Jackson M.J. Micro and Nanomanufacturing
Подождите немного. Документ загружается.


Microgrinding 261
6.2 Grinding Wheels
Grinding wheels are made up of two materials, the abrasive grains
and the bonding material (Fig. 6.3). They are produced by mixing
the appropriate grain size of the abrasive with the required bond and
pressed into shape. The abrasive grains do the actual cutting and the
bond holds the grain together and supports them while they cut. The
cutting action of a grinding wheel is dependent on bonding materi-
als,
the abrasive type, grain size (grit size), wheel grades, and wheel
structure. Selection of the right combination of these features is es-
sential for optimum solution of different grinding tasks.
6.2.1 Bond Materials
Bonds are usually made of different types of raw materials and are
classified as follows [21,22]:
i. Vitrified materials (ceramics consist of glass,
feldspar and clay),
ii.
Resinoid materials (thermoset plastics -
phenolformaldehyde resin),
iii.
Rubber (both natural and synthetic),
iv. Shellac,
v. Metal (sintered powdered metals and electroplated -
bronze, nickel aluminum alloys, zinc, etc.)
vi.
Oxychloride (chemical action of magnesium
chloride and manganese).
vii.
Silicate (sodium silicate NaSiOs or water glass)
viii.
No bond
Vitrified bonds are also called ceramic bonds [23,24], which al-
low porosity of up to 55%, and the rigidity of this bond makes it

262 Micro- and Nanomanufacturing
possible to obtain high stock removal rates. Some cBN wheels are
made from this kind of bond for grinding steel. Compared to vitri-
fied and metal bonds, resinoid bonds furnish more flexibility while
grinding and thus produce a finer surface than the two. Both resin
and metal bonds are commonly used in the manufacture of diamond
and cBN wheels. Because of the resiliency of rubber, it makes them
excellent bond materials for polishing wheels and is used where burr
and burn must be held to a minimum. Rubber bond is also used for
thin flexible cut-off wheel application [21,23,25] and regulating
wheel in centerless grinding [21]. Shellac-bonded wheels are good
for producing high finish required in roll, camshaft, and cutlery
grinding [22]. Oxychloride bonds are considered to be the weakest
bond among the grinding wheel and used particularly for disc grind-
ers.
The wheels are cool cutting and seldom produce burn. Silicate
bond wheel can be used in operations that generate less heat. It is not
as strong as vitrified. Among all, silicate, shellac, and oxychloride
have limited uses [23].
6.2,2. Abrasive Types
Abrasive grains used for grinding wheels are very hard, highly re-
fractory materials and randomly oriented. Although brittle, these
materials can withstand very high temperatures. They have the abil-
ity to fracture into smaller pieces when the cutting force increases.
This phenomenon gives the abrasive its self-sharpening effect. Dur-
ing grinding, whenever dulling begins, the abrasive fractures thus
create new cutting points. The following four types of abrasives are
commonly used:
i. Aluminum oxide, or alumina (AI2O3)
ii.
Silicon carbide (SiC)
iii.
Cubic boron nitride (cBN)
iv. Diamond

Microgrinding 263
Aluminum oxide and silicon carbides are known as conventional
abrasives while cBN and diamond are known as superabrasives.
Aluminum oxide wheel is usually used for grinding metals such as
carbon steel, alloy steel, high-speed steel, annealed malleable iron,
wrought iron and bronzes, and similar metals. On the other hand,
silicon carbide wheel is harder but more brittle than alumina and
commonly used to grind low tensile strength materials such as gray
iron, chilled iron, brass, soft bronze and aluminum, as well as
stone/marble, rubber, leather and other non-ferrous metals [26,27].
Diamond wheels are suitable for machining non-ferrous metal while
cBN is normally good for grinding ferrous metal. However, the lat-
ter also used for grinding titanium alloys and the performance is bet-
ter than SiC and AI2O3 wheels. Aluminum oxide wheel is often re-
placed by cBN for hardened steel (>45HRc), superalloys (nickel,
cobalt, or iron base with hardness greater than 35HRc), high-speed
steels and cast iron. cBN has four times the abrasion resistance of
aluminum oxide. The high thermal conductivity of cBN prevents
heat buildup and associated problems such as wheel glazing and
workpiece metallurgical damage [27]. Some properties of these
abrasives compared to hardened steel and glass are shown in Table
6.2
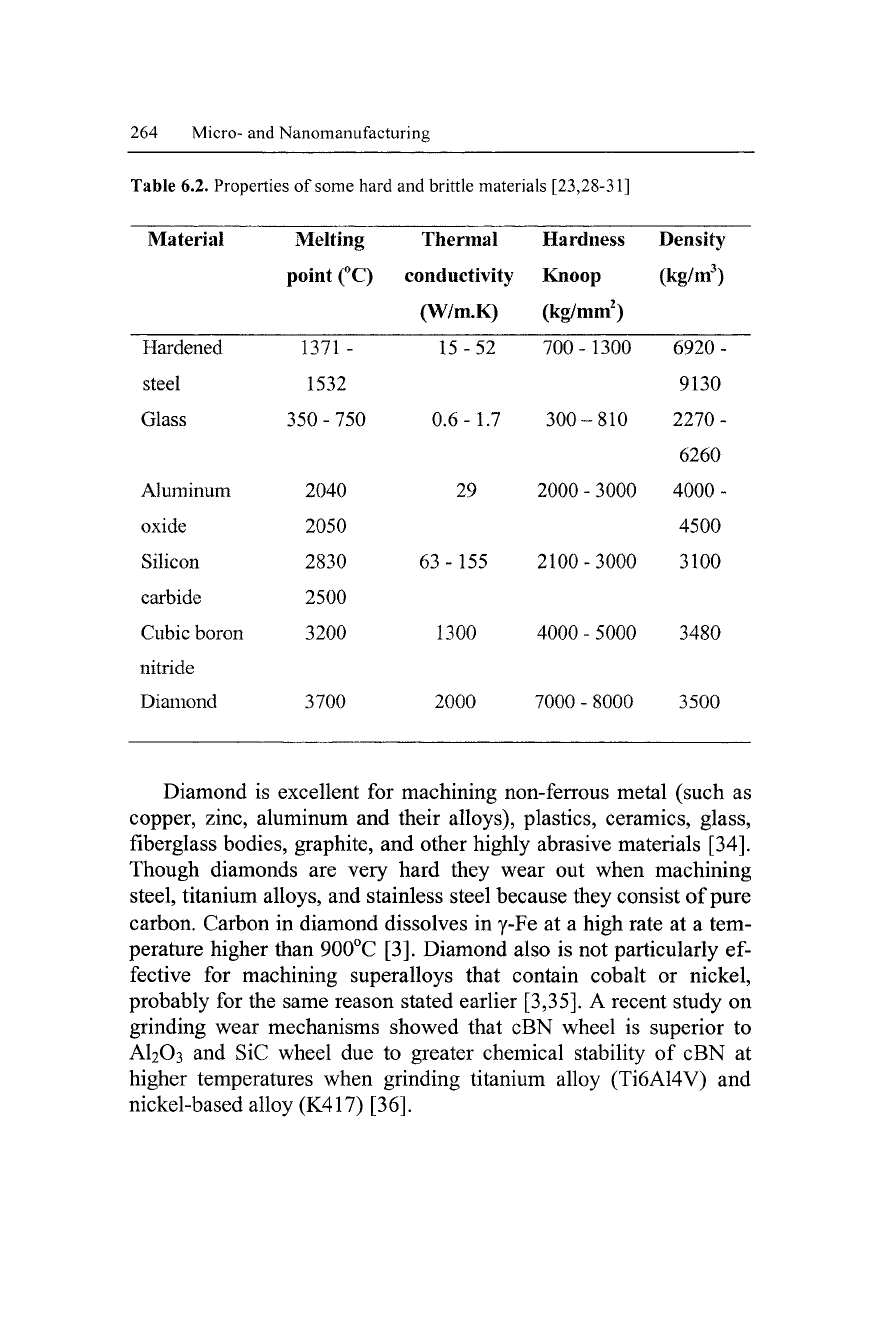
Table 6.2 shows that diamond has promising properties compared
to the three other abrasives. One of the unique properties of diamond
that stands out is its extreme hardness. The hardness of this material
gave it the greatest electrical resistance and thermal conductivity of
all known substances. Also, it is chemically inert. Chemical
inertness normally prevents the diamond from bonding to or reacting
with other substances [32]. For these reasons it is the most desirable
abrasive for many applications, but there are limitations to its
usefulness other than cost. The surface chemistry of diamond limits
it usefulness in certain conditions. Diamond is carbon and at high
enough temperatures will burn, or will react with carbide-forming
metals. If either event occurs to any significant extent, the diamond
is lost. The service conditions that are required to avoid such losses
are low temperatures and avoidance of carbide-forming metals
except at temperatures close to room temperature such as in lapping
and polishing operations. The high thermal conductivity of diamond
helps to relieve the problem by conducting heat away [33].
264 Micro- and Nanomanufacturing
Table 6.2. Properties of some hard and brittle materials [23,28-31]
Material Melting Thermal Hardness Density
point (°C) conductivity Knoop (kg/m
3
)
(W/m.K) (kg/mm
2
)
Hardened
steel
Glass
Aluminum
oxide
Silicon
carbide
Cubic boron
nitride
Diamond
1371-
1532
350 - 750
2040
2050
2830
2500
3200
3700
15-52
0.6-1.7
29
63 -155
1300
2000
700-
300-
2000
2100-
4000-
7000 -
1300
-810
-3000
-3000
-5000
•8000
6920-
9130
2270-
6260
4000-
4500
3100
3480
3500
Diamond is excellent for machining non-ferrous metal (such as
copper, zinc, aluminum and their alloys), plastics, ceramics, glass,
fiberglass bodies, graphite, and other highly abrasive materials [34].
Though diamonds are very hard they wear out when machining
steel, titanium alloys, and stainless steel because they consist of pure
carbon. Carbon in diamond dissolves in y-Fe at a high rate at a tem-
perature higher than 900°C [3]. Diamond also is not particularly ef-
fective for machining superalloys that contain cobalt or nickel,
probably for the same reason stated earlier
[3,35].
A recent study on
grinding wear mechanisms showed that cBN wheel is superior to
AI2O3 and SiC wheel due to greater chemical stability of cBN at
higher temperatures when grinding titanium alloy (Ti6A14V) and
nickel-based alloy (K417) [36].

Microgrinding 265
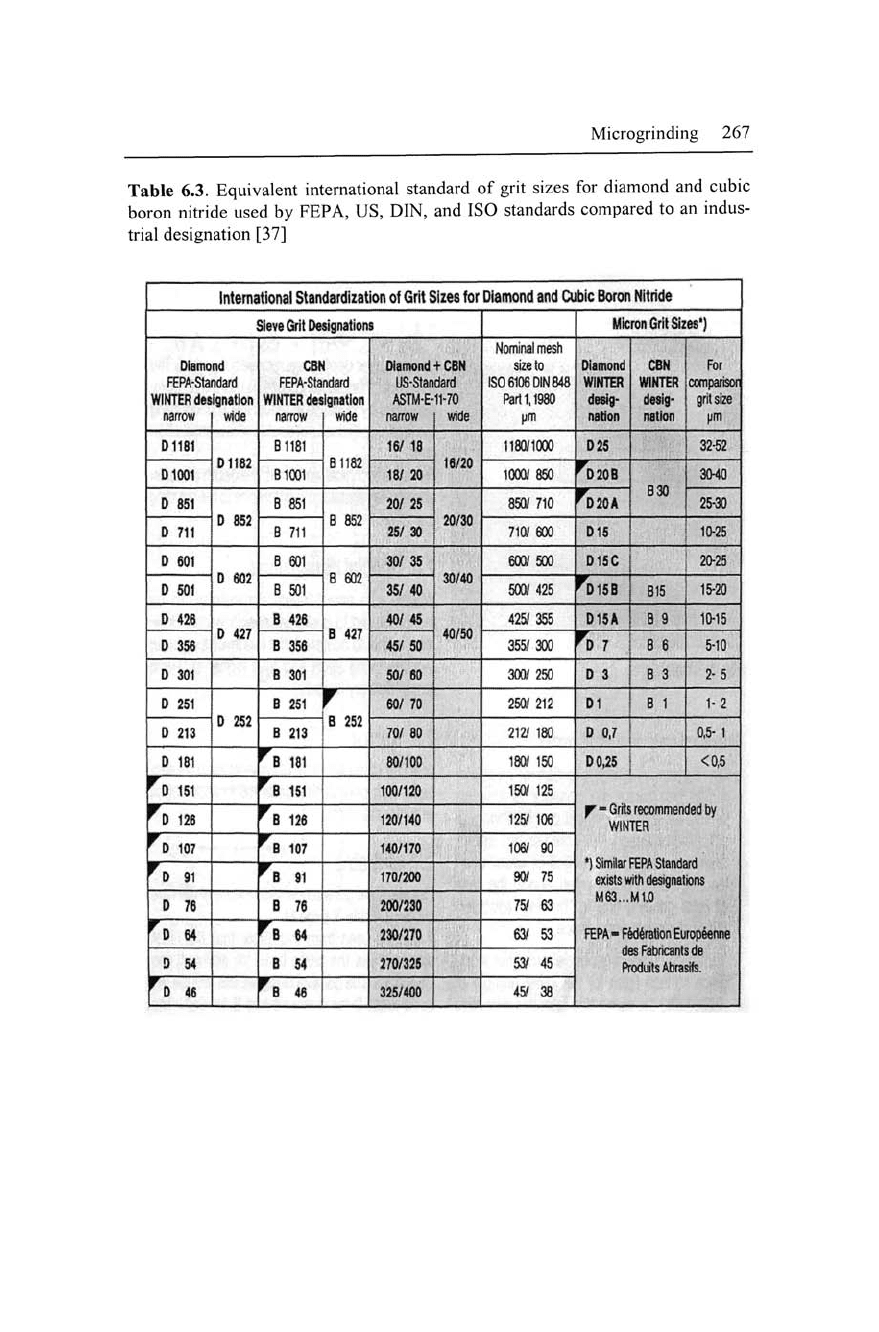
6.2.3 Abrasive Grain Size
The size of an abrasive grain is identified by a number, which nor-
mally is a function of mesh width of the sieve size either in micron
or mesh openings per inch. Table 6.3 shows the equivalent grain
size used by FEPA (micron), ASTM 11 (inches), ISO, and DIN (mi-
cron) standard both for diamond and cBN wheels. In the metric sys-
tem (microgram size), the smaller the number the smaller the grain
size.
However, the coding is reversed in the imperial system in
which smaller number represents coarser grain size.
6.2.4 Mechanical Grade
The grade of a grinding wheel refers to its strength in holding the
abrasive grains in the wheel. This is largely dependent on the
amount of bonding material used. As the amount of bonding mate-
rial is increased, the linking structure between grains becomes lar-
ger, which makes the wheel act harder. A hard wheel has a stronger
bond than a soft wheel. The type and the amount of bond in the
wheel also influence the overall strength. In standard marking sys-
tems,
the grade of the grinding wheel is labeled by A-Z (soft to
hard).
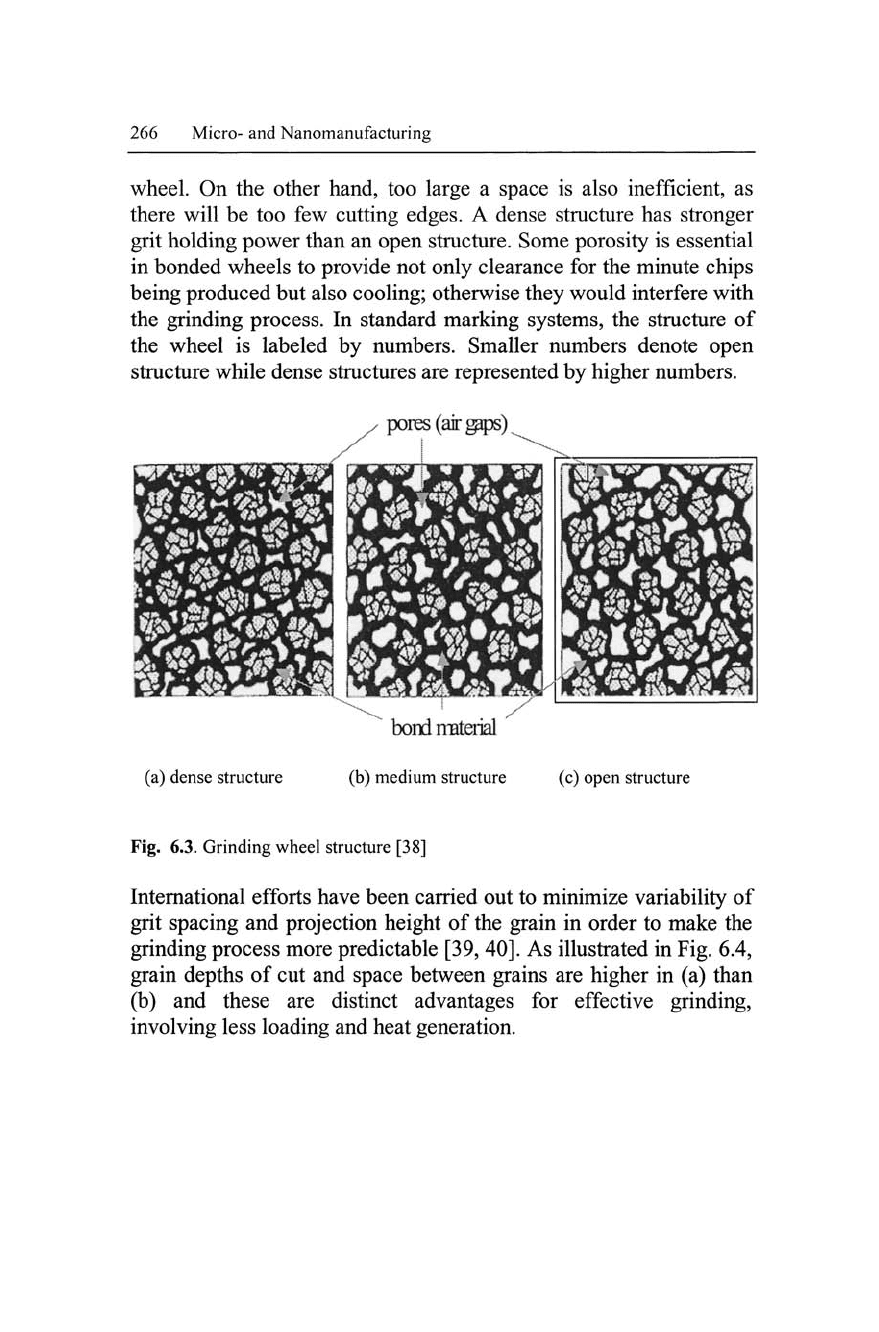
6.2.5 Abrasive Structure
The structure of a grinding wheel represents the grain spacing and is
a measure of the porosity of a bonded abrasive wheel. Figure 6.3 il-
lustrates the structure of a grinding wheel, showing bigger pore areas
(voids) in open structure than medium and dense structure. Porosity
allows clearance space for the grinding chips to be removed for
proper cutting action during grinding operations. This clearance
space must not be too small or the chip will stay in the wheel, caus-
ing what is called wheel loading. A loaded cutting wheel heats up
and is not efficient in its cutting action. When this happens, frequent
dressing is needed to remove loaded workpiece particles off the
266 Micro- and Nanomanufacturing
wheel. On the other hand, too large a space is also inefficient, as
there will be too few cutting edges. A dense structure has stronger
grit holding power than an open structure. Some porosity is essential
in bonded wheels to provide not only clearance for the minute chips
being produced but also cooling; otherwise they would interfere with
the grinding process. In standard marking systems, the structure of
the wheel is labeled by numbers. Smaller numbers denote open
structure while dense structures are represented by higher numbers.
" bond material
(a) dense structure (b) medium structure (c) open structure
Fig. 6.3. Grinding wheel structure [38]
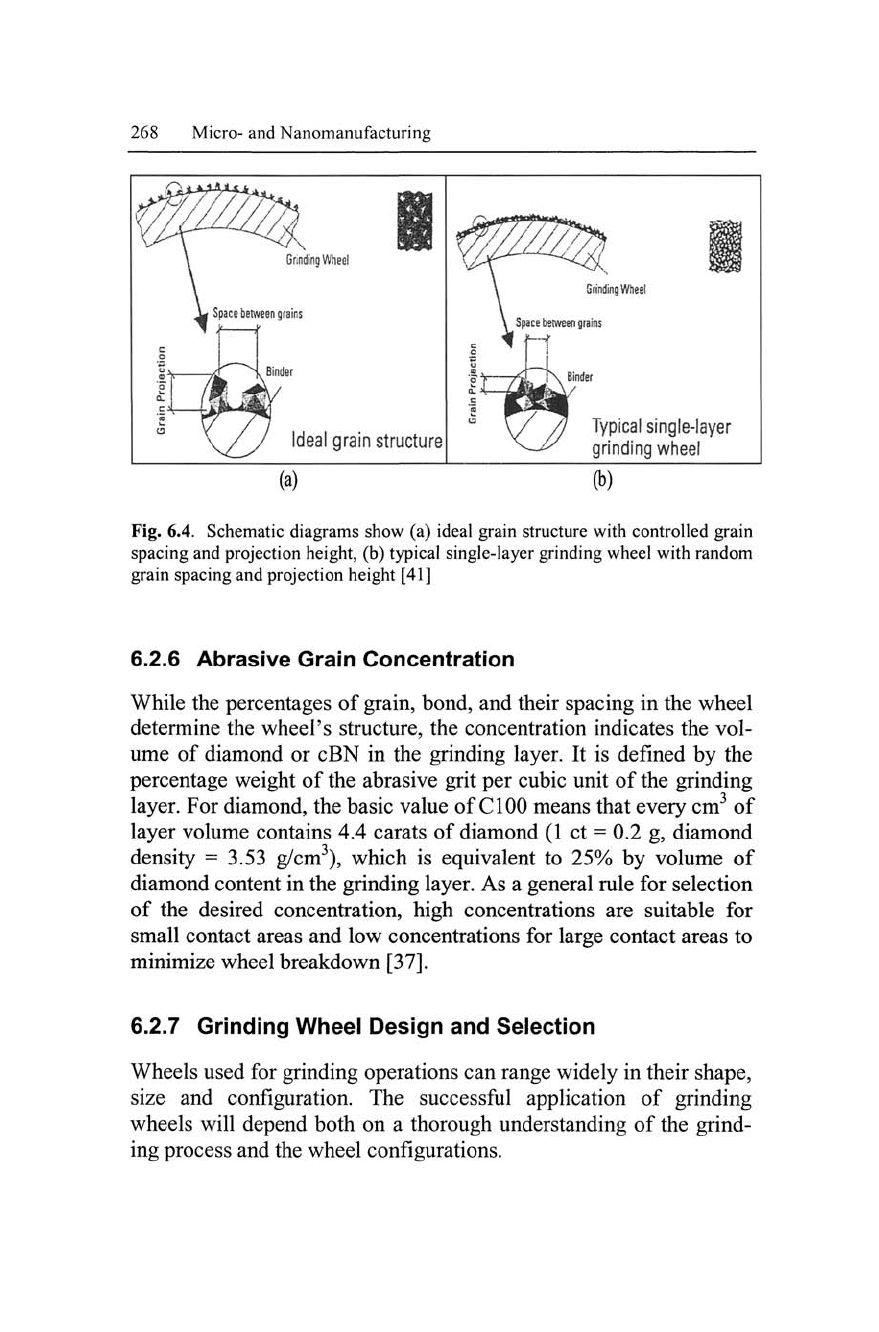
International efforts have been carried out to minimize variability of
grit spacing and projection height of the grain in order to make the
grinding process more predictable [39, 40]. As illustrated in Fig. 6.4,
grain depths of cut and space between grains are higher in (a) than
(b) and these are distinct advantages for effective grinding,
involving less loading and heat generation.
Microgrinding 267
Table 6.3. Equivalent international standard of grit sizes for diamond and cubic
boron nitride used by FEPA, US, DIN, and ISO standards compared to an indus-
trial designation [37]
International Standardization of Grit Sizes for Diamond and Cubic Boron Nitride
Sieve Grit Designations
Diamo
FEPA-Sta
WINTER des
narrow
D1161
D1001
D 851
D 711
D 601
D 501
D 426
D 356
D 301
D 251
D 213
D 181
f D 151
f D 128
Y D 107
TD 91
ID 78
TO 64
| D 54
[D 46
nd
ridard
ignation
wide
D1182
D 852
D 602
D 427
D 252
m
FEPA-Sta
WINTER des
narrow
B
1181
81001
B 851
B 711
B 601
B 501
B 426
B 356
B 301
B 251
B 213
^B 181
^B 151
^B 126
r
B 107
^B 91
B 76
^B 64
B 54
^B 46
1
ndard
ignation
wide
B
1182
B 852
B 602
B 427
f
B 252
Diamond
US-Stan
ASTM-E-
narrow
16/
18
18/
20
20/
25
25/
30
30/35
35/
40
40/
45
45/
50
50/
60
60/
70
70/
80
80/100
100/120
120/140
140/170
170/200
200/230
230/270
270/325
325/400
+ CBN
dsrd
11-70
wide
16/20
20/30
30/40
40/50
Nominal mesh
size
to
ISO 6106 DIN 848
Part
1,1980
ym
1180/1000
1000/
850
850/
710
710/
600
60a'
500
500/
425
42S'
355
im 300
30a'
250
250/
212
Wt 180
180/
150
150/
125
125'
106
106/
90
90/
75
75'
63
63/
53
53/
45
45/
38 |
I
Micron Grit Sizes']
Diamond
WINTHR
desig-
nation
D25
^D20B
^D20A
D15
D15C
^D15B
D15A
?*!
D 3
D1
D 0,7
CBN
WINTER
desig-
nation
B30
B15
B 9
B 6
B 3
B 1
00,25 i
For
I
comparison
grit size
pm
32-52
J
30-40 I
25-30
J
10-25
I
20-25
J
15-20 |
10-15
5-10
J
2-5 |
1-2 |
0,5-
1
I
<0,5 |
f -
Grits
recommended by '
WINTER |
*) Similar
FEPA Standard
!
exists with
designations
M
63...
M 1.0
FEPA
-
Federation
Europeenne
des
Fabncants
de
ProduitsAbrasifs.
268 Micro- and Nanomanufacturing
(a)
(b)
Fig. 6.4. Schematic diagrams show (a) ideal grain structure with controlled grain
spacing and projection height, (b) typical single-layer grinding wheel with random
grain spacing and projection height [41]
6.2.6 Abrasive Grain Concentration
While the percentages of grain, bond, and their spacing in the wheel
determine the wheel's structure, the concentration indicates the vol-
ume of diamond or cBN in the grinding layer. It is defined by the
percentage weight of the abrasive grit per cubic unit of the grinding
layer. For diamond, the basic value of CI00 means that every cm
3
of
layer volume contains 4.4 carats of diamond (1 ct = 0.2 g, diamond
density = 3.53 g/cm
3
), which is equivalent to 25% by volume of
diamond content in the grinding layer. As a general rule for selection
of the desired concentration, high concentrations are suitable for
small contact areas and low concentrations for large contact areas to
minimize wheel breakdown [37].
6.2.7 Grinding Wheel Design and Selection
Wheels used for grinding operations can range widely in their shape,
size and configuration. The successful application of grinding
wheels will depend both on a thorough understanding of the grind-
ing process and the wheel configurations.

Microgrinding 269
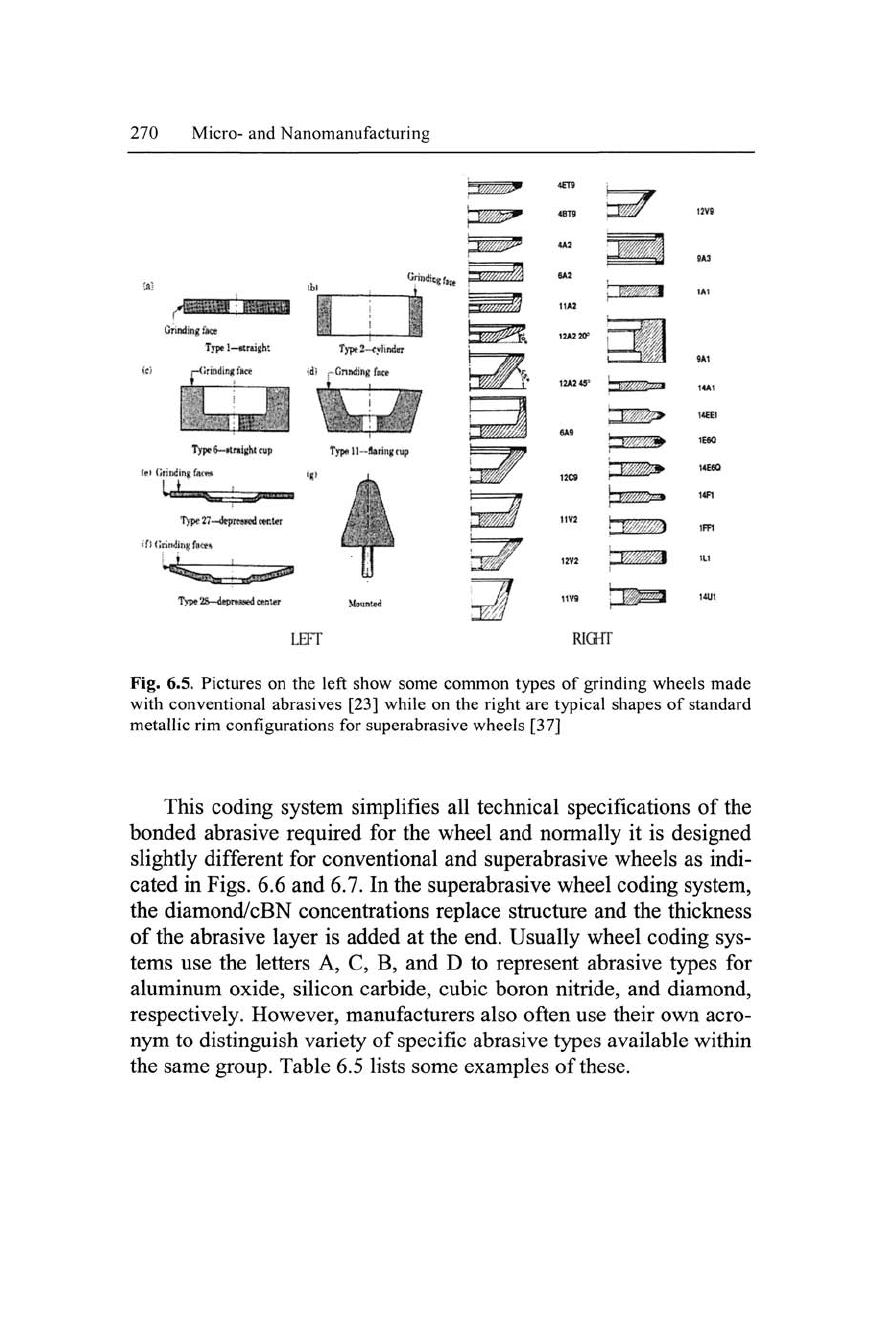
Figure 6.5 shows some of available shapes for conventional (left
side) and superabrasive (right side) wheels, respectively. It is clearly
seen that in this figure the entire shape of conventional wheels is of-
ten made of abrasives, whereas in contrast, only small sections of the
superabrasive wheel contain abrasives.
Since superabrasive wheels are very expensive compared to
conventional abrasive grains, it is not economical to make entire
wheels with abrasives, as is common for conventional wheels.
Hence, superabrasive wheels are often designed with a core section
that does not carry the abrasive. The abrasive forms a layer of a few
millimeters thick, and the outer section of
the
core and their shape is
partly regarded as a standard wheel configuration. Extra care should
be taken when deciding the grinding face during grinding operations
for different wheel configurations as it may damage/break the wheel
if the wrong side is engaged to the workpiece and thus subject the
operator to unsafe conditions. Apart from the wheel shape and con-
figurations, users must also take account of outside diameter, height,
width of
abrasive,
bore size, and other dimensions as necessary.
Bonded abrasives are marked with a standardized system of let-
ters and numbers, indicating the type of abrasive, grain size, grade,
structure, concentration, bond type and layer thickness of abrasive.
Table 6.4. shows one type of marking order in a block diagram that
is generally used for wheel marking system.
Table 6.4. Standard method of wheel marking order [42]
Marking
order
X
Grain/
abrasive
type
X
Grain
size
X
Grade
X
Structure
X
Bond
type
X
Manufacturer's
no.
(optional)
270 Micro- and Nanomanufacturing
Grinding
:kc
Type 1—«trai(jh*
ici p<Jriiidir((
bet
Type ^8—depressed cenler
LEFT
RIGHT
Fig.
6.5.
Pictures
on the
left show some common types
of
grinding wheels made
with conventional abrasives
[23]
while
on the
right
are
typical shapes
of
standard
metallic rim configurations for superabrasive wheels [37]
This coding system simplifies
all
technical specifications
of
the
bonded abrasive required
for the
wheel
and
normally
it is
designed
slightly different
for
conventional
and
superabrasive wheels
as
indi-
cated
in
Figs.
6.6
and 6.7.
In
the superabrasive wheel coding system,
the diamond/cBN concentrations replace structure
and
the thickness
of the abrasive layer
is
added
at
the end. Usually wheel coding sys-
tems
use the
letters
A, C, B, and D to
represent abrasive types
for
aluminum oxide, silicon carbide, cubic boron nitride,
and
diamond,
respectively. However, manufacturers also often use their own acro-
nym
to
distinguish variety
of
specific abrasive types available within
the same group. Table 6.5 lists some examples of these.
