inspection
of
critical brazed
components
c
3.4
Specification for torch brazing
c
3.5
Specification for induction brazing
C
3.6
Specification for furnace brazing
c
3.7
Specification for aluminum brazing
C
3.8
Recommended practices for ultrasonic inspection
ASME Code Section
11,
Part
C
10
SOLDERING
OF
HEAT EXCHANGERS
Soldering
is defined as a process of joining two similar or dissimilar metals with a filler metal
in molten state. Soldering joins materials by heating them in the presence of a filler metal
having a liquidus temperature below
840°F
(450°C). The filler metal is called
solder.
Heating
may be provided by a variety of processes. The filler metal distributes itself between the
closely fitted surfaces of the joint.
10.1
Elements
of
Soldering
Most of the elements
of
soldering, such as joint design, solder selection, fluxing, precleaning
and surface treatment, cleaning after soldering, and inspection, have lot of similarities with
brazing process. Therefore, without going deep into these aspects, only salient features of
certain elements relevant to radiator design are described here. Subsequently, two versions of
the soldering process are described. One involves the conventional soldering of automobile or
locomotive radiators, and the other involves the fluxless ultrasonic soldering of all-aluminum
core for room air conditioners.
Joint Design
Soldered radiator joints are required to perform in a severe environment. They are subjected
to fluctuating temperatures, mechanical vibration, immersion under a water-based fluid, and
contact with dissimilar metals. Added to that the frequent neglect of the quantity and quality
of coolant solution
[83].
Tube Joints
Conventionally, brass flat plates are solder coated and formed into tubes, lock-seam jointed
in
a soldering machine. Recently tubes with a butt-welded joint have been used for the construc-
tion of tubes. This type of radiator tube is stronger and more leak proof than the conventional
roll-formed brass lock-seam product, and provides a material savings of about
25%
through
elimination of the lock seam
[84].

I022
Chupter
15
Tube-to-Header Solder Joints
Solder alloys generally have lower strength properties than the base materials. Joints between
the tubes and header plates are essentially shear loaded in both directions
as
the radiator is
heated, pressurized, and subsequently cooled
[U].
Failure of the tube-to-header solder joint
occurs because of
a
variety of problems
[86]:
weak solder joints due to poor mechanical
assembly and fit-up problems, brittle fracture, creep and fatigue, and external or internal corro-
sion. Possible approaches for increasing the durability of the tube-header solder joints are
discussed by Webb et
al.
[86].
Solders
Solders generally have melting points or melting ranges generally below
800°F
(425°C).
There
is
a
wide range of commercially available solders designed
to
work with most industrial metals
and alloys. Tin-lead solders, improved tin-lead solders, and zinc-aluminum solders are dis-
cussed
in
the following section and their composition is shown
in
Table
7.
More details
on
solder selection can be found
in
Ref.
53.
kad-Based
Solders.
Tin-lead solders are most widely used in the joining of metals,
par-
ticularly conventional automotive radiators. Most commercial fluxes, cleaning methods, and
soldering processes may be used with tin-lead solders.
Mechanicul
Properties.
The requirements of
a
lead-based solder for tube-to-header radiator
joints include the following
[83]:
1.
It
must have
a
minimum amount
of
tin
so
that sufficient
tin
oxide film is formed to
passivate the surface.
2.
It
must have excellent mechanical properties, particularly with regard to creep resistance,
to minimize damage to the protective film.
3.
It
must have good solderability properties.
4.
It
must be cost-competitive with alternate joining materials.
All lead-based solders have
a
very low yield point. Under almost any load, they are
subjected to some plastic deformation. These alloys are particularly sensitive to creep deforma-
t
ion.
Impro\vd
Led-Tin
Solder
Alloy.
For years, the standard radiator solder had been
70
:
30
leadtin. The
70:
30
solder is relatively brittle, and prone to stress cracking
as
a
result of
vibrational stresses built up
in
the radiators. This led to solder-joint failures. This solder has
been replaced almost entirely by high-lead solders containing up to
97%
lead
[87].
This solder,
called soft solder, could better withstand engine vibration;
it
has the following approximate
composition:
97%
lead,
1.5-2.594
tin,
and
0.5-
1.5% silver. The
tin
content was reduced both
Table
7 Solder
Compositions
Sn-Pb Sn-Pb- Ag
Zn-
AI-Cu
Sn
Pb Sn Pb Ag Zn A1 Cu
5
95
5.5
94.0 0.5 95
5
-
10 90 5.0 94.5 0.5
89
7
4
15 85
3.8
95.0
1.2
20 80
2.5
95.0
1.2
25
75
1.0 97.5
1.5
30 70

Heat Exchanger Fabrication
I023
to save money and to improve mechanical properties. Solders containing less tin will form less
eutectic, which will improve the solder’s high-temperature mechanical properties
[83].
At the
same time, a certain concentration of tin in the solder is necessary. If sufficient tin is present
in the solder a tenacious and stable oxide film forms over the solder, which improves the
corrosion resistance of the solder joint. Hence, it is necessary that the solder contain a mini-
mum of
5%
tin. In high-lead solders, where mechanical strength is considered very important,
e.g., for tube-to-header joints, silver is added. Typical of these high-lead solders are 95Pb-5Sn
and 97.OPb-OSAg-2.5Sn (Modine).
Zinc-Based Solders.
Zinc-aluminum solder, the most standard grade 95Zn-5A1 is specifically
used for ultrasonic soldering of aluminum. It develops joints with high strength and good
corrosion resistance.
Cleaning and Descaling
An unclean surface prevents the solder from flowing and makes soldering difficult or impossi-
ble. Solvent or alkaline degreasing is recommended for cleaning oily or greasy surfaces. For
removing rust or scale, pickling or mechanical cleaning may be used.
Soldering Fluxes
With
flux
soldering, the flux helps to break up the tenacious oxide layer on the base metal, by
reacting with or otherwise loosening that film from the base metal surface. Without the action
of the flux, the molten solder cannot wet the surface. Soldering fluxes are divided into three
main groups based on their residues
[85]:
corrosive, intermediate, and noncorrosive. Whenever
possible, use the least corrosive flux to eliminate the potential postsoldering corrosion problems
stemming from the residual flux. Various compositions of fluxes for copperhrass radiator
manufacture include the following:
Zinc chloride, ammonium chloride, and water to make
Zinc chloride, sodium chloride, ammonium chloride, hydrochloric acid, and water to make
Zinc chloride, ammonium chloride, petroleum jelly, and water to make
Soldering Processes
Soldering processes or the heating methods available for heat exchanger manufacture are:
Flame or torch soldering: Hand-held manual torch soldering is most frequently used for repairs
to tube-to-tube-plate joints.
Furnace or oven soldering: Furnace soldering is considered when an entire assembly (e.g.,
radiator) is to be brought to the soldering temperature and when the assembly is compli-
cated in nature, making other heating methods impractical.
Dip soldering: The dip soldering method uses a molten bath of solder to supply both the heat
and the solder necessary to join the work pieces. Tube-to-header joints of radiators are
made by this process.
Ultrasonic soldering: Ultrasonic soldering
can
be considered a form of dip soldering, in which
the workpiece is immersed in a tank of molten solder. Ultrasonic soldering is discussed
separately.
Infrared soldering: This involves focusing infrared light (radiant energy) by means of an optical
system. Typical applications include soldering pretinned interference fit return bends by
Reynolds interference fit.
Various Stages
of
Radiator Manufacture
by
Conventional Soldering Process.
In the soldered
design. a radiator core is formed by soldering the plain fins or corrugated fins
to
flattened heat
exchanger tubes and the core is connected to the upper and the lower tanks via a pair of header
plates. Radiator tubes are normally made by fabricating a lock seam and joining this seam
