Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

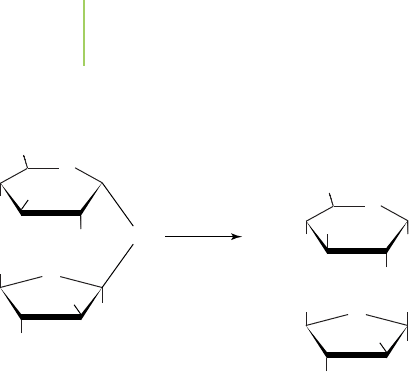
b. Because both reactions involve the hydrolysis, the enzyme may
be able to catalyze the reaction; however, many enzymes have a very
specific active site and therefore would not be able to catalyze the
reaction; c. Lysine can be coded by the AAA or AAG codon on
mRNA and by the UUU or UUC anticodon on tRNA; d. 9.50 kg;
e. 8.16 kg; f. Nonedible fibers could be converted into edible food.
A48 Answers to Practice Exercises and Selected Exercises
49. a.
Fructose
Glucose
OH
OH
HO
HOH
2
C
CH
2
OH
O
OH
OH
HO
OH
HOH
2
C
O
Invertase
O
OH
HO
HOH
2
C
CH
2
OH
O
Sucrose
OH
HO
OH
HOH
2
C
O
Credits
Text and Illustrations
Figures 1.1 and 1.2, p. 2: Reprinted with permission. Copyright 2002
American Chemical Society. Figure 2.23, p. 62: Reproduced with per-
mission. Courtesy of Agilent Technologies, Inc. Figure 2.26, p.63: “The
Fingerprint of a Flavor” from “A Fragrant Feast” by Mark S. Lesney.
Today’s Chemist at Work, June 2002. Copyright © 2002 American
Chemical Society. Figure 5.8, p. 173: Reprinted by permission of
Dr. David Webb. Figure 5.22, p. 193: U.S. Department of Energy
National Energy Technology Laboratory. National Methane Hydrate
Program. Figure 5.25, p. 197: http://www.whitehouse.gov/news/
release/2001/06/energyinit.html. Figure 10.26, p. 430: Reprinted
courtesy of Forsyth County Environmental Affairs Department.
Figure 10.27, p. 432: http://www.gcrio.org/ipcc/aa/05.html. United
Nations Environment Programme World Meteorological Organiza-
tion. Figures 11.1 and 11.2, p. 441: Reprinted by permission of
UNESCO. Figure 15.1, p. 621: http://pubs.usgs.gov/circ/circ1151/
nawqa91.6.html. Figure 16.7, p. 679: Reprinted courtesy of Robert
Graves, Chromatographic Instruments Company. Figure 17.1,
p. 718: http://www.census.gov/const/www/newresconstindex.html.
Figure 17.2, p. 720: Science Magazine, May 21, 2004. Reprinted cour-
tesy of Kenneth D. Jordan and Richard A. Christie. Table 18.6,
p. 812: Reprinted by permission of The Dow Chemical Company.
Figure 21.12, p. 921: http://www.iaea.org/programmes/a2/index.html.
Photos
Chapter 1
1: Jerry Walsh. 3: (top) Paul Kelter. (left center) Teflon® is a registered
trademark of DuPont. (right center ) © Houghton Mifflin Company.
All rights reserved. (bottom) Robert Bruschini, Bristol-Myers Squibb.
4: (top left) Stockybyte Platinum/Getty Images. (top center) Photo
Disc/Getty Images. (top right) © Houghton Mifflin Company. All
rights reserved. (bottom) © Argus Fotoarchiv/Peter Arnold, Inc.
6: © Houghton Mifflin Company. All rights reserved. 7: (top) ©
Houghton Mifflin Company. All rights reserved. (bottom) NASA.
9: (top left) Don Farrall/Photodic Green/Getty Images. (bottom left
and center) © Houghton Mifflin Company. All rights reserved. (top
right) Photodisc Green/Getty Images. (bottom right) Comstock Im-
ages/Alamy. 10: © Bojan Brecelj/CORBIS. 12: (left and center) ©
Houghton Mifflin Company. All rights reserved. (right) © Jim
Olive/Peter Arnold, Inc. 13: NASA. 14: Hulton Archive/Getty Images.
15: Courtesy of Bayer Healthcare LLC. 17: (both) © Houghton
Mifflin Company. All rights reserved. 18: (top) © Houghton Mifflin
Company. All rights reserved. (bottom) © Houghton Mifflin Com-
pany. All rights reserved. 19: © Bettmann/CORBIS. 20: (both) ©
Houghton Mifflin Company. All rights reserved. 21: Burke/Triolo
Productions/Brand X Pictures/Getty Images. 23: (all) © Houghton
Mifflin Company. All rights reserved. 24: © Houghton Mifflin Com-
pany. All rights reserved. 25: © Houghton Mifflin Company. All
rights reserved. 29: © Houghton Mifflin Company. All rights re-
served. 31: © Houghton Mifflin Company. All rights reserved.
35: (top) © Richard Feldman/Phototake. (bottom) Jorge Uzon/Getty
Images. 36: (top) NASA. (bottom) © Institute for Molecular Manu-
facturing www.imm.org.
Chapter 2
45: © James L. Amos/CORBIS. 46: NASA. 47: (top) Hulton
Archive/Getty Images. (center) Edgar Fahs Smith Collection,
University of Pennsylvania Library. (bottom) Edgar Fahs Smith
A49
Collection,University of Pennsylvania Library. 48:© Houghton Mifflin
Company. All rights reserved. 49: Courtesy of Manchester Literary
and Philosophical Society. 50: (left) Edgar Fahs Smith Collection,
University of Pennsylvania Library. (right) © Houghton Mifflin
Company. All rights reserved. 52: (top) Canterbury Archaeological
Trust. (bottom) © Paul A. Souders/CORBIS. 53: (top) Keystone/
Getty Images. (bottom) © Bettmann/CORBIS. 62: (top) Courtesy
of PerkinElmer Inc., Shelton, Connecticut. (bottom) Courtesy of
PerkinElmer Inc., Shelton, Connecticut. 63: Reprinted with permis-
sion from Culp, R. A., J. M. Legato, and E. Otero, 1998. Carbon iso-
tope composition of selected flavoring compounds for the determina-
tion of natural origin by GC/IRMS. In C. J. Mussinan and M. J.
Morello, eds., Flavor Analysis—Developments in Isolation and Char-
acterization. American Chemical Society Symposium Series 705.
260–287. Copyright 1998 American Chemical Society. 65: (all) ©
Houghton Mifflin Company. All rights reserved. 68: © Houghton
Mifflin Company. All rights reserved. 70: (top) Charles Winters/
Photo Researchers, Inc. 74: © Houghton Mifflin Company. All rights
reserved. 81: © 1993 Richard Megna, Fundamental Photographs,
NYC. 85: Reprinted from R. C. Wiens, “Radiometric Dating: A Chris-
tian Perspective,” http://www.asa3.org/ASA/resources/wiens.html.
Original data from Stuiver et al., Radiocarbon 40, 1041–1083, 1998
and Beck et al., Science 292, 2453–2458. 2001.
Chapter 3
86: Will & Deni McIntyre/Photo Researchers, Inc. 89: (both) ©
Houghton Mifflin Company. All rights reserved. 90: (all) © Houghton
Mifflin Company. All rights reserved. 91: Edgar Fahs Smith Collec-
tion, University of Pennsylvania Library. 92: © Houghton Mifflin
Company. All rights reserved. 93: © Houghton Mifflin Company. All
rights reserved. 97: © Houghton Mifflin Company. All rights re-
served. 99: (left) © Bob Krist/CORBIS. (top center) Isabelle Rozen-
baum & Frederic Cirou/Photo Alto/Getty Images. (bottom center)
Berit Myrekrok/Digial Vision/Getty Images. (right) Image Source/
Getty Images. 101: PDB ID: 1A3N; J. Tame, B. Vallone, 1998.
113: © Houghton Mifflin Company. All rights reserved.
116: (left) © Houghton Mifflin Company. All rights reserved.
(right) © Royalty-Free/Corbis.
Chapter 4
124: Creatas Royalty Free/ Fotosearch Stock Photography.
125: (top) © Royalty-Free/Corbis. (bottom left) AP/Wide World
Photos. (bottom right) Eye of Science/Photo Researchers, Inc.
126: (both) © Houghton Mifflin Company. All rights reserved.
129: © Houghton Mifflin Company. All rights reserved. 130: (all)
Photo by Ken O’Donoghue. © Houghton Mifflin Company. All rights
reserved. 135: David Woodfall/Stone/Getty Images. 138: (all) ©
Houghton Mifflin Company. All rights reserved. 140: © Houghton
Mifflin Company. All rights reserved. 141: (all) © Houghton Mifflin
Company. All rights reserved. 142: (top) Michael Mosher. (center)
Gordon M. Grant/Alamy. (bottom) Denver Instruments. 145: Simon
Fraser/Science Photo Library/Photo Researchers, Inc. 146: (top)
Nathan Miller/Center for Lithospheric Studies, University of Texas
at Dallas. (bottom) © Houghton Mifflin Company. All rights re-
served. 149: © Houghton Mifflin Company. All rights reserved.
153: (left) Jeff LePore/Photo Researchers. (right) Ross Barnett/Lonely
Planet Images/Getty Images. 158: (top left) USGS photo.
(top right) Photograph by Michele A. Justice. (center) Brookhaven
National Laboratory. (bottom) blinkwinkel/Alamy.
Chapter 5
167: NASA. 168: NASA. 172: (top) Achim Sass/Westend61/Alamy.
(bottom) NASA. 174: AP/WideWorld Photos. 175: (all) © Houghton
Mifflin Company. All rights reserved. 177: NASA. 178: NASA. 179:
(top) Douglas Pulsipher/Alamy. (bottom) © Houghton Mifflin Com-
pany. All rights reserved. 180: (both) © Houghton Mifflin Company.
All rights reserved. 181: © Houghton Mifflin Company. All rights re-
served. 187:(top) Mark Baker/Reuters/Landov. (bottom) © Houghton
Mifflin Company. All rights reserved. 188: Kim Heacox/Peter Arnold
Photography. 193: AP Photo/Dean J. Koepfler. 196: Archive of the
Goldschmidt Gmbh. 197: Goh Cha Hin/AFP/ Getty Images. 198:
(top) Peter Frischmuth/ Peter Arnold Photography. (bottom left) FPL
Energy. (bottom right) Corbis Royalty-Free. 199: (top) © Reuters
CORBIS (bottom) John A. Turner/National Renewable Energy
Laboratory.
Chapter 6
209: Courtesy: IBM Research, Almaden Research Center. Unautho-
rized use prohibited. 210: Photo 24/Brand X Pictures/Getty Images.
212: Courtesy of Casio. 213: Science Photo Library/Photo Re-
searchers, Inc. 214: (bottom) National Telecommunications and
Information Administration Office of Spectrum Management.
(top) Junko Kimura/Getty Images. 215: © Alfred Pasieka/Peter
Arnold, Inc. 217: (left) © Houghton Mifflin Company. All rights re-
served. (right) © Richard Megna/Fundamental Photographs, NYC.
221: Post Danmark. 224: (top) Index Stock/Alamy. (bottom) ©
B. Runk/S. Schoenberger/Grant Heilman Photography. 225: (top) ©
Peter Beck/CORBIS. (bottom) Science Photo Library/Photo Re-
searchers Inc. 226: © Royalty-Free/Corbis. 228: © Bettmann/
CORBIS. 229: NIST. 230: © George D. Lepp/CORBIS. 232: (top) ©
Bettmann/CORBIS. (bottom) © Hulton-Deutsch Collection/ CORBIS.
233: © Royalty-Free/Corbis. 237: Courtesy: IBM Research, Almaden
Research Center. Unauthorized use prohibited. 242: (top) ©
Bettmann/CORBIS. (bottom) © Gabe Palmer/CORBIS. 243: Science
Photo Library/Photo Researchers, Inc. 255: © Royalty-Free/Corbis.
Chapter 7
259: Getty Images. 260: (top) © Royalty-Free/Corbis. (bottom) Pho-
todisc Green/Getty Images. 261: © PEMCO-Webster & Stevens Col-
lection; Museum of History and Industry, Seattle/CORBIS. 262: (all)
© Houghton Mifflin Company. All rights reserved. 264: (top) ©
Houghton Mifflin Company. All rights reserved. (bottom left) Uni-
versity of Chicago Library. (bottom right) Edgar Fahs Smith Collec-
tion, University of Pennsylvania Library. 265: © Houghton Mifflin
Company. All rights reserved. 266: © Royalty-Free/Corbis.
267: (left) The Art Archive/British Library. (right) Mark Joseph/
Digital Vision/Getty Images. 268: (left right) © Houghton Mifflin
Company. All rights reserved. 270: (left and right) © Houghton
Mifflin Company. All rights reserved. 271: (all photos of metals) ©
Houghton Mifflin Company. All rights reserved. (bottom left)
AP/Wide World Photos. 272: (all) © Houghton Mifflin Company. All
rights reserved. 273: (top left and right) © Houghton Mifflin Com-
pany. All rights reserved. (bottom) © Roger Ressmeyer/CORBIS.
274: (top photos, bottom left) © Houghton Mifflin Company. All
rights reserved. (bottom right) © 1993 Richard Megna, Fundamental
Photographs, NYC. 275: (top) Photodisc Blue/Getty Images.
(center) David Buffington/Photodisc Green/Getty Images.
(bottom) © Houghton Mifflin Company. All rights reserved.
276: (top) Modified from the protein data bank structure, 1AG6.
Primary reference: Xue, Y., Okvist, M., Hansson, O., Young, S.“Crystal
structure of spinach plastocyanin at 1.7 A resolution,” Protein Sci.,
A50 Credits
1998, 7, 2099. (bottom left and right) © Houghton Mifflin Company.
All rights reserved. 277: Elementree © Fernando Dufour. Photogra-
pher Joseph Donahue, Montreal, Quebec, Canada. 278: Scott
Camazine/Photo Researchers, Inc. 283: (all) © Houghton Mifflin
Company. All rights reserved. 289: Wonderfile. 291: Modified from
the protein data bank structure, 1A3N and 1GZX. Primary reference:
Paoli, M., Liddington, R., Tame, J., Wilkinson, A., Dodson, G.“Crystal
Structure of T State Haemoglobin with Oxygen Bound at All Four
Haems,” J. Mol. Biol., 1996, 256, 775. 292: (left) Chuck Pefley/Alamy.
(right) David Toase/Photodisc Green/Getty Images. 293: © Houghton
Mifflin Company. All rights reserved. 299: © Royalty-Free/Corbis.
301: © Houghton Mifflin Company. All rights reserved.
Chapter 8
302: © Houghton Mifflin Company. All rights reserved. 304: Lewis,
Gilbert Newton. Valence and the Structure of Atoms and Molecules.
New York: The Chemical Catalog Company, Inc., 1923. Othmer Li-
brary of Chemical History, Chemical Heritage Foundation, Philadel-
phia, PA. 307: (top) Wonderfile. (chalk) Stockbyte Gold/Alamy.
(salt) © Houghton Mifflin Company. All rights reserved. (pigment)
Photograph by James Scherer. © Houghton Mifflin Company. All
rights reserved. (Milk of Magnesia) Photograph by James Scherer.
© Houghton Mifflin Company. All rights reserved. (glass) Ragnar
Schmuck/fStop/Getty Images. (baking soda) © Houghton Mifflin
Company. All rights reserved. (salt) © Royalty-Free/Corbis. (tooth-
paste) Ingram Publishing/Alamy. 310: Science Photo Library/Photo
Researchers, Inc. 316: (top) Stockfood. (bottom) © Houghton
Mifflin Company. All rights reserved. 317: (left) © Houghton Mifflin
Company. All rights reserved. (top center) Don Farrall/Photodisc
Green/Getty Images. (bottom center) Robert Sullivan/Getty Im-
ages. (right) © Houghton Mifflin Company. All rights reserved.
318: Bryan Mullennix/Getty Images. 323: Collection of The
Corning Museum of Glass, Corning, NY, gift of Mr. and Mrs.
Richard Barons. 329: © Houghton Mifflin Company. All rights re-
served. 338: © Houghton Mifflin Company. All rights reserved.
Chapter 9
355: Omikron/Photo Researchers, Inc. 363: (left) Andrew Lawson
Photography/Alamy. (center) Andrew Cowin/Alamy. (right) © Eric
Crichton/CORBIS. 364: © Houghton Mifflin Company. All rights
reserved. 380: © Richard Megna/Fundamental Photographs, NYC.
382: (left) Library of Congress. (right) Courtesy of Eastman Kodak
Company.
Chapter 10
394: Kevin Kelley/Getty Images. 396: © Air Products and Chemi-
cals, Inc. 400: © Houghton Mifflin Company. All rights reserved.
401: (left) © Houghton Mifflin Company. All rights reserved.
(right) Courtesy of Cryovac, Inc. 402: © Houghton Mifflin Com-
pany. All rights reserved. 403: © Shepard Sherbell/CORBIS SABA.
404: (all) © Houghton Mifflin Company. All rights reserved. 406:
(left) Courtesy of the Chemical Heritage Foundation Image Archives.
The Shannon Portrait of Hon. Robert Boyle F.R.S. (1627–1691), 1689,
Johann Kerseboom, oil on canvas. Chemical Heritage Foundation
Collections, Philadelphia, Pennsylvania, U.S.A. Photo by Will Brown.
(center) Courtesy of Carlos Castro-Acuna. (left) Michael Mosher.
407: Michael Mosher. 408: © Houghton Mifflin Company. All rights
reserved. 409: (top) Musee Lambinet, Versailles, France/Bridgeman
Art Library. (bottom) Edgar Fahs Smith Collection, University of
Pennsylvania Library. 419: Patti McConville/Getty Images.
421: (left) Bert Hardy/Getty Images. (right) © SSPL/The Image
Works. 427: © Houghton Mifflin Company. All rights reserved.
429: NASA. 430: BananaStock/Alamy. 431: (left) © Royalty-
Free/Corbis. (right) The source of this material is the Cooperative
Program for Operational Meteorology, Education, and Training
(COMET
®
) Website at http://meted.ucar.edu/ of the University Cor-
poration for Atmospheric Research (UCAR) pursuant to a Coopera-
tive Agreement with National Oceanic and Atmospheric Administra-
tion. © 2000 University Corporation for Atmospheric Research. All
Rights Reserved. 432: New York Times Graphics. 436: © Houghton
Mifflin Company. All rights reserved. 439: © Houghton Mifflin
Company. All rights reserved.
Chapter 11
440: © Julio Etchart/Peter Arnold, Inc. 442: Map entitled “Freshwater
Withdrawal by Sector in 2000,” from World Resources 2000–2001, Peo-
ple and Ecosystems: The Fraying Web of Life, WRI, 2000. 443: USGS;
aquaculture photo courtesy of Clear Springs Foods, Inc. 445, 452,
455, 456: © Houghton Mifflin Company. All rights reserved.
459: NASA/JPL-Caltech. 462: (top center) © Houghton Mifflin
Company. All rights reserved. (bottom) imagebroker/Alamy.
463: (top)© Royalty-Free/Corbis.(bottom left and right) © Houghton
Mifflin Company. All rights reserved. 465: © Houghton Mifflin
Company. All rights reserved. 472: (top) DigitalVues/Alamy.
(bottom) © Houghton Mifflin Company. All rights reserved.
473: Art Vandalay/Photodisc Red/Getty Images. 476: Edgar Fahs
Smith Collection, University of PennsylvaniaLibrary. 477:© Houghton
Mifflin Company. All rights reserved. 480: © Leonard Lessin/Peter
Arnold, Inc. 481: (top) Mark Richards/PhotoEdit. (center) David
Young Wolff/PhotoEdit. (bottom) Christine Osborne Pictures/
Alamy.
Chapter 12
491: © Karen Kasmauski/CORBIS. 492: (top) ZEE/Alamy.
(bottom) Hugh Threlfall/Alamy. 493: © Royalty-Free/Corbis.
494: AP/Wide World Photos. 495: Andrew Lambert/Photo Re-
searchers, Inc. 496: (left) © Amos Nachoum/CORBIS. (center)
Shape’n’color/Alamy. (right) B.A.E. Inc./Alamy. 501: Stockbyte
Platinum/Alamy. 506: © Royalty-Free/Corbis. 507: Courtesy of
Agilent. 515: © Houghton Mifflin Company. All rights reserved.
524: © Houghton Mifflin Company.All rights reserved.
Chapter 13
539: Don Tremain/Photodisc Green/Getty Images. 540: © Ari J.
Bauman/Phototake. 541: © Royalty-Free/Corbis. 545: (left) Edward
Kinsman/Photo Researchers, Inc. (right) Courtesy of CCLRC.
546: N. Vogelaar, Virginia Tech Cyrstallography Lab. 555: © Reuters/
CORBIS. 557: (top) Mauro Fermariello/Photo Researchers, Inc.
(bottom) Mauro Fermariello/Photo Researchers, Inc. 558: (left)
Winfred Evers/Getty Images. (right) Courtesy of Lehigh Northwest
Cement Ltd. 559: © James L. Amos/CORBIS. 560: Phanie/Photo Re-
searchers, Inc. 561: © Gabe Palmer/CORBIS. 562: (left) Courtesy of
Bruker. (right) Courtesy of Bruker. 567: AP/Wide World Photos.
568: Scott Camazine/Photo Researchers, Inc. 569: © Houghton
Mifflin Company. All rights reserved. 570: AP/Wide World Photos.
572: (top) Clive Freeman/Biosym Technologies/Photo Researchers,
Inc. (bottom) Lawrence Berkeley National Lab.
Chapter 14
579: © Houghton Mifflin Company. All rights reserved. 582: John
Noel Photographic Collection. 586: (top) © Houghton Mifflin
Company. All rights reserved. (bottom) © David G. Houser/Post-
Houserstock/Corbis. 591: imagebroker/Alamy. 593: (left) Panoramic
Images/Getty Images. (right) V. Tunicliffe, University of Victoria.
594: Philip & Karen Smith/Getty Images. 608: © Galen Rowell/
CORBIS. 614: © Houghton Mifflin Company. All rights reserved.
Credits A51
Chapter 15
619: Photolibrarycom/Getty Images. 622: © Reuters/CORBIS.
623: AP/Wide World Photos. 631: © Nicole Duplaix/CORBIS.
632: Courtesy of Brookhaven National Laboratory. 639: Courtesy
of Delphi. 642: © Still Pictures/Peter Arnold, Inc. 656: Courtesy
of Engelhard. 657: (top) © Houghton Mifflin Company. All rights
reserved. (bottom) PDB ID: 1W2T; F. Alberto, B. Henrissat, M.
Czjzek, 2004.
Chapter 16
668: K-Photos/Alamy. 669: (top) AP/Wide World Photos.
(bottom) Modified from the protein data bank structure, 1A3N
and 1GZX. Primary reference: Paoli, M., Liddington, R., Tame, J.,
Wilkinson, A., Dodson, G.“Crystal Structure of T State Haemoglobin
with Oxygen Bound at All Four Haems,” J. Mol. Biol., 1996, 256, 775.
676: (left) Steve Dunwell/Getty Images. (right) Photolink/Photodisc
Green/Getty Images. 679: Science Photo Library/Photo Researchers,
Inc. 700: Edgar Fahs Smith Collection, University of Pennsylvania
Library. 701: AP/Wide World Photos. 704: Image courtesy Altair
Nanotechnologies. 709: Photograph by James Scherer. © Houghton
Mifflin Company. All rights reserved. 714: © Houghton Mifflin
Company. All rights reserved.
Chapter 17
717: © Bob Daemmrich. 719: © Houghton Mifflin Company. All
rights reserved. 720: Edgar Fahs Smith Collection, University of
Pennsylvania Library. 723: © Todd Gipstein/CORBIS. 727: © Pete
Stone/CORBIS. 748: (both) © Houghton Mifflin Company. All rights
reserved. 756: Photograph of isoelectrically focused barley proteins is
by permission of the Department of Primary Industries and Fisheries,
Queensland, Australia. 757: David Woodfall/Getty Images.
758: National Atmospheric Deposition Program.
Chapter 18
765: © Peticolas/Megna/Fundamental Photographs, NYC. 766: ©
Houghton Mifflin Company. All rights reserved. 767: © Houghton
Mifflin Company. All rights reserved. 772: (both) © Houghton
Mifflin Company. All rights reserved. 787: (left) © Houghton Mifflin
Company. All rights reserved. (right) Courtesy of Dispersion Tech-
nology, Inc. 799: (all) © Houghton Mifflin Company. All rights
reserved. 802: © Jonathan Blair/CORBIS. 807: © Houghton Mifflin
Company. All rights reserved. 809: Jonathan A. Meyers/Photo
Researchers, Inc.
Chapter 19
823: Maximilian Stock Ltd./Photo Researchers, Inc. 825: (top) Siman
Fraser/Photo Researchers, Inc. (bottom) AP/Wide World Photos.
826: (left) © Charles O’Rear/CORBIS. (right) Edgar Fahs Smith Col-
lection, University of Pennsylvania Library. 827: Joe Raedle/Getty
Images. 834: (all) © Houghton Mifflin Company. All rights reserved.
840: (both) © Houghton Mifflin Company. All rights reserved. 845:
(both) © Houghton Mifflin Company. All rights reserved. 849: ©
Royalty-Free/Corbis. 850: (both) © Houghton Mifflin Company. All
rights reserved. 852: (all) © Houghton Mifflin Company. All rights
reserved. 853: © Houghton Mifflin Company. All rights reserved.
859: Stockdisc Classic/Getty Images. 860: © Houghton Mifflin Com-
pany. All rights reserved.
Chapter 20
868: Erich Lessing/Art Resource, NY. 873: © E. R. Degginger/Color-
Pic, Inc. 876: From Coste et al.,“Crystal structure of a double-stranded

DNA containing a cisplatin interstrand cross-link at 1.63 A resolu-
tion: hydration at the platinated site,” from Nucleic Acids Research,
Vol. 27 (8) 1999. By permission of Oxford University Press and Dr.
Franck Coste. 877: National Atmospheric Deposition Program.
884: Digital Image © The Museum of Modern Art/Licensed by
SCALA/Art Resource, NY. 889 (both) © Houghton Mifflin Company.
All rights reserved. 890: © Houghton Mifflin Company. All rights re-
served. 893: (all) © Houghton Mifflin Company. All rights reserved.
Chapter 21
900: Fermilab. 903:© Houghton Mifflin Company. All rights reserved.
907: (both) © Houghton Mifflin Company. All rights reserved.
910: © Tom Steward/CORBIS. 913: Photograph courtesy of As-
traZeneca Oncology. 914: © Tom & Deen Ann McCarthy/CORBIS.
917: © Houghton Mifflin Company. All rights reserved. 920: AIP
Emilio Segre Visual Archives, Brattle Books Collection. 925: Courtesy
of Brookhaven National Laboratory. 926: (top left and right) ©
Dr. A. Leger/ISM/Phototake. (bottom) © ISM/Phototake. 927: ©
Sovereign/ISM/Phototake. 930: Courtesy of John DeArmond.
931: © H. Windeck/UNEP/Peter Arnold, Inc. 933: © Houghton
Mifflin Company. All rights reserved.
A52 Credits
Chapter 22
934: LSHTM/Photo Researchers, Inc. 937: (left) © Jewish Chronicle
Ltd/HIP/The Image Works. (center) Omikron/Photo Researchers,
Inc. (right) Michael Steele/Getty Images. 946: (top) PDB ID: 1LDG;
C. Dunn, M. Banfield, J. Barker, C. Higham, K. Moreton, D. Turgut-
Balik, L. Brady, J. J. Holbrook, Nat. Struct. Biol., 1996, 3, 11.
(bottom) Based on PDB ID: 1BKV; R. Z. Kramer, J. Bella, P. Mayville,
B. Brodsky, H. M. Berman, Nat. Struct. Biol., 1999, 6, 454.
947: (left) Kenneth Eward/BioGrafx/Photo Researchers, Inc.
(right) PDB ID: 1MBN; H.C. Watson, Prog. Stereochem., 1969, 4, 299.
949: PDB ID: 1TKA; S. Koenig, A. Schellenberger, H. Neef, G.
Schneider, J. Biol. Chem., 1994, 269, 10879. 953: PDB ID: 1BPH; O.
Gursky, J. Badger, Y. Li, D.L.D. Caspar, BIOPHYS. J., 1992, 63, 1210.
959: From Michal/Biochemical Pathways/0471331309. Reprinted
with permission of John Wiley & Sons. 960: Smithsonian
Institution Photo. 963: Courtesy of West-Ward Pharmaceuticals.
967: (top) PDB ID: 1C1G; F. G. Whitby, G. N. Phillips Jr., Proteins:
Struct., Funct., 2000, 38, 49. (bottom) © CORBIS.
Index/Glossary
Page numbers followed by f refer to figures. Page numbers followed by t refer to tables.
Acid–base reactions The reaction between
an acid and a base. The products are
water and an ionic compound, 144,
148–152
conjugate acid–base pairs, 720–722, 753
stoichiometry of, 151–152
strong acids and bases, 148–149
weak acids and bases, 150
Acid–base titrations, 779, 787–801
applications for, 787
buffer regions in, 793–794
equivalence point of, 789–790
pH indicators for, 799–801
strong-acid–strong-base, 787–792
titration curves, 790
titration endpoint, 801
titration midpoint, 794
weak-acid–strong-base, 792–796
weak-base–strong-acid, 796–798
Acid deposition The precipitation of acidic
compounds from the atmosphere. This
includes wet deposition as rain and dry
deposition of particles, 757–758
Acid dissociation constant (K
a
) The
equilibrium constant for the dissociation
of an acid, 724–726, A13
Acidic Having a pH less than 7.0 in aqueous
solution at 24°C, 735
Acidic solutions
balancing redox reactions in, 836–840
common-ion effect in, 737–738
equilibrium constant for, 724–726
equilibrium hydrogen ion concentrations,
729–730
with mixture of monoprotic acids,
742–743
pH of strong, 736–738
pH of weak, 738–742
with polyprotic acids, 749–752
salts, 752–757
strong and weak acids, 724–730
Acid-neutralizing capacity The capacity of
a solution, such as lake water, to
neutralize acidity, 758
Acidophiles, 592t, 593
Acids A compound that produces hydrogen
ions (H
+
) when dissolved in water.
According to the Arrhenius model, a
species that produces hydrogen ions in
solution. Compare the definitions of
Brønsted–Lowry acid and Lewis acid,
128, 148
Arrhenius, 719–720
Brønsted–Lowry, 720–722
characteristics of, 723
conjugate, 720–722, 753
diprotic, 736
importance of, 718–719
Lewis, 722–723
list of common, 719t
molecular structure of, 728–729
monoprotic, 736, 742–743
polyprotic, 747–752
strength of, 130, 149–150, 723–730
superacids, 729
triprotic, 747
See also Acid–base entries; Aqueous
equilibria
Acrylonitrile, 516t, 567
Actinides The second set of inner transition
elements. The actinides include elements
90–103, 275–276
Activated complex The unstable collection
of atoms that can break up either to
form the products or to reform the
reactants, 651
Activation energy The energy that is required
to initiate a chemical reaction. The
minimum energy of collision that
reactants must have in order to
successfully create the activated complex,
174, 630, 651–654
Active site The location on an enzyme where
the reaction of the substrate is catalyzed,
335, 948
Activity The effective concentration of a
solute in solution, 675, 706
Activity series A series of elements ranked in
order of reactivity. Also known as a
reactivity series, 291–292, 849–850
Actual yield The experimental quantity,
in grams, of product obtained in a
reaction, 115
Addition polymer A polymer formed by the
process of addition polymerization,
515–516
Addition polymerization A type of
polymerization involving addition
reactions among the monomers involved,
515–516, 522
Addition reaction A type of reaction in
which atoms are added to a double or
triple bond, 514–515
Adenine, 935, 936, 937f, 942
Adenosine diphosphate (ADP), 607–608
Adenosine monophosphate (AMP),
607–608
Adenosine triphosphate (ATP), 545, 585,
595, 607–608, 611
Adhesion The interaction of a compound
with the surface of another compound,
such as the interaction of water with the
surface of a drinking glass, 463–464
Adrenaline (epinephrine), 87–88, 89
A53
Absolute zero The temperature obtained by
extrapolation of a plot of gas volume
versus temperature to the “zero volume”
point. The lowest temperature possible,
which is 0 K, or −273.15°C, 409
Absorb To be associated, through
intermolecular forces of attraction, by
being taken up or mixing into a
substance, 656
Absorption spectroscopy The measurement
of how atoms and molecules absorb
electromagnetic radiation as a function
of the wavelength or frequency of the
radiation, 216
Accuracy The closeness of a measurement to
the actual value, 29–31
Acetaldehyde. See Ethanal
Acetanilide, 623–624, 625–626
Acetic acid (ethanoic acid)
dehydration of, 104–105, 108–109
formation of, 103–104, 519–520
ionization of, 688–689, 691–693,
724–727
in solution with hydrochloric acid,
742–743
titration with sodium hydroxide,
684–685, 792–795
uses of, 520
as weak acid, 150, 724, 725
as weak electrolyte, 129, 130f
Acetic acid–acetate buffer system,
770–771, 778
Acetone (propanone)
boiling-point elevation of, 476t
formation of, 519
vapor pressure of, 454
Acetonitrile, 420, 513t
Acetylcellulose, 340
Acetylene (ethyne), 512t
combustion of, 358
formation of, 420, 421
pi bonds in, 370
uses of, 420, 501
Acetylsalicylic acid. See Aspirin
Achiral A term used to describe molecules
that have a superimposable mirror
image, 525
Acid anhydrides Binary compounds formed
between nonmetals and oxygen that
react with water to form acids, 757
Acid–base indicator A compound that
changes color on the basis of the pH of
the solution in which it is dissolved. The
color change is often a result of structural
changes due to protonation or
deprotonation of acidic groups within
the compound, 799–801

Adsorb To be associated, through
intermolecular forces of attraction, with
a surface, 656
Aerogel A silica glass whose structure is
mostly air; also known as ceramic foam,
572–573
Agriculture
future applications, 35
pesticides, 620–621
use of chelates in, 812t
Alachlor (Lasso), 623–624, 625–626
Alanine, 605–606, 755–756, 940f
Alcohols A chemical containing the hydroxyl
(OOH) group, 512t, 514
ethanol, 514, 516–517
list of selected, 518t
types of, 517
Aldehydes Compounds that carry a carbonyl
functional group (CPO) with at least
one H atom bonded to the carbonyl
carbon atom, 512t, 517–519, 872–873
Aliphatic compounds Compounds in which
all of the carbon atoms are sp
3
hybridized and contain only single
bonds, 495t, 496
Alkali, 148
Alkali metals The highly reactive metals
found in Group IA of the periodic table,
which form alkalis upon reaction with
water, 66, 270
commercial uses of, 270t
crystal lattice in, 545
electron configurations of, 250
ionization energies for, 282–283
properties of, 270, 279
Alkaline battery, 846, 848–849
Alkaline earth metals The metals found
in Group IIA of the periodic table,
66t, 270
commercial uses of, 271t
electron configurations of, 250
in ionic compounds, 68, 74
properties of, 270–271
Alkaliphiles, 592t, 593
Alkaloids Nitrogen-containing bases found
in vegetables and other plants, 746–747
Alkanes Saturated hydrocarbons, 496–500
branched-chain, 498–499
cyclic, 500, 502
naming of, 499–500
normal, 496–497
typical reactions of, 510–511
Alkenes Hydrocarbons containing at least
one CPC double bond, 500–501,
502, 512t
formation of, 510–511
geometric isomers, 502–504
naming of, 502
Alkoxy anion An anion that contains an
alkyl group attached to an oxygen anion,
such as CH
3
O
−
or CH
3
CH
2
O
−
, 520
Alkyl group A hydrocarbon group, such as
OCH
3
or OC
2
H
5
, within the structure
of an organic compound, 504–506
Alkyl halide A functional group in which a
halogen atom is bonded to an alkyl
group, 511
Alkynes Hydrocarbons containing a CqC
bond, 501, 512t
formulas for, 101
naming of, 502
Allotropes Forms of an element that have
very different chemical and physical
properties, such as the allotropes O
2
and
O
3
, 71, 493
Alloys A homogeneous mixture of metals. A
solution of two or more metals, 266, 267,
556, 559t
Allred–Rochow electronegativity scale, 320
Alpha decay A type of radioactive decay
wherein an alpha particle is emitted
from the nucleus of a radioactive
nuclide. Common for elements whose
nuclei are larger than bismuth, alpha
decay is often accompanied by the release
of a gamma ray, 905–906
Alpha helix (α helix), 945
Alpha particles (α particles) Particles
emitted from the nucleus of a radioactive
element during the process of alpha
decay. They are helium nuclei
(2 protons, 2 neutrons), with a +2
charge, 54–55, 905–906, 919t
penetration capability of, 909–910
Aluminosilicate, 560t
Aluminum
density of, 23
gravimetric analysis of, 807
as Lewis acid, 722, 723f
metallic bonding of, 304
oxidation of, 168, 586t
production of, 826, 857–858
reactivity of, 291–292, 857
sources and uses of, 265t, 267, 271
specific heat of, 181t
in thermite reaction, 196–197
thermodynamic data for, A9
Aluminum chloride, lattice enthalpy of, 316
Aluminum hydride
hybrid orbitals in, 367
molecular geometry of, 368
Aluminum hydroxide, 147
Aluminum perchlorate, 168
Aluminum sulfate
in paper formation, 719, 723
in water treatment, 808
Amalgam A solution made from a metal
dissolved in mercury, 557, 856
American Academy of Orthopedic
Surgeons, 557
American Chemical Society, 2
Amide bond A bond formed by a
condensation reaction between an amino
group (ONH
2
) and a carboxylic acid
group (OCOOH), 523, 940
Amides, 513t
Amines A compound containing the ONH
2
functional group, 512t, 523, 940
Amino acids, 588
chirality of, 960
found in living cells, 940f
protein formation and, 940–945
water solubility of, 755–756
Amino functional group A functional group
containing an ––NH
2
attached to an sp
3
hybridized carbon atom, 523
Aminopolycarboxylic acid chelating
agents, 811t
Ammonia
in aqueous solution, 722
as base, 150, 722, 723, 743–744
complex with zinc, 809–810
hybrid orbitals in, 367
hydrogen bonds in, 450f
mass-action expression for, 673–674
molecular geometry of, 340, 341, 368
nicotine and, 743, 746
oxidation states for, 831
pH of precipitation and, 758
pH of solution of, 745
production of, 156, 597, 625, 702–703,
704, 722
titration with hydrochloric acid,
796–798
uses of, 340, 395t, 718t, 719t, 743
Ammonia–ammonium ion buffer system,
767–770, 775–777, 782–783
Ammonium chloride, reaction with barium
hydroxide, 597
Ammonium cyanide, 755
Ammonium nitrate, 97–98, 175
Ammonium phosphate, 47, 75
Ammonium sulfate, 97–98
Amorphous solid A solid whose atoms,
ions, or molecules occupy fairly rigid
and fixed locations but that lacks a high
degree of order over the long term, 541
Amount of substance, as SI base unit,
17t,20
Ampere (A) The SI base unit of electric
current,17t,20
Amphiprotic Having the ability to act as
either an acid or a base in different
circumstances, 733, 755
Amplitude The distance between the highest
point and the midpoint (zero) of a
wave, 212
m-Amsacrine, 962
Anabolism The metabolic process of
constructing molecules from smaller
molecules using high-energy molecules
that have been made by catabolism, 958
Analgesic drugs, 14–15, 335
Analyte A solute whose concentration is to be
measured by a laboratory test, 766
Anastas, Paul, 571
Angstrom, Anders, 217
Angstrom A unit of distance equal to
10
−10
m. It is symbolized by Å, 211
Angular As a function of the angles that
describe the orientation of the radius in
spherical polar coordinates, 234
A54 Index/Glossary

Angular momentum quantum number
The quantum number, l, that describes
the shape of the orbital; l can be any
whole-number value from zero to n – 1,
235, 243t
Anhydrides, in aqueous solution, 757–758
Anions Ions with a negative charge, 56,
286, 289
acidity of salt and, 756–757
alkoxy, 520
carboxylate, 520
electron configurations of, 305
Lewis dot symbols for, 305
size of, 311
Anode The electrode at which oxidation
takes place, 826, 845
sacrificial, 850
Antacids, 784–786
Anthocyanins A naturally occurring class of
compounds responsible for many of the
colors of plants. These compounds often
act as acid–base indicators, 801
Anthracene, 505f
Antibiotics Substances that kill, harm, or
retard the growth of microorganisms,
961–962
Antibodies Large biological molecules that
are produced during an immune
response, travel to the site of infection,
bind to foreign material, and signal
the destruction of the foreign
material, 954
Antibonding orbitals An orbital that
indicates a lack of electron density
between adjacent nuclei (no bond exists
when the orbital is occupied). The
antibonding orbital arises from the
subtraction of two overlapping atomic
orbitals, 375, 376, 378
in metals, 551–552
Anticodon The three-nucleic-acid sequence
on a tRNA polymer that recognizes and
binds to the codon on an mRNA
polymer, 943
Antifreeze, 462, 477, 478
Antilogarithm, A4
Antimatter Particles that have the same mass
as, but charges opposite to, corresponding
matter. Antimatter particles such as the
positron and antineutrino are similar to
the electron and neutrino, respectively,
but have opposite characteristics, 905
Antimony, 272t,A9
Antineutrino A subatomic particle produced
in beta-minus decay that has no charge,
has essentially no mass, and interacts
only rarely with matter, 904–905,
909–910
Antlerite, 586
Aqua regia, 844
Aquatic systems
acid concentrations and, 729–730, 733
calcium carbonate dissociation, 802–804
impact of mercury on, 876–877
Aqueous Water-based; also implies that a
dissolved substance has a sphere of
hydration, 126
Aqueous equilibria
acid–base titrations, 787–801
acid strength and, 724–730
buffers and, 767–786
common-ion effect in, 737–738, 768–771
complex-ion equilibria, 809–815
Henderson–Hasselbalch equation and,
777–779
pH of acidic solutions and, 738–743
pH of basic solutions and, 744–745
pH of polyprotic acids and, 749–752
in salt solutions, 752–756
solubility equilibria, 802–809
See also Equilibrium; Solubility equilibria
Aqueous solutions A homogeneous mixture
of a solute dissolved in the solvent water,
107, 465
anhydrides in, 757–758
dissolution process, 465–466
hydration, 466, 479
pH of, 735–746
solubility of substances in, 467–468
See also Solution stoichiometry; Solutions
Aramid, 567
Arginine, 940f
Argon, 903
lasers, 224t
thermodynamic data for, A9
uses of, 274t, 275, 312, 401
ArK excimer lasers, 224t
Arnolfini Wedding (van Eyck), 868
Aromatic hydrocarbons Hydrocarbons that
contain one or more aromatic rings,
495t, 504
Arrhenius, Svante, 52, 652, 719
Arrhenius equation, 652
Arsenic
in drinking water, 135, 471
thermodynamic data for, A9
uses of, 272t
Arthrobacter psychrolactophilus, 592
Ascorbic acid. See Vitamin C
Asparagine, 940f
Aspartate, 940f
Aspirin (acetylsalicylic acid), 87, 719
t
buffers in, 784, 786f
chemical formula for, 89, 90f, 303
as covalent bond, 304
formula mass of, 88, 90–91
Lewis dot structure for, 328
molar mass of, 92
properties of, 303, 723–724
synthesis of, 15, 103–104, 322
Astatine, 274t
Atmosphere, 292f, 394
changes in ozone concentration, 427–432
composition of, 71, 293–294, 394, 395
partial pressures in, 402–403
Atmosphere (atm) A unit used to measure
pressure. 1 atm = 760 mm Hg, 399, 400t
Atomic bomb, 920
Atomic force microscopy (AFM), 570
Atomic mass units (amu) The arbitrary
unit of mass for elements based on the
isotope carbon-12. One atom of
carbon-12 is defined to have a mass of
exactly 12.0000 amu, 60–61, 87
Atomic number (Z) The number of protons
in the nucleus of an atom, 56, 58
in nuclear equations, 904
nuclear stability and, 917–918
periodicity of, 264
Atomic orbitals. See Orbitals
Atomic radius Half the distance between the
nuclei in a molecule consisting of
identical atoms. Also known as the
covalent radius, 280–281, 311
Atoms The smallest identifying unit of an
element,5
atomic number of, 56
Bohr’s model of, 220–224
Dalton’s atomic theory, 49–51
determining mass of, 59–63, 87–88
isotopes, 57–58
mass number of, 57
origins of word, 47
periodic table, 64–66
radioactivity and, 53–55
size of, 21, 280–281, 311
structure of, 51–57
total number in universe, A1, A2
ATP. See Adenosine triphosphate
Atrazine, 620–621, 636–637, 645, 654
Aufbau principle As protons are added to
the nucleus increasing the atomic
number of the atom, electrons are added
successively to the next highest energy
orbital. The method of filling hydrogen
atomic orbitals to get the ground-state
electron configuration of a multielectron
atom by adding the electrons one at a
time until all are accommodated; from
the German phrase for “building up,”
245, 250, 261, 279
Automobiles
air bags, 419–420
catalytic converters, 277, 317, 430,
638–639, 656
gasoline, 317–318, 508–509
and increase of surface ozone, 430
solar-powered, 555
Autoprotolysis Proton transfer between
molecules of the same chemical species,
as in the autoprotolysis of water, 733,
737, 738
Average rate The rate of a reaction measured
over a long period of time, 622–627
Average speed The sum of the molecular
speeds of each molecule divided by the
number of molecules, 424
Avogadro, Amadeo, 91, 404
Avogadro’s law Equal amounts of gases
occupy the same volume at constant
temperature and pressure, 404–405,
413t, 423
Index/Glossary A55

Avogadro’s number (N
A
) The number of
particles (6.022 × 10
23
) of a substance
in 1 mol of that substance. By definition,
Avogadro’s number is equal to the
number of carbon-12 atoms in exactly
12.0000 g of carbon-12, 91, 92
Axial position The position of a group when
it is aligned along the z axis of a
molecule, 340
Baekeland, Leo, 563
Bakelite, 563
Balanced Appropriate coefficients have been
added such that the number of atoms of
each element are the same in both
reactants and products, 103
Balances, 47, 668, 806
Balmer, Johann J., 217, 218
Balmer series, 218–219, 223
Band gap A small energy gap that exists
between the valence band and the
conduction band, 553–554
Band theory A metal is a lattice of metal
cations spaced throughout a sea of
delocalized electrons, 551–553
Bar A unit used to measure pressure.
1.01325 bar = 1 atmosphere, 399
Barium, 271t,A9
Barium hydroxide
reaction with ammonium chloride, 597
reaction with magnesium sulfate, 147
Barometer, 399
Bartlett, Neil, 329
Base hydrolysis The process in which a base
reacts with water to produce its
conjugate acid and hydroxide ion, 753
Base pair A pair of nucleic acids that are
intermolecularly bound to each other
through noncovalent forces of attraction,
for example, the combination of A and T
or of G and C in a DNA duplex, 936,
937f, 942
Bases According to the Arrhenius model, a
species that produces hydroxide ions in
solution. Compare the definitions of
Brønsted–Lowry base and Lewis base,
129, 148
Arrhenius, 719–720
Brønsted–Lowry, 720–722
characteristics of, 723
conjugate, 720–722, 753
equilibrium constants for, 725, A14
importance of, 718–719
Lewis, 722–723, 870
list of common, 719t
strength of, 130, 149–150
See also Acid–base entries;
Aqueous equilibria
Base units The set of seven fundamental
units of the International System,
17–20
Basic anhydrides Binary compounds that
are formed between metals with very low
electronegativity and oxygen and that
react vigorously with water, 757
Basic Having a pH greater than 7.0 in
aqueous solution at 24°C, 735
Basic solutions
balancing redox reactions in, 840–841
determining pH of, 743–746
salts, 752–757
Batteries Two or more voltaic cells joined in
series, 826, 847–849
chemistry of common, 848–849
decreasing potentials in, 854
list of selected, 848t
rechargeable, 848, 849, 854
See also Electrochemical cells
Bauxite, 271, 420, 857, 858
Bazemore, Whit, 622, 623f
Becquerel (Bq) An SI unit of activity
equivalent to one nuclear disintegration
per second, 911
Bent geometry, 336–337, 340
Benzene, 71, 505f
boiling-point elevation of, 476t
freezing-point depression for, 478t
miscibility of, 466
molecular orbital for, 384–385
properties of, 385, 467
shorthand notations for, 385f
Beryllium
electron configuration of, 246
electronegativity of, 289
Lewis dot symbol for, 305
thermodynamic data for, A9
uses of, 271t
Beryllium chloride, molecular geometry
of, 337
Beryllium hydride, hybrid orbitals in, 366
Beta-galactosidase, 592
Beta-particle emission A naturally occurring
type of radioactive decay wherein an
electron is ejected at high speed from the
nucleus, typical of nuclei that have too
many neutrons to be energetically stable.
An antineutrino accompanies this
emission, and sometimes one or more
gamma rays as well. Also known as beta
emission, 904–905, 919t
Beta particles (β particles) Particles emitted
from the nucleus of a radioactive atom
during the process of beta decay. These
particles are high-speed electrons,
54–55, 904
penetration capability of, 909–910
Beta pleated sheet (β sheet), 945
Beta-plus emission A type of radioactive
decay wherein a positron is ejected at
high speed from the nucleus, typical of
nuclides that have too few neutrons. A
neutrino accompanies this emission, and
usually one or gamma rays as well. Also
known as positron emission, 918
Bidentate Capable of forming two coordinate
covalent bonds to the same metal
center, 874
Bimolecular reaction A reaction involving
the collision of two molecules in the
rate-determining step, 648
Binary covalent compounds Compounds
composed of only two nonmetal
elements, 72–73
Binary ionic compounds Compounds typi-
cally composed of a metal and nonmetal
element, which have ions that interact via
electrostatic attractions,73–75
Binding energy The energy released when a
nucleus is formed from protons and
neutrons. Binding energies are expressed
as a positive number, 915–917
Binding rate constant The rate constant the
indicates the association of two
molecules, 670
Biochemical pathway A sequence of
biochemical reactions that lead to the
consumption or production of a
particular metabolite, 958–959
Bioenergetics The study of the energy changes
that occur within a living cell, 580
Biological processes. See Chemistry of life
Biomass conversion The release of energy
from the chemical conversion—often
simply the burning—of plants and
trees, 198
Biopolymer A polymer of biological
materials, such as DNA or
cellulose, 572
Bismuth, 272t,A9
Blood
chemicals in, 126, 260
color of, 890–891
hemoglobin, 260, 291, 869–870
as isotonic solution, 480
Body-centered cubic (bcc) unit cell A unit
cell built via the addition of an atom,
ion, or molecule at the center of the
simple cubic unit cell, 543, 546, 549
Bohr, Niels, 220, 221f, 222
Bohr frequency condition, 222
Bohr’s model of atomic structure, 220–224,
227–228, 234–235
Boiler scale A buildup of calcium and
magnesium salts within pipes and water
heaters. Typically composed of calcium
carbonate and magnesium carbonate,
309–310, 767
Boiling The process that occurs when the
vapor pressure of a liquid is equal to the
external vapor pressure, 455
Boiling point The temperature at which the
pressure of a liquid’s vapor is equal to the
surrounding pressure, 455
hydrogen bonds and, 449–451
impact of altitude on, 455–456
molar mass and, 446–448, 506
of selected compounds, 446t
Boiling-point elevation The increase in the
boiling point due to the presence of a
dissolved solute, 475–477, A13
Boltzmann, Ludwig, 582–583
A56 Index/Glossary

Bomb calorimeter Apparatus in which a
chemical reaction occurs in a closed
container, allowing the energy released
or absorbed to be measured, 184
Bond angles, 340, 357
of coordination complexes, 876–877
factors effecting, 341–342
of hybrid orbitals, 367, 372
Bond dipole The polarization of electrons in
a bond that results in a separation of
partial charges in the bond, 343–344
Bond dissociation energy The energy
required to break 1 mol of bonds in a
gaseous species. Also known as the
enthalpy of bond dissociation, 332–333
Bond energies
covalent bonds, 331–334
electronegativity and, 287
ionic bonds, 313–316
in valence bond model, 360–362
Bonding electron pairs Pairs of electrons
involved in a covalent bond. Also known
as bonding pairs, 340
Bonding orbitals An orbital that indicates
the presence of electron density between
adjacent nuclei (a bond exists when the
orbital is occupied). The bonding orbital
arises from the addition of two over-
lapping atomic orbitals, 375, 376, 378
in metals, 551–552
Bonding pairs Pairs of electrons involved in
a covalent bond. Also known as bonding
electron pairs, 322
multiple bonds, 325–327
in VSEPR model, 337, 340, 341–342
Bond length The average distance between
the nuclei of bonded atoms, 319, 332, 341
of hybrid orbitals, 367t, 371–372
in valence bond model, 360–362
Bond order The number of electrons in
bonding orbitals minus the number of
electrons in antibonding orbitals, divided
by 2. The bond order indicates the degree
of constructive overlap between two
atoms, 377, 378
Borane, 723
Boric acid, 785
Borkenstein, Robert, 329
Born, Max, 313
Born–Haber cycle A diagrammatic
representation of the formation of an
ionic crystalline solid using Hess’s law.
The cycle reveals the lattice enthalpy,
which is difficult to obtain by direct
measurement, 313–314
Boron, 50f
electron configuration of, 246, 365
Lewis dot symbol for, 305
uses of, 271t
Boron trifluoride, 330
hybrid orbitals in, 365–366
molecular geometry of, 338–339
Borosilicate, 560t
Bovine F1 ATP synthase, 545
Boyer, Paul D., 545
Boyle, Robert, 16, 406f
Boyle’s law The volume of a fixed amount of
gas is inversely proportional to its
pressure at constant temperature, 15–16,
405–408, 413t, 422
Bragg, William Henry, 546
Bragg, William Lawrence, 546
Branched-chain alkanes, 498–499
Branched molecules, 447
Brass, 586t
Breathalyzer, 329
Breeder reactors, 922
Brochantite, 586
Brock, Thomas D., 592
Bromine
boiling point of, 446
thermodynamic data for, A9
uses of, 274t
Bromine pentafluoride, molecular geometry
of, 342
Bromochlorofluoromethane, 525
Brønsted, Johannes Nicolaus, 720
Brønsted–Lowry acids Any species that
donates a hydrogen ion (proton) to
another species, 720–722, 723
Brønsted–Lowry bases Any species that
accepts a hydrogen ion (proton) from
another species, 720–722
Bronze, 586t
Buckminsterfullerene, 493–494
Buffer capacity The degree to which a buffer
can “absorb” added acid or base without
changing pH, 779–784
Buffer region The region of a titration
indicated by the presence of a weak acid
or base and its conjugate. The pH
changes little within this region,
793–794, 796
Buffers A solution containing a weak acid
and its conjugate base or a weak base
and its conjugate acid. Buffers resist
changes in pH upon the addition of acid
or base or by dilution, 767–786
adding strong acid or base to, 780–782
calibration, 772–773
capacity of, 779–784
common-ion effect in, 768–771
criteria for suitable, 784
defined, 767
Henderson–Hasselbalch equation and,
777–779
in medicine and biology, 784–786
pH of, 767–771
preparation of, 772–777
Buret A piece of laboratory glassware used to
measure volume and to add discrete
amounts of solution in a repetitive
fashion, 140, 463–464
Butadiene, 357
1,3-Butadiene, 372, 381
Butane, 497t
bond length in, 372
combustion of, 106–107, 597
Butene, 502–503
1-Butene, 372, 501f, 502, 503
2-Butene, 503
Butylamine, 512t
Butyne, 101f
1-Butyne, 501f
Cadaverine, 523
Cadmium sulfide, 555
Calcareous oozes The calcium-containing
detritus from dead single-celled,
calcium-based sea life, 802
Calcium
atomic mass of, 91
gravimetric analysis of, 807
in milk, 309
reactivity of, 283f
sources and uses of, 265t, 271t
thermodynamic data for, A10
titration with EDTA, 681, 767, 810, 813,
814–815
Calcium carbide, 420, 421f
Calcium carbonate, 748
color of, 885
as ionic compound, 307t
in mortar, 558
naming of, 75
solubility of, 802–804
Calcium channel blockers, 331
Calcium chloride
formation of, 309
formula for, 69
as ionic compound, 307t
lattice enthalpy of, 315–316
Calcium dihydrogen phosphate, 98
Calcium fluoride
formula for, 68
lattice enthalpy of, 315–316
Calcium hydroxide, 558, 808
Calcium ion, 67, 261
Calcium oxide, 68, 271, 718t
Calcium phosphate, 748
Calcium sulfate, 719, 748, 804
Calcium sulfate hemihydrate,
557, 558
Calcium supplements, 748
Calorie (Cal) (Note capital C) Unit of energy
equal to 1000 calories (1 kilocalorie) and
to 4184 joules, 179–181
calorie (cal) (Note lowercase c) Unit
of energy equal to 4.184
joules, 180
Calorimeter An apparatus in which
quantities of heat can be measured,
183–185
Calorimetry The study of the transfer of heat
in a process, 182–185
Calotype, 382
Cancer treatment, 528–530, 876, 901,
912, 962
Candela (cd) The SI base unit of luminous
intensity,17t,20
Capacitors, thin film, 568
Index/Glossary A57
