Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

A28 Answers to Practice Exercises and Selected Exercises
N
2
O = 418 kJ/mol; N
2
H
6
= 160 kJ/mol
51. Na
3
PO
4
:
CaCO
3
:
Fe(NO
3
)
2
:
The ionic bonding occurs between the separate ions, whereas the
covalent bonding is happening between the atoms of the polyatomic
anions. 53. C—C < HOC < NOO < CaOH < CaON
55. a. 0; b. no change; c. 0; d. –1 57.
59. The structure of NO
2
is . In this diagram, the (
∗
)
are lone-pair electrons, (:) are bonding electrons, and (°) is a radical
electron. The nonzero formal charges are labeled within the
circles. 61. Because the formal charge of –1 is on the
carbon, H
+
with its positive charge should attach to carbon and its
negative charge.
63. The formal charge on sulfur is 0
(F.C. = 6 – 4 – 2 = 0), and the oxidation number on sulfur is +4. In
the calculation of the oxidation number, oxygen is taken at –2 and
hydrogen at +1, giving the sulfur a +4 oxidation number, but in the
calculation of formal charges, both oxygen and hydrogen have a
value of zero.
65. ,
67.
HCN
H
H
C
H
O
O
H
HCN
H
H
C
H
O
O
H
0
ONO
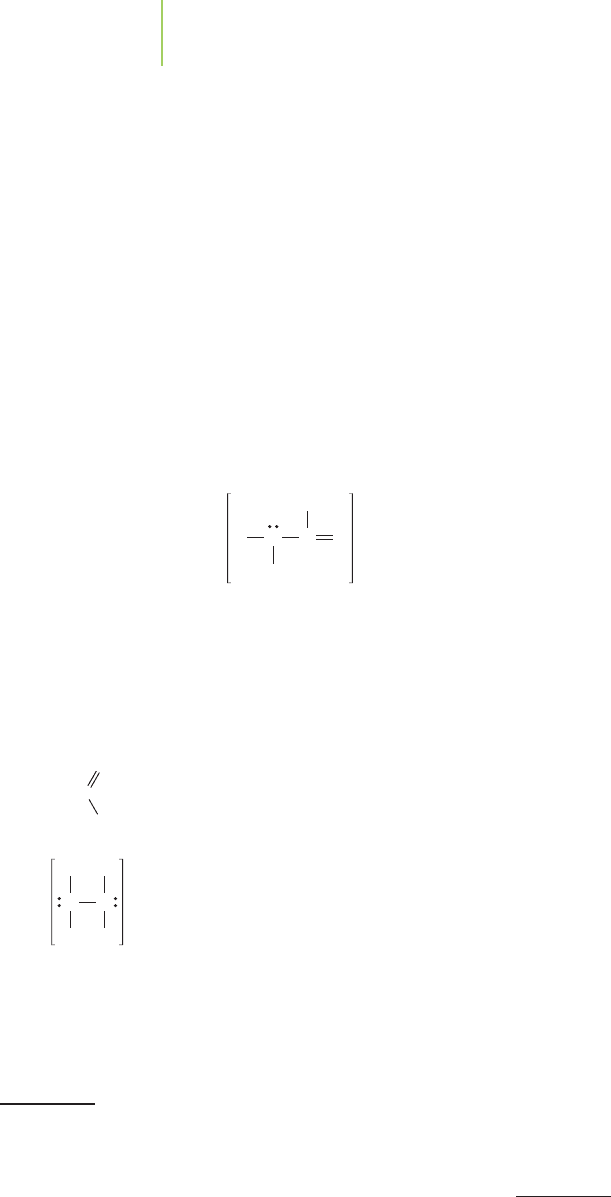
ON
O
O
0
OS
OH
HO
NC
0
OON O
HN
H
H
H Cl
[Fe]
2
O
N
O
O
O
N
O
O
Ionic
bonding
Covalent
bonding
[Ca]
O
C
O
O
Covalent
bonding
Ionic
bonding
2
2
P
O
O
[Na]
[Na]
[Na]
OO
Covalent
bonding
Ionic
bonding
3
69. C
2
H
6
:,C
2
H
4
:,
C
2
H
2
:[HOCqCOH] The number of bonds between carbon
increases from one to two to three, as shown above. The greater
number of bonds between two atoms, the stronger the bond and the
shorter the bond length. C
2
H
2
will have the strongest (and shortest)
bond, and C
2
H
6
will have the longest (and weakest) bond. 71. H =
–2674 kJ/mol. This value is different from the direct determination
because the values for the bond energies are taken from the average
of the bond in several molecules and may not be exactly the same as
the bond energies in the molecules of this reaction.
73. H(H
2
O) = –254 kJ/mol, H(H
2
O
2
) = –153 kJ/mol. Because
water releases more energy than hydrogen peroxide in its forma-
tion, it will be the more stable of the two molecules. 75. H
2
O: bent;
NO
2
: bent; PCl
2
: bent; NBR
3
: trigonal pyramidal 77. NO
2
−
, bent;
PO
4
3−
: tetrahedral; SO
3
2−
: trigonal pyramidal; CO
3
2−
: trigonal
planar; BrO
4
−
: tetrahedral 79. C
2
H
6
: tetrahedral at each; C
3
H
6
:
tetrahedral on left, trigonal planar at the other two; C
2
H
4
: trigonal
planar at each, C
3
H
8
: tetrahedral at each; C
4
H
10
: tetrahedral at each
81. The Lewis structure of ClO
2
is (note the radical
electron). At Cl, it has four electron groups (tetrahedral) but two
lone pairs/radicals, making the molecular geometry bent.
83. BeCl
2
> AlCl
3
> CCl
4
> NCl
3
> XeCl
4
85. , see-saw
87. In N
2
H
2
, each nitrogen has three electron groups (trigonal
planar) but one lone pair, so the molecular geometry is bent. The
H—N—N bond angle will be slightly less than the normal 120°
because of the additional lone-pair repulsion. In N
2
H
2
2+
,each
nitrogen has only two electron groups and no lone pairs, making the
geometry linear. The bond angle is then expanded from 120° in
N
2
H
2
to 180° in N
2
H
2
2+
.
89. CmH < CnN = CmB = CnCl < CnO < CmMg
(arrow denote dipoles)
91. The structures are SF
6
and
SF
5
.SF
6
is octahedral (six electron groups
around S) and nonpolar because all the S—F dipoles cancel. SF
5
has
six electron groups but one lone pair, making the geometry square
pyramidal. Removing one S—F dipole that helped make SF
6
nonpo-
lar makes SF
5
polar, because not all the S—F dipoles cancel out.
93. A: ionic, B: nonpolar, C: polar 95. The structure of H
2
O
2
is
and is bent (see Problem 76). The O—H
bonds are polar with an electronegativity difference of 1.4. Because
the molecule is bent, even though the dipoles point in nearly
opposite directions, the dipoles do not cancel and the molecule is
polar. If the molecule were linear, the dipoles would cancel out,
because they would be aligned and in opposite directions. The linear
molecule would be nonpolar. 97. From left to right: H
+
,H
−
, and H
99. Because N
2
has a triple bond, its bond energy is very large
OH O H
S F
F F
FF
FSF
F F
FF
Cl Se Cl
Cl Cl
Cl OO
CC
H H
HH
HHC
H
H
C
H
H
Chapter 9 A29
(941 kJ/mol). Breaking this bond in order to cause a reaction is very
difficult and therefore unlikely. N
2
will emerge just as it entered.
101. There is only a single choice for the anion (−2), so our choice
will be based on which cation will have a weaker interaction.
The weakest lattice attractions will come from ion pairs that are
widely separated and have smaller charges. This set of ions (the small
+1 and the larger +3) have these trends in opposition. The ion with
the +3 charge has roughly twice the diameter, which is not a large
enough increase in size to offset the larger charge. The +3 ion will
have a larger ionic attraction. Therefore, the weakest interaction will
be between the −2 anion and +1 cation. 103. +3 cation with
−2 anion 105. a. The attraction that two charges particles have for
one another is larger as the charges on each become larger, and the
attraction is larger as the particles get closer to one another. Large at-
tractive forces lead to high melting points. Neutral molecular species
at most have partial charges from the dipoles, limiting the strength
of attraction to other molecules. The lower attraction leads to lower
melting points; b. For a high melting point, we should choose species
that are small and have high charges. For a low melting point, we
should choose low charges and large radii. By far the highest charge
and lowest size belong to B
3+
. For the anion we could choose N
3−
or
O
2−
. Nitride has the higher charge but is bigger (171 pm versus 140
pm), but it is less than 1.25 times larger whereas its charge is 1.5
times larger than that of oxide. Highest melting point: BN. The
largest ions are I
−
and Cs
+
; CsI should have the lowest melting
point. 107. a. Very few molecules can be found in only a diatomic
form allowing for direct measurement of the bond energy. Inside
larger molecules, the strength of the bond can vary depending on the
other atoms and on how well the electrons are distributed within the
molecule. In these cases, several measurements are made, and the av-
erage value of the bond energy is placed in the table; b. The presence
of the three fluorine atoms with their high electronegativity will tend
to shift the electrons from the other bonds toward the fluorine
atoms. This has the potential to weaken the carbon–carbon bond.
109. a. 2Fe + 3O
2
→ 2Fe
2
O
3
; b. 5 unpaired; c. 69.94% 111. a. No;
b. 12.00%; c. 113. a. 346.3 g/mol;
b. c. The N in the ring
114. a. or ;
b. or ;
c. Molecule in part a; d. ; e. Yes, all bonds are nonpolar.;
f. 0.57 g
CC
H
H
H
H
Cl
CC
HCl
H
H
H
CCH Cl
H
H
Cl
H
H
CC
Cl Cl
H
H
H
CCCl Cl
H
H
H
H
O
N
O
O
O
H
N
O
O
OCO
Chapter 9
P9.1 The configuration of the valence electrons in fluorine is 2s
2
2p
5
.
Overlap of one of the 2p orbitals from each fluorine allows the elec-
trons to be shared between the two atoms. The valence bond model
of FOF shows a 2p–2p orbital overlap that constitutes the covalent
bond.
P9.2 The bond formed in F
2
is from the overlap of two 2p orbitals,
in HCl from 1s and 3p orbitals, and in Cl
2
from two 3p orbitals. Be-
cause both 2p orbitals are smaller than the 3p orbitals, the bond in
F
2
is shorter than Cl
2
and because the 1s orbital is much smaller
than the 3p orbital, the HCl bond is shorter than the Cl
2
bond.
Cl
2
has the longest bond.
P9.3 a. Lewis dot structure model for OF
2
,,
indicates that the central oxygen has two bonds and two lone pairs.
Mixing the four orbitals (one 2s and three 2p) on the oxygen atom
allows us to have two lone pairs of equal energy and two bonds
to the adjacent fluorine atoms. The oxygen atom possesses sp
3
hybridized orbitals. b. Lewis dot structure model for H
2
S,
, indicates that the central sulfur has two bonds
and two lone pairs. Mixing the four orbitals (one 3s and three 3p) on
the sulfur atom allows us to have two lone pairs of equal energy and
two bonds to the adjacent hydrogen atoms. The sulfur atom pos-
sesses sp
3
hybridized orbitals. c. Lewis dot structure model for
NH
4
+
, , indicates that the central nitrogen has four
bonds. Mixing the four orbitals (one 2s and three 2p) on the nitro-
gen atom allows us to have four bonds to the adjacent hydrogen
atoms. The nitrogen atom possesses sp
3
hybridized orbitals.
P9.4 a. OF
2
geometry: sp
3
hybridized atoms adopt a tetrahedral
geometry. Because two of the sp
3
orbitals contain lone pairs, the
VSEPR model indicates that the molecule has an overall bent geome-
try. The bond angles should be less than 109.5° because the lone
pairs repel each other more than the bonding pairs. b. H
2
S geome-
try: sp
3
hybridized atoms adopt a tetrahedral geometry. Because two
of the sp
3
orbitals contain lone pairs, the VSEPR model indicates that
the molecule has an overall bent geometry. The bond angles should
be less than 109.5° because the lone pairs repel each other more than
the bonding pairs. c. NH
4
+
geometry: sp
3
hybridized atoms adopt a
tetrahedral geometry. The bond angles should be 109.5°.
P9.5 A quick look at the Lewis dot structure for
CH
2
O, , shows that C will need three sigma bonds
(one to O and two to H) and will use sp
2
hybridization to create the
bonds. Oxygen also will be sp
2
hybridized to create the sigma bond
to carbon and for the two lone pairs. Both carbon and oxygen have a
half-filled p orbital that can be used to create a pi bond to complete
the double bond (one sigma bond, one pi bond) between C and O.
Formaldehyde has a total of one pi bond and three sigma bonds.
P9.6 To answer the question, a quick look at the Lewis dot
structures will help:
CH
2
NOH:
CH
3
NHOH:
HCN
H
H H
O
H
HCN
H
O
H
HCH
O
HN
H
H
H
S HH
O FF
A30 Answers to Practice Exercises and Selected Exercises
The shorter bond will be found in CH
2
NOH as the NO bond is
formed from the combination of sp
2
(on N) and sp
3
(on O) hy-
bridizations. In CH
3
NHOH, both N and O have sp
3
hydridization.
We can further explain by noting that we can write a resonance
structure for CH
3
NOH in which a double bond exists between N
and O, making the bond shorter:
P9.7 We can get an idea of the shapes by first looking at the Lewis
dot structures:
a. b.
c.
Pentane (structure a) has a straight chain structure and will be able
to pack more closely together. It should have the most intermolecu-
lar attraction and the highest boiling temperature. The other two
molecules are progressively more branched and can pack less tightly.
They will boil at lower temperatures.
P9.8 The molecular orbital diagram for He
2
is shown here, including
only the valence electrons (1s
2
) from each He atom. Note that all the
molecular orbitals are filled; equal numbers of bonding and anti-
bonding orbitals are filled. The bond order will be zero, and He
2
is
not stable.
P9.9 The molecular configuration for H
2
shows two valence elec-
trons in the bonding σ
1s
and none in the antibonding σ
∗
1s
. The bond
order for H
2
is only 1. Through promotion of a single bonding elec-
tron to an antibonding orbital, the bond order would be zero and
the molecular bond would be cleaved. The molecular configuration
for O
2
(using only the valence 2s and 2p electrons, like the F
2
dia-
gram) shows eight bonding electrons and four antibonding elec-
trons, giving a bond order of 2. There are several possibilities for
single photon absorptions. A transition from the π
∗
to σ
∗
MO causes
no change in the bond order. A transition from the π to π
∗
MO de-
creases the bond order to 1, because this transition moves a bonding
electron into an antibonding orbital. In either case, the bond order
in oxygen remains higher.
1. a. The dashed line doesn’t tell anything about the bond length,
strength, or energy; it merely indicates that the connected atoms are
bound in some manner. (It also doesn’t tell anything about specific
bond angles.); b. Using better models for bonding allows chemists to
understand more fully the interaction between chemicals and how
molecular shapes, bond strength, and polarity play a role in those
interactions. 3. 1s
2
2s
2
2p
6
3s
2
3p
2
; two bonds, not consistent
σ
1s
σ
1s
1s 1s
*
HC
H
H
H H
C
C
C
H
H
H
H
C
H
H
H
HC
H
H
C
H
H
C
H
H
CH
H
H
CH
H
HHC
H
H
C
H
H
C
H
H
C
H
H
C
H
H
HCN
H
O
H
5. Cl: 1s
2
2s
2
2p
6
3s
2
3p
5
, one; Se: 1s
2
2s
2
2p
6
3s
2
3p
6
4s
2
3d
10
4p
4
,two;
B: 1s
2
2s
2
2p
1
,one 7. 1s
2
2s
2
2p
2
; Carbon has only two unpaired elec-
trons (2p
x
1
2p
y
1
) to use in the formation of two covalent bonds. With
only two electrons to form bonds, carbon cannot form the four
bonds in CCl
4
. 9. a. H: 1s
1
;Br:1s
2
2s
2
2p
6
3s
2
3p
6
4s
2
3d
10
4p
5
; b. H: 1s;
Br: 4p; c. This bond should be weaker than the HOF bond because
the overlap of the much larger Br orbital with the small H orbital
creates a weaker interaction and a weaker bond. 11. Because each
chlorine atom has a configuration of 1s
2
2s
2
2p
6
3s
2
3p
5
, one 3p orbital
from each Cl is involved in creating the covalent bond. These orbitals
are exactly the same size and energy, so the amount of overlap in the
bond is substantial. 13. Cl
2
;Br
2
;HBr 15. The 1s orbital on
H(1s
1
), the 3p orbital on Cl (1s
2
2s
2
2p
6
3s
2
3p
5
), and the 2p orbitals on
O (1s
2
2s
2
2p
4
) are likely candidates for bonding in HOCl. (Using the
hybridization model, we realize that the sp
3
orbitals on oxygen are
used in the molecule.) Because the same bonds are used by O in the
bonds with H and Cl, the size of the orbitals on H (smaller) and
Cl (larger) predict a larger OOCl bond. 17. KH; KH should have
the shorter and stronger bond because the 4s orbital on K is smaller
than the 6s orbital on Cs. 19. The milk represents the s orbital and
is involved in all hybridization schemes. The eggs represent the p or-
bitals and can be mixed with the milk such that one part milk com-
bines with one, two, or three eggs.The character of the omelet repre-
senting the hybrid orbitals is a little different in each case, but all are
similar in that each is still an omelet. Also, the size of the omelet in-
creases (as does the size of the hybrid orbital) as the number of eggs
(p orbitals) increases. 21. a. six; b. We will still finish with generally
the same shape for the hybrid orbital as for sp
3
; however, the pres-
ence of more orbitals causes the angles between them to decrease to
90°, with an overall octahedral shape. Additionally, because the d or-
bitals are larger than the s or p orbitals, the sp
3
d
2
hybrid orbitals will
tend to be longer than any of the sp hybrid types. 23. a. , sp
3
;
b. tetrahedral 25. H
2
O = ~104.5°; NH
3
= ~107°; CH
4
= 109.5°
27. The 3p orbitals used in the bonding by Cl are longer and larger
than the 1s orbitals in H. The lengthening of the three COCl bonds
relative to the COH bonds changes the tetrahedral shape. Addition-
ally, the ClOCOCl bond angles may increase slightly to accommo-
date the larger Cl atoms relative to H. 29. two 31. two, three, four
33. a. sp
3
; b. sp
3
; c. sp
2
; d. sp 35. a. linear; b. trigonal planar;
c. tetrahedron; d. trigonal bypyramid; e. octahedron
37.
p orbital
forms
π bond
sp
2
hybrids
form σ bonds
C
2
H
4
Two p orbitals
form
π bonds
sp hybrids
form σ bonds
C
2
H
2
Si
Chapter 9 A31
The least amount of energy is required to break the bond in C
2
H
6
because it has only a single bond between the carbon atoms.
39. a. sp
2
, trigonal planar; b. sp
3
, trigonal pyramidal; c. sp
3
d
2
, octahe-
dral; d. sp
3
, ring/crown 41. a. sp
3
, tetrahedral; b. sp
3
d
2
, square pla-
nar; c. sp
3
d, see-saw; d. sp
2
,bent 43. For atoms bound to more than
one other atom: O, sp
3
; left C, sp
2
; right C, sp
3
;N,sp
3
45. Both Li
2
and K
2
will have the same bonding (because both have the same va-
lence configuration of one s electron); however, the orbitals on K are
larger. The overlap of the atomic orbitals from K in forming the co-
valent bond will be less than that on Li. K
2
would have the weaker
bond. 47. sp
3
, <109.5° 49. six, sp
2
, 120° 51. left N: one lone pair,
120°, sp
2
; right N: one lone pair, 109.5°, sp
3
53. sp
3
, 109.5°
55. a.
b. two; c. five; d. two
57.
59. Because the p orbitals on sulfur are larger, the bond distance in S
2
is larger. Because the atoms are further apart than oxygen, the p
orbitals are not able to create a good overlap to create the pi bond.
61. The greatest difference between bonding and antibonding or-
bitals is that bonding orbitals do not have a node perpendicular to
the bond axis, whereas antibonding orbitals do. The bonding orbitals
also have lower energies than antibonding orbitals made from the
same atomic orbitals. 63. a. LCAO stands for linear combination of
atomic orbitals. It is the method by which the molecular orbital the-
ory creates its orbitals. The electron densities of the atomic orbitals
S
2
O
2
Model Scientist General Summary
VSEPR G. Lewis Distribute bonding and
nonbonding electron pairs.
VB L. Pauling Overlap and hybridization
create new orbitals.
MO E. Schrödinger Subtract and add overlap to
create π and σ bonds.
C C
H
C
C
CH
3
O
sp
3
CH
C
H
N
H
C
HHHH
C
H
C
HH
CCH
3
CH
H
C
CH
3
C
C
O
H
C
HH H
HO
sp
3
hybrids
form σ bonds
C
2
H
6
can be added or subtracted to create the new molecular orbitals;
b. HOMO stands for highest-energy occupied molecular orbital. It is
the orbital that has the highest energy among those orbitals that con-
tain electrons; c. LUMO stands for lowest-energy unoccupied molec-
ular orbital. It is the orbital that has the lowest energy among those
orbitals that do not contain electrons. 65. a. The bond orders for
the molecules shown below are as follows: N
2
−
, 2.5; N
2
, 3; and N
2
+
,
2.5; b. N
2
−
and N
2
+
; c. N
2
< N
2
−
≈ N
2
+
67. a. Ne; b. paramagnetic; c. 1.5 69. seven sigma bonds, two pi
bonds 71. a. 1; b. 1.5; c.1 73. a. 3; b. none 75. a. Orbital over-
lap occurs when the orbitals on two adjacent atoms share some space
in common. When this occurs, the orbitals are free to combine and
make bonds (or antibonds);
b.
77. a. The Lewis dot structures (or skeleton structures) cannot show
the single average structure that truly exists for molecules that have
resonance structures. Some molecules will have bonds of order 1.5
or 1.33 that are difficult to draw without the resonance structures;
b. This resonance structure has alternating positions for the double
bonds inside the ring.
79. sp 80. 1-2-3 and 2-3-4 are both 180°. 81. 3 pi bonds, thirteen
sigma bonds 82. carbon atoms 1 and 6 87. a. The carbons in the
ring and the carbon attached to the ring have delocalized electrons;
b. sp
2
; c. p orbitals 89. All carbon atoms are sp
2
.
C
C
C
C
H
H
C
C
H
H H
H
C
H
C
C
C
H
C
C
C
C
O
H
H
C
O
C
O
H
H
C
H
O
C
C
H
H
H
s
–
s
overla
p
s
–
p
–
overla
p
p
–
p
–
overla
p
σ
2p
σ
2p
σ
2s
σ
2s
*
*
π
2p
π
2p
*
N
2
–
N
2
N
2
+
A32 Answers to Practice Exercises and Selected Exercises
91. In the minimum-energy states: a. two; b. none; c. two; d. four
93. a. sp
3
hybridization, tetrahedral shape for orbitals, molecule has
trigonal pyramidal geometry; b. sp
3
d
2
hybridization, octahedral
shape; c. sp
3
hybridization, tetrahedral shape for orbitals, molecule
has bent geometry; d. sp
3
d hybridization, trigonal bipyramid shape
95. NO
2
−
: Nitrogen in both has sp
2
hybridization. Because nitrite has
a lone pair in one of the sp
2
orbitals, the O—N—O bond angle in ni-
trite is reduced from the normal 120° that is found in the nitrate ion.
97. Each carbon in octane will be sp
3
hybridized with tetrahedral
geometry and form a straight chain of carbons. Each C—C bond will
be free to rotate, and the separate molecules can arrange themselves
to closely associate with each other, making the formation of a solid
more likely. 2,2,3,3-Tetramethylbutane has a branched structure.
Each carbon is still sp
3
hybridized, but the center two carbons have
three additional carbons attached to each. The molecule will be like a
large ball, with little possible change in its geometry. This geometry
makes close association between different molecules more difficult
and the process of boiling easier. Octane will not boil as easily as
2,2,3,3-tetramethylbutane.
99. The structure of NH
2
CHO is . The carbon
is sp
2
hybridized (three sigma bonds, no lone pairs) and will be trig-
onal planar. Nitrogen is sp
3
hybridized (three sigma bonds, one lone
pair) and will be tetrahedral. Because the carbon is sp
2
hybridized,
the atoms connected to carbon lie in a single plane, but nitrogen has
tetrahedral geometry, so its two hydrogens are not in the same plane
as the rest of the molecule. The overall molecule is not planar and
will be puckered. 101. a. COC; b. 3.98 × 10
−19
J; c. 239 kJ/mol;
d. blue-green. 103. a. C
18
H
34
O
2
+ H
2
n C
18
H
36
O
2
; b. 3.2 g; c. The
polar end ( ) will interact with the polar water and point down.
104. a.
b. trigonal pyramidal
around each N; c. sp
3
; d. This compound is unlikely to have a color
because it does not have a low-lying orbital to which an electron may
make a transition. The sp
3
hybridization scheme has all four orbitals
used in bond making or holding an electron pair. No empty pi or pi
∗
are present; e. N
2
H
4
+ O
2
n N
2
+ 2H
2
O
H = –590 kJ/mol.
Chapter 10
P10.1 6.7 m/s
2
P10.2 0.979 atm; 744 torr; 0.992 bar; 9.92 ×10
4
Pa
P10.3 1.2 ×10
2
torr P10.4 0.75 mol P10.5 1.3 L P10.6 522 K
P10.7 It is polar and experiences attractive intermolecular forces.
P10.8 0.34 L P10.9 0.036 mol P10.10 0.734 atm P10.11 O
2
:
ideal, 24.4 atm; van der Waals, 23.9 atm; NH
3
: ideal, 24.4 atm,
van der Waals, 21.2 atm P10.12 46.1 g/mol P10.13 53.7 g
P10.14 18.5 kg P10.15 If the masses of two particles are different,
but the particles have the same kinetic energy, their velocities must
be different to offset the difference in mass. As the mass increases,
the velocity must decrease in order for the kinetic energy to remain
the same. P10.16 F
2
P10.17 H
2
effuses 1.4 times as rapidly
as He.
1. These forces are collectively known as van der Waals forces and are
very small because of the large distances between the molecules and
the rapid velocities. 3. High temperature and low pressure
N
H
H
N
H
H
C
OH
O
HN
H
H
C O
5. 8.7 N 7. 8.4 × 10
2
N 9. 1.06 × 10
5
Pa; 106 kPa; 1.05 atm;
797 torr; 1.06 bar; 31.4 in Hg 11. 4.9 lb 13. 3.25 × 10
3
lb
15. 32.2 psi, 2.19 atm 17. 5.5 × 10
2
mm Hg 19. N
2
: 0.656 atm;
O
2
: 0.574 atm 21. 758 mm Hg 23. Ne: 0.306 mm Hg; He: 3.12
mm Hg 25. 9.0 mol, 1.8 mol 27. 5.32 L, 10.1 L 29. 0.847 L, 1.34 L
31. 10.5 L 33. 594 K 35. 0.12 L 37. 0.144 mL
39. a. 0.08205 L·atm·mol
−1
·K
−1
, 8314 L·Pa·mol
−1
·K
−1
; b. 62.36
L·mm·Hg·mol
−1
K
−1
; c. 1.206 L·psi·mol
−1
·K
−1
41. 3.8 × 10
7
L
43. 22.4 L, 51.2 L 45. 0.193 mol 47. 17.1 g/mol 49. 0.755 g/L
51. 2.06 × 10
21
molecules 53. 114 g/mol 55. 1.41 ×10
3
g/mol, No
57. 271 × 10
4
K 59. 8.47 L 61. 12.6 g/L 63. 0.306 atm
65. H
2
is the limiting reagent, 1.33 L 67. 33.9 g/mol 69. F
2
71. 529 L 73. According to the kinetic-molecular theory, the aver-
age kinetic energy of a particle is directly proportional to the tem-
perature. The pressure of a gas inside the cylinder depends on both
the number of collisions with the walls of the container and the
speed of the molecules. As the temperature increases, the speed
increases and both the number and the velocity of the collisions
increase; therefore, the pressure is directly proportional to the
temperature. 75. a. same; b. Cl
2
< ClO
2
; c. ClO
2
; d. ClO
2
77. a. 4.4 × 10
−20
J; b. 1.7 × 10
−20
J; c. 2.0 × 10
−20
J 79. Ar < C
2
H
6
< H
2
81. 411 m/s 83. CO
2
: 376 m/s; H
2
O: 588 m/s 85. Point 1:
The marbles do not have negligible size relative either to the con-
tainer or to the distance between marbles. Point 2: Unless the mar-
bles are dirty and sticky, this should be true because the marbles will
not be strongly attracted to each other. Point 3: If the marbles are
continuously shaken during the demonstration, this should be true.
Point 4: This should also be true during the demonstration if the
marbles are continuously shaken. As the piston is moved, the num-
ber of particle collisions per surface area will change. A smaller vol-
ume will correspond to larger pressures. Point 5: This will be approx-
imately correct. There will be some small energy loss (due to friction
and collisional heating) as the marbles move and collide with each
other and the container. Point 6: This will not be true in the analogy,
but more violent shaking could be applied to show higher tempera-
tures. 87. Xe < SO
3
< CO
2
< N
2
89. 72.7 g/mol 91. Ar 93. Be-
cause the masses are not exactly the same (CO
2
= 44.01 g/mol and
C
3
H
8
= 44.09 g/mol), there will be a slight difference in the effusion
rates. It would be possible, though impractical, to try to separate
these molecules via effusion. 95. 16 cm from the HCl end
97. 0.57% faster 99. a. 1.57 × 10
13
L; b. 1.97 × 10
13
L; c. 1.43 × 10
6
metric tons; d. 1.09 × 10
12
mol 101. 4.75 × 10
24
molecules
103. ideal: 21.8 atm; van der Waals: 22.1 atm 105. ∆T = 894 K;
V
final
= 0.916 V
initial
107. 91.4 g; 338 balloons 109. One of the
considerations is which property is easier to manage: a temperature
of only 20 K or a high pressure of 400 atm. Each requires a different
infrastructure to handle. High pressures require thick walls with
excellent welding, whereas low temperatures require vacuum dewers
(like a thermos), and any variation in temperature causes the hydro-
gen to boil and be lost. 110. a. dispersion only; b. hydrocarbons
behave ideally except at high pressures or low temperatures;
c. 0.0123 mol; d. C
3
H
8
; e. 0.453 L
Chapter 11
P11.1 The size of the London dispersion force between the mole-
cules increases as the size of the molecule increases. Therefore, the
forces between the molecules of dodecane are much larger than the
forces between hexane molecules, which in turn are much larger
than the forces between propane molecules. The larger the force, the
closer the molecules and the stronger they will be held together.
Dodecane is so strongly held that it forms a solid, and hexane is held
strongly enough to cause it to condense to a liquid at room tempera-
ture. P11.2 Propylene glycol has a higher boiling point because the
intermolecular forces will be higher. P11.3 Any molecule with
greater forces will have a higher boiling point and a lower vapor
Chapter 11 A33
pressure. Examples include ethylene glycol and propylene glycol.
P11.4 Hexane should have lower overall forces than acetone and
is likely to have the higher vapor pressure. P11.5 440 kJ
P11.6 80°C; Yes, lower external pressures make for lower boiling
temperatures. P11.7 CCl
4
and I
2
P11.8 47.1 g KOH,
χ
water
= 0.975 P11.9 1.33 × 10
−7
M P11.10 No, 6.4 mL
P11.11 122.5 torr P11.12 a. 101.0°C; b. 100.7°C; c. 67.1°C
P11.13 a. 2.45 atm; b. 27.5 atm; c. 91.7 atm
1. Intermolecular forces are between molecules, while intramole-
cular forces are between atoms in a molecule and are stronger.
3. Intermolecular forces are due to the attraction of positive and
negative charges, partial to full in magnitude, between molecules.
5. S—H
—
—
C—H < N—H < O—H 7. a. intermolecular;
b. intramolecular; c. intermolecular; d. intramolecular,
intermolecular 9. 0.101 nm, stronger and intramolecular; 0.175,
intermolecular 11. Larger forces lead to a higher boiling point.
a. pentane, less compact and larger dispersion forces; b. cyclohexane,
larger dispersion forces on rings than on chains; c. hexane, larger
mass and dispersion forces; d. water, stronger forces (hydrogen bond-
ing); e. pentane, larger mass and dispersion forces 13. a. B → O;
b. P → Cl; c. H → O 15. a. dispersion, dipole–dipole, hydrogen
bonding; b. dispersion; c. dispersion; d. dispersion, dipole–dipole,
hydrogen bonding 17. a. CF
4
; b. H
2
O 19. a. yes; b. yes; c. no,
lacks O, F, or N; d. no, lacks H bound to O, F, or N 21. a. C—O
bonds; b. dispersion, dipole–dipole 23. hexane, because lower in-
termolecular forces (only dispersion versus hydrogen bonding in
methanol) give a higher vapor pressure 25. Kr, because it has the
largest mass (and number of electrons) and is most polarizable, giv-
ing larger dispersion forces 27. a. As the central atom becomes
larger and more polarizable, the intermolecular forces increase.
Higher forces lead to increasing boiling point, which is the trend
seen down the group; b. It is the most polar and can hydrogen-bond.
29. The forces in water, including hydrogen bonding, are much
larger than in methane, leading to higher energy requirements.
31. 2.5 × 10
6
J 33. a. dispersion; b. gas; c. It is pressurized. 35.
The H—Te bond is not polar enough to allow for hydrogen bonding.
37. 48.0 kJ 39. a. 231 K; b. 0.75 atm, 224 K; c. gas 41. gas to solid
43. liquid to solid 45. As the pressure increases, the gas will become
a liquid at 1.4 atm. 47. See the accompanying diagram; note that
the pressure is plotted on a logarithmic scale.
49. Surface tension is a measure of how strongly the molecules of a
substance interact and “pull” the surface molecules toward the cen-
ter. Viscosity is a measure of how strongly the molecules interact and
prevent the flowing of one molecule past another. Capillary action is
a balance of two interactions: that of the substance with its container
and that of the substance with itself. Molecules capable of strong
intermolecular interactions often have strong interactions with other
surfaces. 51. The figure should show a convex surface (curved
upward). 53. From least to most viscous: gasoline, water, honey.
0.0001
0.001
0.01
0.1
1
10
100
–200 –100 0 100
Temperature (°C)
Ethylene Phase Diagram
Pressure (atm)
Solid
Liquid
Gas
The order is the same as the increase in intermolecular forces—
larger forces make for higher viscosity. 55. The forces (and viscos-
ity) are high because the molecules are large, with correspondingly
large dispersion forces. 57. a. positive; b. 49 g per100 g water
59. Water is able to dissolve many different sizes and shapes of mole-
cules, from very small salts to very large protein molecules.
61. a. yes; b. There is additional stability as a consequence of mixing.
63. A-water; B-heptane 65. a. Solubility has limits, whereas misci-
bility is possible in any combination; b. They share type and size of
intermolecular forces. 67. a. 2.10 M; b. 0.222 M; c. 4.16 M
69. a. 2.01 m; b. 0.310 m; c. 0.217 m 71. a. 0.266; b. 0.0555;
c. 0.00318 73. NH
4
Cl: 0.0424, 2.46 M; KNO
3
: 1.96 g, 0.00278;
C
6
H
12
O
6
: 325 g, 7.21 M 75. 0.037%, 2.1 × 10
−3
M,
1.9 × 10
−3
m 77. 0.873 M 79. a. 0.0130%; b. 1.30 × 10
5
ppb
81. a. left; b. right 83. 5.1 × 10
−4
M 85. 0.00300 M
87. pentane > water > glycerol 89. a. 100.76°C; b. 100.02°C;
c. 100.39°C; d. 89.1°C 91. a. –0.93°C; b. –0.20°C; c. 42.1°C;
d. –2.23°C 93. a. 3.66 torr; b. 3.9 torr; c. 4.01 torr; d. 4.39 torr
95. The vapor pressure is the equilibrium position at a specific tem-
perature and does not depend on the surface area of the liquid. The
area of the liquid affects how quickly that equilibrium can be
reached, but the final value of the vapor pressure will be the same
whether the liquid is in a cup or forms an ocean, as long as the con-
tainer is closed. 97. All these solutions have the same number of
particles dissolved, whereas electrolytes may produce more or fewer
ions than others. 99. 5340 g
101. a.
b. If the solution and body fluid do not have the same osmotic pres-
sure, cell damage from shrinkage or rupture will result. 103. A hy-
drogen bond is formed between a hydrogen on O or N and a second
O or N without a hydrogen.
105. 0.868 g 107. CHBr
3
because of its larger dispersion forces and
dipole–dipole interactions 109. Boiling occurs when the vapor
pressure of the liquid matches the external pressure. Turning up the
gas will not change the external pressure but only cause the boiling
to be more vigorous.
111. 5.73 M 113. a. 6; b. Yes, C
n
H
2n+2
; c. dispersion only
115. a. vaporization of water; b. 3.9 × 10
2
L; c. 688 kJ
116. a.
b. dispersion forces;
c. yes, because the dispersion forces are small; d. no; e. 0.301 mol;
f. The gas is under pressure, raising its boiling point; g. Pentane boils
higher than butane, and propane boils lower than butane.
HCC
H
H
C
H
H
C
H
H
H
H
H
H
C
O
N
C
NH
CH
N
H
H
C
H
O
C
C
N
C
N
H
N
H
N
HC
N
H
A34 Answers to Practice Exercises and Selected Exercises
Chapter 12
P12.1
P12.2
C
CC
C
CC
H
H
H
H
H
H
H
H
H
H
H
H
CH CH
2
CH
3
CH
2
CH
3
CH
3
CH
2
CCH
2
CH
3
CH
2
CH
3
CH
3
CH
3
CHCH
2
CH
3
CH
3
CH
2
CH
2
CH
3
CCH
2
CH
3
CH
3
CH
2
CH
3
CH
3
CHCH
3
CH
3
CH
3
CH
2
CH CH
3
CHCH
3
CH
3
CH
2
CH
2
CH
2
CH
3
CCHCH
3
CH
3
CH
3
CH
3
CH
3
CH CHCH
3
CH
3
CH
3
CH
2
CH
3
CH
2
CH
3
CH
2
CH
2
CH
2
CH
2
CH
3
P12.3
P12.4
P12.5
P12.6
Benzyl ethanoate
CH
2
CH
C
CH
3
O
C
CH
CH
CH
CH
O
Ethyl benzoate
C
O
CO
CH
2
CH
3
CH
CH
CH
CHCH
H
NH
2
H
CC
HH
C
C
H
H
C
CHO
OH
Carboxylic
acid
Aromatic ring
A
lcohol
Amine
C
O
CC
H
C
CH
CH
2
CH
2
CH
3
CH
3
CH
3
CH
2
CH
2
CH
3
CH
2
CH
2
CH
3
CH
2
CH
2
C
CH
3
CH
3
CH
3
C
CH
CH
3
CH
3
CH
2
CH
CH
CH
3
CH
3
CH
2
CH
2
CH
CH
3
CH
2
cis
CH
3
CH
CH
CH
3
CH
2
CH
3
CH
CH
CH
3
CH
2
trans
Chapter 12 A35
P12.7 Left molecule: second carbon from left; right molecule: carbon
at the lower right P12.8 Yes, m-amsacrine will attack the DNA in
healthy cells also.
P12.9
1. diamond (sp
3
), graphite (sp
2
), fullerenes (sp
2
) 3. In diamond, all
of the carbons are sp
3
hybridized (tetrahedral) and form an extensive
three-dimensional network of carbon atoms bound together. The
carbon atoms in graphite are all sp
2
(recall that sp
2
atoms form trigo-
nal planes) hybridized and form extended planar surfaces that are
only weakly bound via van der Waals forces between the planes.
5. 2.01 × 10
22
atoms 7. Aliphatic compounds are those with no
double or triple bonds, while the aromatics are characterized by
structures with several multiple bonds. 9. Because many biological
compounds (proteins and others) contain sulfur and the source of
the crude oil is those biological materials, many compounds result-
ing from the breakdown of this material also contain sulfur.
11. a. 2-methyloctane; b. 2-methylpentane; c. 3-ethylpentane;
d. pentane
13. a.
b. c.
d.
15.
Alkane Alkene Alkyne
COC CPCCqC
sp
3
sp
2
sp
17. a.
C
CH
3
CH
3
CH
3
CH
3
2,2-Dimethylpropane
2-Methylbutane
CH
3
CH
2
CH
CH
3
CH
3
CH
3
CH
2
CH
2
CH
2
CH
3
Pentane
CH
2
CH
3
CH
2
CH
2
CH
3
CH
2
CH
3
CH
2
CH
2
CH
CH
3
CH
3
CH
2
CH
3
CH
CH
2
CH
3
CH
3
CH
2
CH
2
CH
2
CH
2
CH
CH
2
CH
3
CH
3
CH
3
O
O
O
S
N
OH O
OH
Ketone
Alcohol
Alcohol
Ester
Alkene
Ether
Each molecule is C
5
H
12
; b. pentane (36°C), 2-methylbutane (28°C),
2,2-dimethylpropane (9.5°C) 19. C
28
H
58
21.
23. C
6
H
12
and C
7
H
14
25. left: trans; right: cis 27. CaC
2
+ 2H
2
O →
Ca(OH)
2
+ C
2
H
2
29. Cyclohexane: a. 12 H; b. sp
3
; c. 109.5°;
d. no double bonds; benzene: a. 6 H; b. sp
2
; c. 120°; d. three double
bonds;
e.
31. During a “run” of the gas chromatograph, a sample is injected
into the instruments and heated to vaporize the sample. A carrier gas
takes the sample into and through a column. Inside the column, the
different molecules interact to varying extents with the materials
C
CC
C
CC
HH
HH
C
CHHC
C
CC
HH
HH
HH
CH
3
CH
2
CH CH
CH
2
CH
3
CH
3
CH
3
CH
3
CH
2
CH CH
2
CH
CH
3
CH
3
CH
3
CH
3
CH
CH CH
2
CH
2
CH
3
CH
3
CH
3
CH
3
CH
2
CCH
2
CH
2
CH
3
CH
3
CH
3
CH
3
CH
CH
2
CH
3
CH
3
CH
2
CH
3
CH
3
C
CH
2
CH
2
CH
3
CH
2
CH
3
CH
3
CH
3
CH
CH
2
CH
2
CH
2
CH
2
CH
3
CH
3
A36 Answers to Practice Exercises and Selected Exercises
inside the column. The greater the interaction, the more slowly the
molecules move through the column. The molecules are separated
by being selectively slowed on the basis of their properties and are
later detected upon exiting the column. 33. Boiling temperature;
Larger and straighter molecules have higher boiling points.
35. C
8
H
18
;2C
8
H
18
+ 25O
2
→ 16CO
2
+ 18H
2
O 37. C
7
H
16
39. 87% isooctane and 13% heptane 41. CH
4
+ Br
2
→CH
2
Br
2
+ H
2
43.
45. During complete combustion: H
2
O and CO
2
47. −2.10 ×10
3
kJ
49. a. b.
CH
3
OH, CH
2
O,
c. d.
C
2
H
4
,CH
3
COCH
3
,
51.
53.
C
H
H
H
H
CC
H
C
H
OC
C
O
H H
C
H
C
H
O
O
O
C
O
H
H
H
H
H
H
C
PS
S
H
H
H
C
COHNC
C
CC
HH
HH
CC
H
H
HO
H
Amide
Aromatic
Alcohol
C
O
CH
3
CH
3
CC
H
H
H
H
CO
H
H
COH
H
H
H
Hexachloroethane
C
Cl
C
Cl
Cl
Cl
Cl
Cl
Pentachloroethane
C
Cl
C
Cl
Cl
H
Cl
Cl
1,1,2,2-Tetrachloroethane
C
Cl
C
H
Cl
H
Cl
Cl
1,1,1,2-Tetrachloroethane
C
Cl
C
Cl
Cl
H
Cl
H
1,1,2-Trichloroethane
C
Cl
C
H
Cl
H
H
Cl
1,1,1-Trichloroethane
C
Cl
C
Cl
Cl
H
H
H
Cl
1,2-Dichloroethane
C
Cl
C
H
H
H
H
C
Cl
C
H
1,1-Dichloroethane
H
H
H
Cl
C
Cl
C
H
H
H
H
H
1-Chloroethane
55. a. none; b. three; c. seven
57. a.
b.
59. Alkane, C
2
H
6
61. a. b. C
3
H
6
+ H
2
O → C
3
H
7
OH
63. a.
b.
65. The HDPE units are long and unbranched, allowing close
packing and high density, whereas the LDPE units are shorter
and more branched, leading to loose packing and low density.
67. 1,4-Pentanediol
69.
71.
73.
,
75.
, Propanal:
77.
, 2-Butanol:
79. CH
3
CH
2
COOH + H
2
O → CH
3
CH
2
COO
−
+ H
3
O
+
CH
3
CH
CH
2
OH
CH
3
CH
3
C
CH
2
O
CH
3
CH
3
CH
2
CH
O
CH
3
CH
2
C
O
OH
CH
3
CH
2
CH
O
CH
3
CH
2
CH
2
OH
C
COHHC
C
HH
CC
HH
CH
2
CH
2
CH
2
CH
CH
3
Secondary alcohol
HO
OH
Primary alcohol
CH
CH
2
CH
2
CH
3
CH
CH
2
CH
3
CH
CH
2
CH
3
CH
CH
3
CH
2
CH
3
CH
CH
2
CH
3
CH
CH
3
CH
CH C
CH
2
CH
2
CH
3
CH
2
CH
3
CH
3
CH
2
CH
3
C
CH CH
2
CH
3
CH
3
Chapter 13 A37
81.
83.
85.
87.
,H
2
O
89.
,NaCl
S
CH
2
CH
2
CH
2
O
CH
2
S
O
CH
2
CH
2
S
CH
2
CH
2
S
S
OCH
2
CH
2
CH
2
CH
2
S
C
O
NHCH
2
H
2
N C
O
OHC
O
CH
2
NHCH
2
CH
3
CH
2
CH
2
CH
2
CH
2
CH
CH
2
CH
2
CH
CH
CH
CH
2
CH
2
CH
2
CH
2
CCH
2
CH
2
OH
O
CH
3
CH
2
CH
2
CH
2
OH
CH
2
CH
3
CH
2
H
2
O
OH
C
CH
2
O
CH
3
CH
2
CH
2
CH
2
O
CH
2
CH
2
C
CH
2
CH
3
O
CH
3
CH
CH
2
CH
3
OH
CH
2
OH
C
CH
3
O
H
2
O
CH
3
CH
CH
2
CH
3
O
CH
2
C
CH
3
O
91. dimethyl ether and butyl methyl ether 93. a. Yes with designs, no without; b. yes; c. yes; d. no 95. In each, the carbon bound to the Br is
the chiral center. 97. 63% 99. Because of the different temperatures, the volatility of the different components changes. In order to maintain
similar vaporization and combustion characteristics, the components in the winter need to be more volatile. In the summer the volatility is de-
creased to prevent “vapor lock” problems that could arise if some of the formulation “boiled” in the gas lines.
101. a. 39 gal; b. 103. a. , pentane; , hexane; b. compounds are not ethers 105. a. reacts
with fibers, color; b. 600–630 nm; c. 1.9 × 10
2
− 2.0 × 10
2
kJ/mol
OH CH
3
2H
2
O
O
CH
3
O
CH
3
CC
O
O
CH
2
CH
2
CH
3
OH
CH
2
CH
2
CC
OH
O
OH
O
OO
O
CH
2
OCC
O
O
O
CC
O
O
CC
O
O
O O
CC
O
O
CH
2
CH
2
O
CH
2
CH
2
CH
2
CH
2
CH
2
106. a.
b. Polar molecule, hydrogen bonding, solid,
acidic; c. 61.6%;
d.
e.
CC
HO
O
OH
O
Chapter 13
P13.1 a. b.
c.
P13.2 47.6% P13.3 11.35 g/cm
3
P13.4 1306 nm
P13.5 metal, glass/ceramic, ceramic, metal (or alloy)
P13.6 composite (magnetic media on plastic), plastic, composite
(glass and plastic) P13.7 4.11 × 10
−7
mol; 4.15 × 10
4
layers for
1 g, 8.30 × 10
5
layers for 20 g; no
1. Crystalline solids have a regular arrangement of particles,
whereas amorphous solids exhibit a random arrangement of
particles. 3. A simple unit cell consists of atoms arranged within a
parallelepiped (often at the corners, centered inside or on the faces
and edges). The crystal lattice consists of rows and columns of the
parallelepiped (unit cells) connected at the corners.
5.
