Hoffman D.M., Singh B., Thomas J.H. (Eds). Handbook of Vacuum Science and Technology
Подождите немного. Документ загружается.

442
Chapter 4.3: Magnetic-Fluid-Sealed Rotary Motion Feedthroughs
Fig.
5.
Quartz Crucible-
Moiten Silicon-
Vacuum
SiO evaporates from
surface
of
molten silicon
Lip Seal Protects Fluid
Seal from Dust
Atmosphere
M
Magnetic Fluid Seal—'
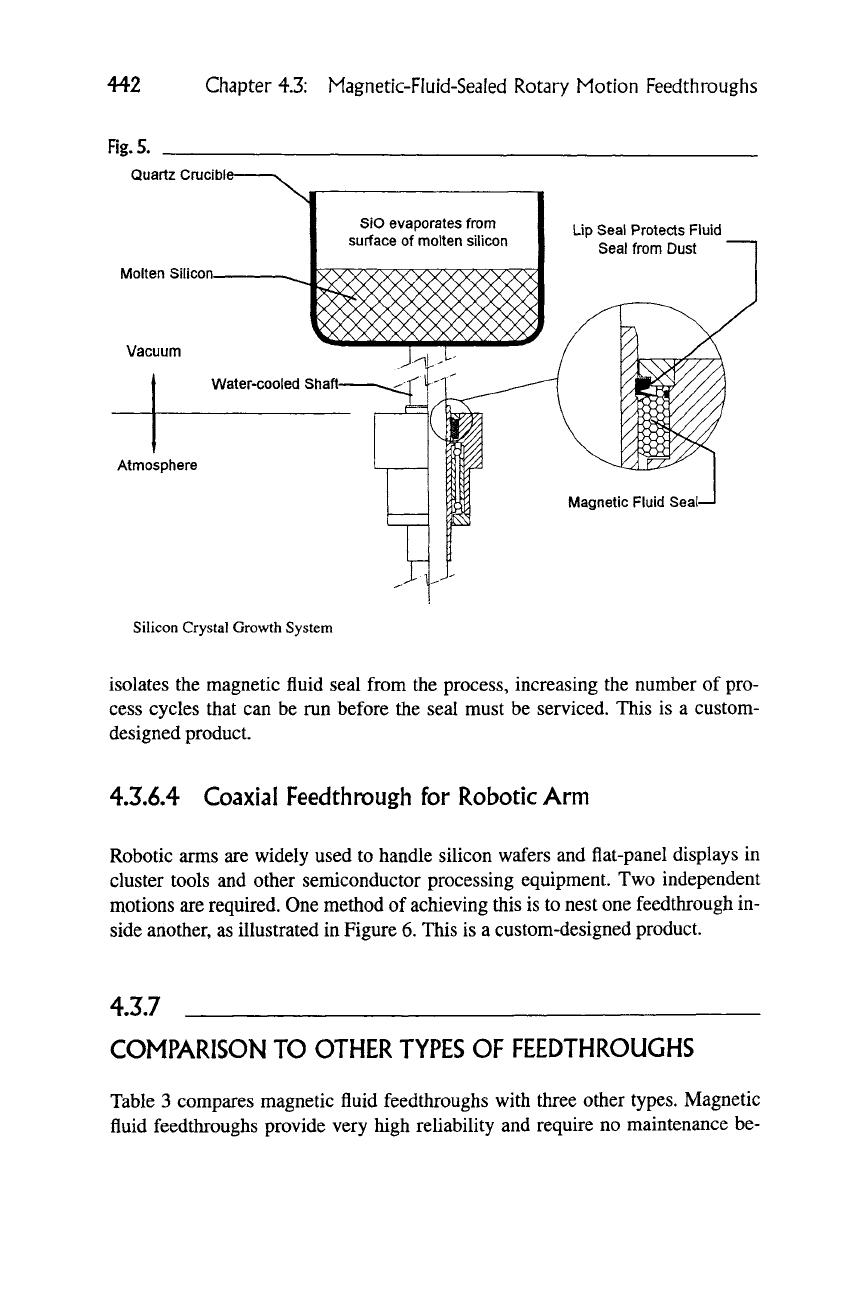
Silicon Crystal Growth System
isolates the magnetic fluid seal from the process, increasing the number
of
pro-
cess cycles that can
be
run before the seal must
be
serviced. This
is a
custom-
designed product.
4.3.6.4 Coaxial Feedthrough
for
Robotic Arm
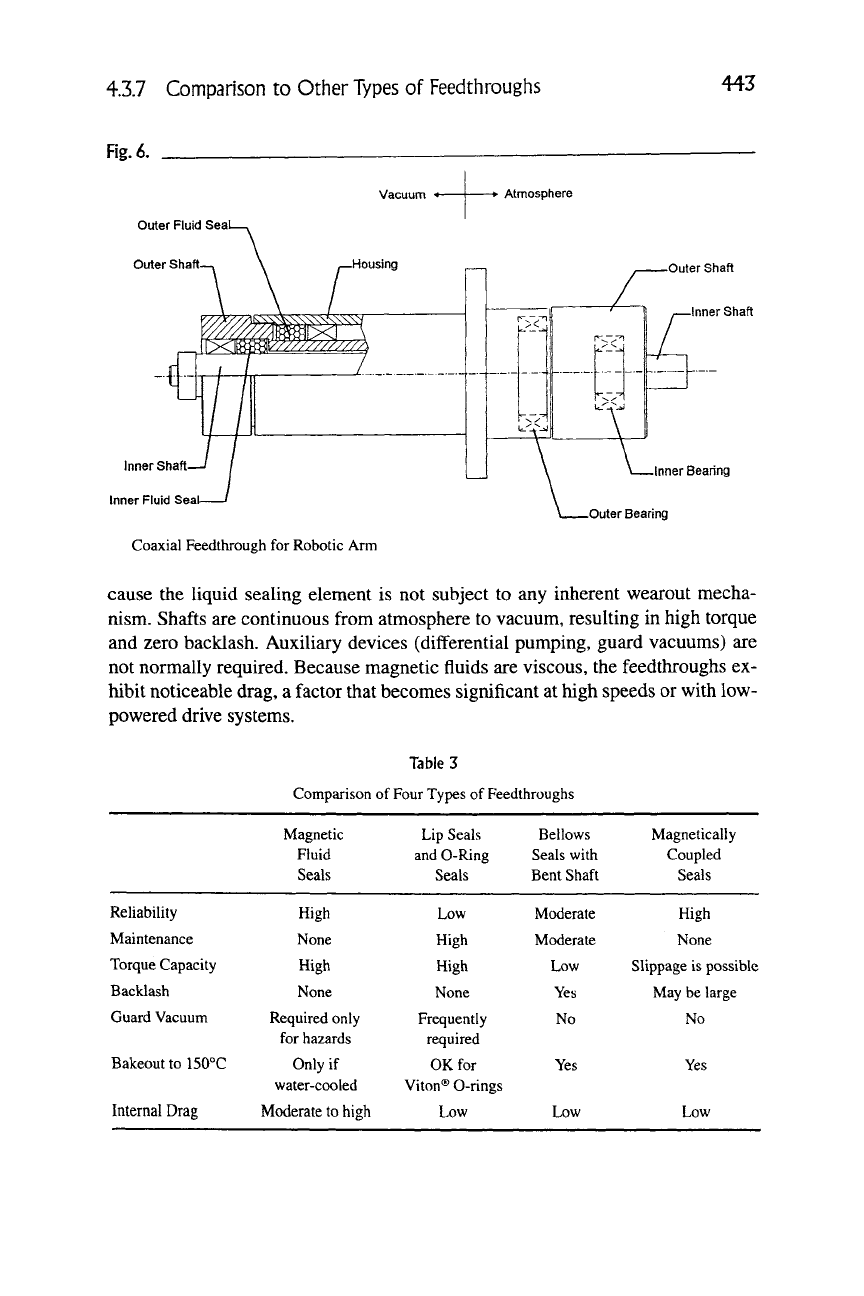
Robotic arms are widely used to handle silicon wafers and
flat-panel
displays
in
cluster tools and other semiconductor processing equipment. Two independent
motions are required. One method
of
achieving this is to nest one feedthrough in-
side another, as illustrated in Figure 6. This
is
a custom-designed product.
4.3.7
COMPARISON TO OTHER TYPES OF FEEDTHROUGHS
Table
3
compares magnetic fluid feedthroughs with three other types. Magnetic
fluid feedthroughs provide very high reliability and require no maintenance be-
4.3.7 Comparison
to
Other Types
of
Feedthroughs
443
Rg.6.
• Atmosphere
Outer Fluid Seal
Outer Shaft-
Outer Shaft
Inner Shaft
Inner Shaft
Inner Fluid Seal
Coaxial Feedthrough for Robotic Arm
Inner Bearing
Outer Bearing
cause the liquid sealing element is not subject to any inherent wearout mecha-
nism. Shafts are continuous from atmosphere to vacuum, resulting in high torque
and zero backlash. Auxiliary devices (differential pumping, guard vacuums) are
not normally required. Because magnetic fluids are viscous, the feedthroughs ex-
hibit noticeable drag, a factor that becomes significant at high speeds or with low-
powered drive systems.
Table 3
Comparison of Four Types of Feedthroughs
Reliability
Maintenance
Torque Capacity
Backlash
Guard Vacuum
Bakeouttol50°C
Internal Drag
Magnetic
Fluid
Seals
High
None
High
None
Required only
for hazards
Only if
water-cooled
Moderate to high
Lip Seals
and
O-Ring
Seals
Low
High
High
None
Frequently
required
OK
for
Viton®
0-rings
Low
Bellows
Seals with
Bent Shaft
Moderate
Moderate
Low
Yes
No
Yes
Low
Magnetically
Coupled
Seals
High
None
Slippage is possible
May be large
No
Yes
Low
CHAPTER
4.4
Viewports
Chuck Kraft
Larson
Electronic
Class
4.4.1
MATERIALS
The three most common viewport materials used in high- and ultra-high-vacuum
systems are glass, quartz, and sapphire. The choice of which to use often depends
on cost, transmission characteristics, bakeout temperature, style, and materials
used for the viewport seal.
Glass viewports, the most widely used and least expensive of the three choices,
are available in a wide variety of diameters. The transmission is approximately
90%
between 0.32 and 2.6 microns. Glass viewports are bakeable to
400""
Celsius
(centigrade). Zero-length viewports, where the surface of the glass is below the
surface of the flange, are Kovar®' sealed. Also available are nonmagnetic view-
ports with the glass sealed to the end of
a
stainless steel tube. The viewport is nor-
mally mounted with the tube extending out from the vacuum chamber. In diame-
ters up to 4 inches, the tube can be inverted to place the glass close to an object
within the chamber. Tubular Kovar® viewports can be made the same way.
Quartz viewports have a transmission of approximately 90% in the range of 0.3
to 2.5 microns and 0.2 to 2.0 microns for UV-grade materials. They are available
in both zero-length and standard-length. A standard-length quartz viewport has
the quartz disk mounted on a tube. Quartz viewports are sealed to stainless steel
' Kovar® is a registered trademark of Carpenter Technology. Kovar is an iron, nickel, and cobalt al-
loy used for glass sealing.
ISBN 0-12-325065-7 Copyright © 1998 by Academic Press
$25.00 All rights of reproduction in any form reserved.
444

4.4.1 Materials 445
frames with a low-temperature brazing alloy. The bakeout temperature is limited
to 200° Celsius.
Sapphire viewports have the broadest transmission range. The transmission is
above 80% at 0.3 to 4.0 microns and 0.25 to 4.5 microns for UV-grade sapphire.
These viewports are sealed with a high-temperature braze material and can with-
stand bakeout temperatures as high as 450° Celsius. The frame is Kovar®.
4.4.2
MOUNTING SYSTEMS AND PRECAUTIONS
Viewports of all three types are available with a variety of mounting flanges, and,
with the exception of the glass and quartz zero-length types, without flanges. In
use,
it is important not to induce stress into the seal, which could cause cracking
or breakage. The two types of stress are mechanical and thermal. Mechanical
stress is created from uneven pressure applied to the viewport frame. Flange bolts
should be tightened in a cross pattern, and foreign objects should not be allowed
to make contact with the frame or the glass. Thermal stress is the result of uneven
heat being applied to the frame and/or the glass during welding, bakeout, or in ac-
tual use on the system. Viewports are designed for vacuum use. The zero-length
glass viewports should be mounted in the normal configuration. Nonmagnetic
glass viewports up to 4 inches in diameter, and quartz and sapphire viewports can
be mounted with vacuum on either
side.
Precautions should be taken to avoid pres-
sure differentials greater than
1
atmosphere. In some applications, safety pressure
valves or shielding should be considered to protect operators and equipment.

CHAPTER 4.5
Construction Materials
janda K. Panitz
Sandi3
National
Laboratories
4.5.1
PROPERTIES DEFINING MATERIAL PERFORMANCE
The gas load in a vacuum system critically depends on the materials used to
build the system and how they are prepared and maintained. Materials add to gas
load in two ways: (1) evaporation, sorption and outgassing, and (2) diffusion and
permeation
4.5.1.1
Evaporation, Adsorption, Absorption, and Outgassing
EVAPORATION
Vapor pressure can be defined as the pressure at which a material's evaporation or
sublimation rate equals its condensation
rate.
If you pump a system below a mater-
ial's vapor pressure, the material sublimes or evaporates. As a general rule, vapor
pressure increases with temperature. At a given temperature and pressure, the sub-
limation or evaporation rate can be predicted from vapor pressure using thermo-
dynamic theory [1].
It is desirable to use low-vapor-pressure materials, e.g., stainless steel, glass,
and polyimide, as opposed to high-vapor-pressure materials, e.g., zinc, ice cubes,
and camphor, at practical operating temperatures. Selected information is pre-
ISBN
0-12-325065-7
Copyright © 1998 by Academic Press
$25.00 All rights of reproduction in any form reserved.
446

4.5.1 Properties Defining Material Performance 447
sented in Tables
1
to 4. These data can be used for guidance in selecting or reject-
ing metals, alloys, and selected compounds.
ADSORPTION, ABSORPTION; AND OUTGASSING
Adsoq)tion can be defined as the condensation of molecularly thin films on a
solid surface. Absorption is condensation that extends into the near-surface re-
gion of a solid. Outgassing occurs when high-vapor-pressure-adsorbed or -ab-
sorbed species evaporate as the vacuum system is pumped down and/or heated.
Sometimes, high-vapor-pressure solid, liquid, or gas films chemically bond to a
surface ("chemisorption"). If this occurs, the pumpdown rate to the high-vacuum
regime and below can be substantially slowed as the film is slowly wrested from
its substrate.
Water, which readily chemically bonds to many metal and ceramic surfaces
when vacuum system components are exposed to lab air, is a particularly ubiqui-
tous and persistent absorbed species. Water is an especially troublesome species
when it outgasses because low partial pressures of water strongly interfere with
many processes and measurements performed in practical vacuum systems. The
problem can be reduced by venting a system with dry nitrogen to blanket surfaces
with a gas species that adheres less tenaciously than chemisorbed water during
pumpdown.
Using smooth, nonporous, relatively chemically inert materials that are easy to
clean and adapting appropriate cleaning techniques will reduce the gas load added
by outgassing. A material's performance depends not only on its intrinsic proper-
ties but also on on how well it is cleaned. Section 4.9, "Preparation and Cleaning
of Vacuum Systems," is critically linked to this section on vacuum material se-
lection. Depending on whether high, ultra-high, and extra-ultra-high vacuums are
desired, progressively aggressive cleaning and system maintenance procedures can
be used to remove high-vapor-pressure contaminants. (Tools, including gloved
hands,
coming into contact with vacuum materials must also be clean.)
4.5.1.2 Solubility, Permeation, and Diffusion
Solubility, diffusion, and permeation relate to a material's chemical properties,
lattice spacing, and microstructure. Gases and certain other high-vapor-pressure
species readily dissolve in many solids. If these materials are used in a vacuum
system, dissolved high-vapor-pressure species can diffuse to the surface and add
to the gas load. If these materials are used to build a vacuum chamber, soluble
gasses can permeate through the walls and add to the gas load [2]. For example,
large amounts of hydrogen can be dissolved in palladium, platinum, and nickel.
Significant amounts of hydrogen can diffuse out of vacuum fixturing made from
44«
Chapter 4.S: Construction Materials
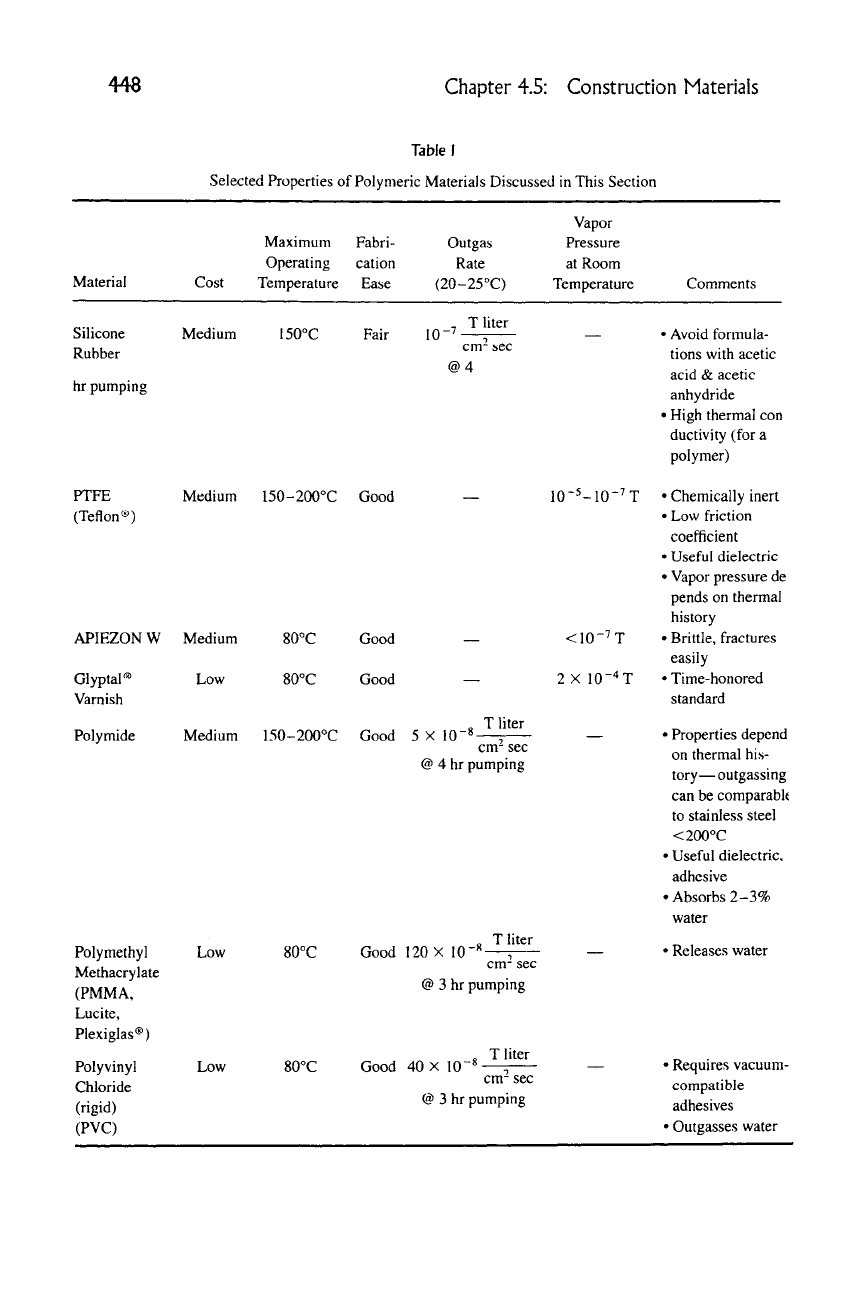
Table I
Selected Properties of Polymeric Materials Discussed in This Section
Material
Maximum Fabri- Outgas
Operating cation Rate
Cost Temperature Ease (20-25°C)
Vapor
Pressure
at Room
Temperature
Comments
Silicone
Rubber
hr pumping
PTFE
(Teflon®)
Medium
150°C
Fair
10"
T liter
cm-
sec
M
Medium 150-200°C Good
APIEZON
Glyptar
Varnish
Polymide
[W Medium
Low
Medium
80°C
SOX
150-200°C
Good
Good
Good
—
, T liter
5 X 10-«—-,
cm-
sec
@ 4 hr pumping
Polymethyl
Methacrylate
(PMMA,
Lucite,
Plexiglas®)
Polyvinyl
Chloride
(rigid)
(PVC)
Low
SOT
Good 120 X 10"
T liter
10-5-10-^T
cm-
sec
@ 3 hr pumping
Low 80°C Good 40 X 10"^
T liter
cm-
sec
@ 3 hr pumping
<10-^T
2X lO'^T
• Avoid formula-
tions with acetic
acid & acetic
anhydride
• High thermal con
ductivity (for a
polymer)
• Chemically inert
• Low friction
coefficient
• Useful dielectric
• Vapor pressure de
pends on thermal
history
• Brittle, fractures
easily
• Time-honored
standard
• Properties depend
on thermal his-
tory—outgassing
can be comparable
to stainless steel
<200°C
• Useful dielectric,
adhesive
• Absorbs 2-3%
water
• Releases water
• Requires vacuum-
compatible
adhesives
• Outgasses water
4.5.1 Properties Defining Material Performance
449
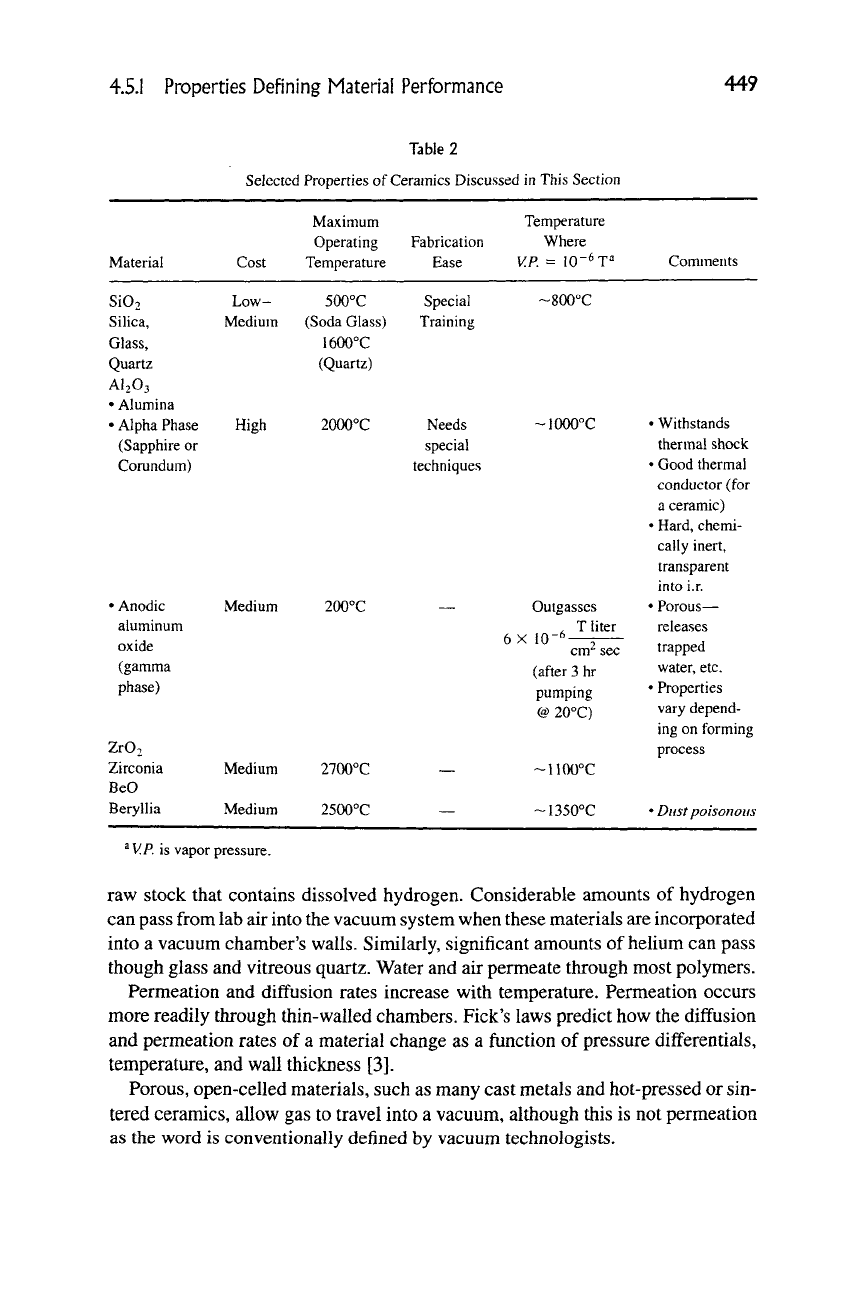
Table 2
Selected Properties of Ceramics Discussed in This Section
Material
Maximum
Operating Fabrication
Cost Temperature Ease
Temperature
Where
V.P.
=
10"^
T=*
Comments
SiO.
Silica,
Glass,
Quartz
AI2O3
• Alumina
• Alpha Phase
(Sapphire or
Corundum)
Low-
Medium
High
500°C
(Soda Glass)
1600°C
(Quartz)
2000°C
Special
Training
Needs
special
techniques
~800"C
• Anodic
aluminum
oxide
(gamma
phase)
Zr02
Zirconia
BeO
Beryllia
Medium
Medium
Medium
200°C
2700°C
2500°C
-lOOOX
Outgasses
. T liter
10-6—^
cm" sec
(after 3 hr
pumping
@ 20°C)
~1100°C
~1350°C
• Withstands
thermal shock
• Good thermal
conductor (for
a ceramic)
• Hard, chemi-
cally inert.
transparent
mto i.r.
• Porous—
releases
trapped
water, etc.
• Properties
vary depend-
ing on forming
process
• Dust poisonous
^
V.P.
is vapor pressure.
raw stock that contains dissolved hydrogen. Considerable amounts of hydrogen
can pass from
lab
air into the vacuum system v^hen these materials are incorporated
into a vacuum chamber's walls. Similarly, significant amounts of helium can pass
though glass and vitreous quartz. Water and air permeate through most polymers.
Permeation and diffusion rates increase with temperature. Permeation occurs
more readily through thin-walled chambers. Pick's laws predict how the diffusion
and permeation rates of a material change as a function of pressure differentials,
temperature, and wall thickness [3].
Porous, open-celled materials, such as many cast metals and hot-pressed or sin-
tered ceramics, allow gas to travel into a vacuum, although this is not permeation
as the word is conventionally defined by vacuum technologists.
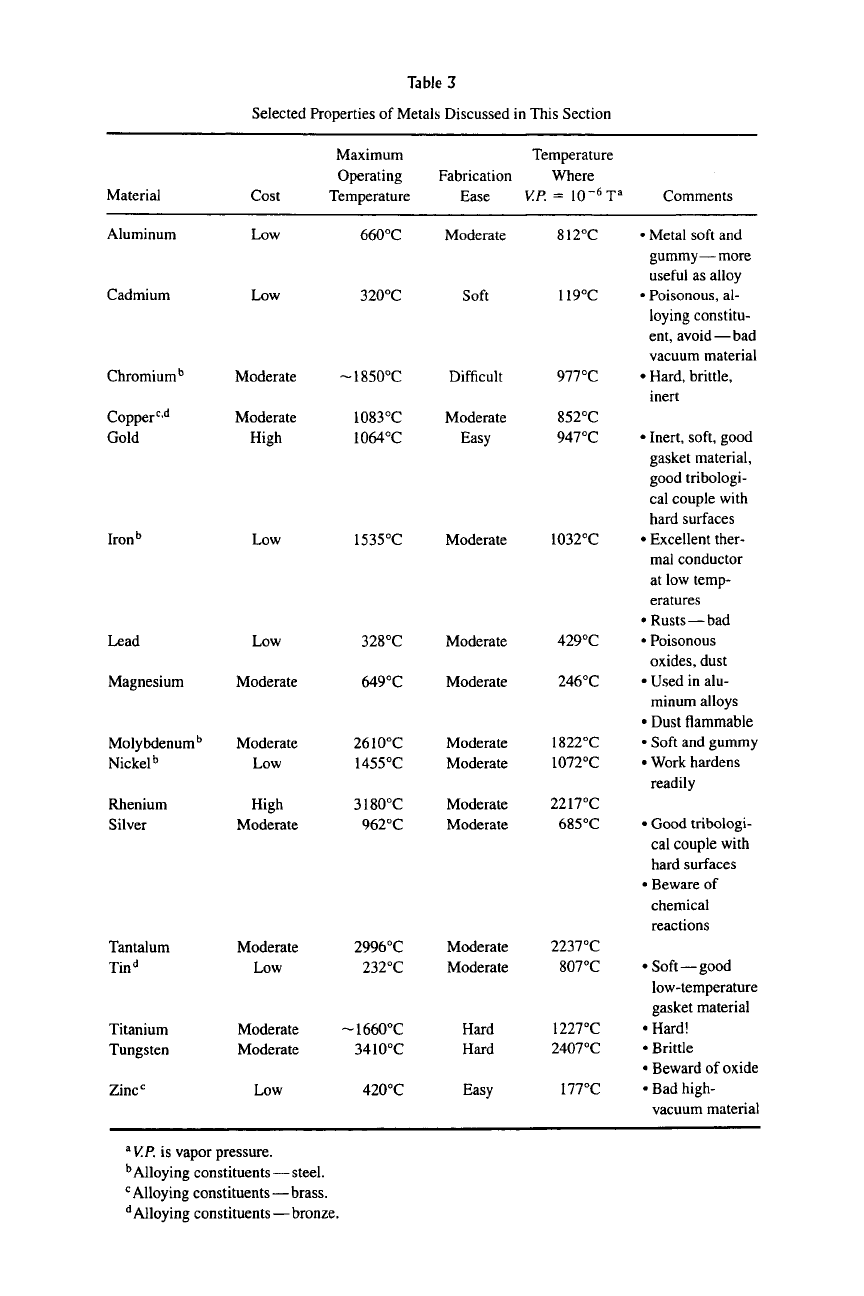
Table 3
Selected Properties of Metals Discussed in This Section
Material
Aluminum
Cadmium
Chromium''
Copper'^'^
Gold
Iron''
Lead
Magnesium
Molybdenum^
Nickel^
Rhenium
Silver
Tantalum
Tin^
Titanium
Tungsten
Zinc*^
Cost
Low
Low
Moderate
Moderate
High
Low
Low
Moderate
Moderate
Low
High
Moderate
Moderate
Low
Moderate
Moderate
Low
Maximum
Operating
Temperature
660°C
320°C
~1850°C
1083°C
i064°C
1535°C
328°C
649°C
2610°C
1455°C
3i80°C
962°C
2996°C
232°C
~1660°C
3410°C
420°C
Fabrication
Ease
Moderate
Soft
Difficult
Moderate
Easy
Moderate
Moderate
Moderate
Moderate
Moderate
Moderate
Moderate
Moderate
Moderate
Hard
Hard
Easy
Temperature
Where
V.P. = 10-^T"
812°C
119°C
977°C
852°C
947°C
1032°C
429°C
246°C
1822°C
1072°C
2217°C
685°C
2237°C
807°C
1227°C
2407°C
177°C
Comments
• Metal soft and
gummy—more
useful as alloy
• Poisonous, al-
loying constitu-
ent, avoid — bad
vacuum material
• Hard, brittle,
inert
• Inert, soft, good
gasket material,
good tribologi-
cal couple with
hard surfaces
• Excellent ther-
mal conductor
at low temp-
eratures
• Rusts—bad
• Poisonous
oxides, dust
• Used in alu-
minum alloys
• Dust flammable
• Soft and gummy
• Work hardens
readily
• Good tribologi-
cal couple with
hard surfaces
• Beware of
chemical
reactions
• Soft—good
low-temperature
gasket material
•Hard!
•Brittle
• Beward of oxide
• Bad high-
vacuum material
^
V.P.
is vapor pressure.
^ Alloying constituents — steel.
^
Alloying constituents—brass.
'^ Alloying constituents—bronze.
4.5.2 Vacuum Chamber Materials
451
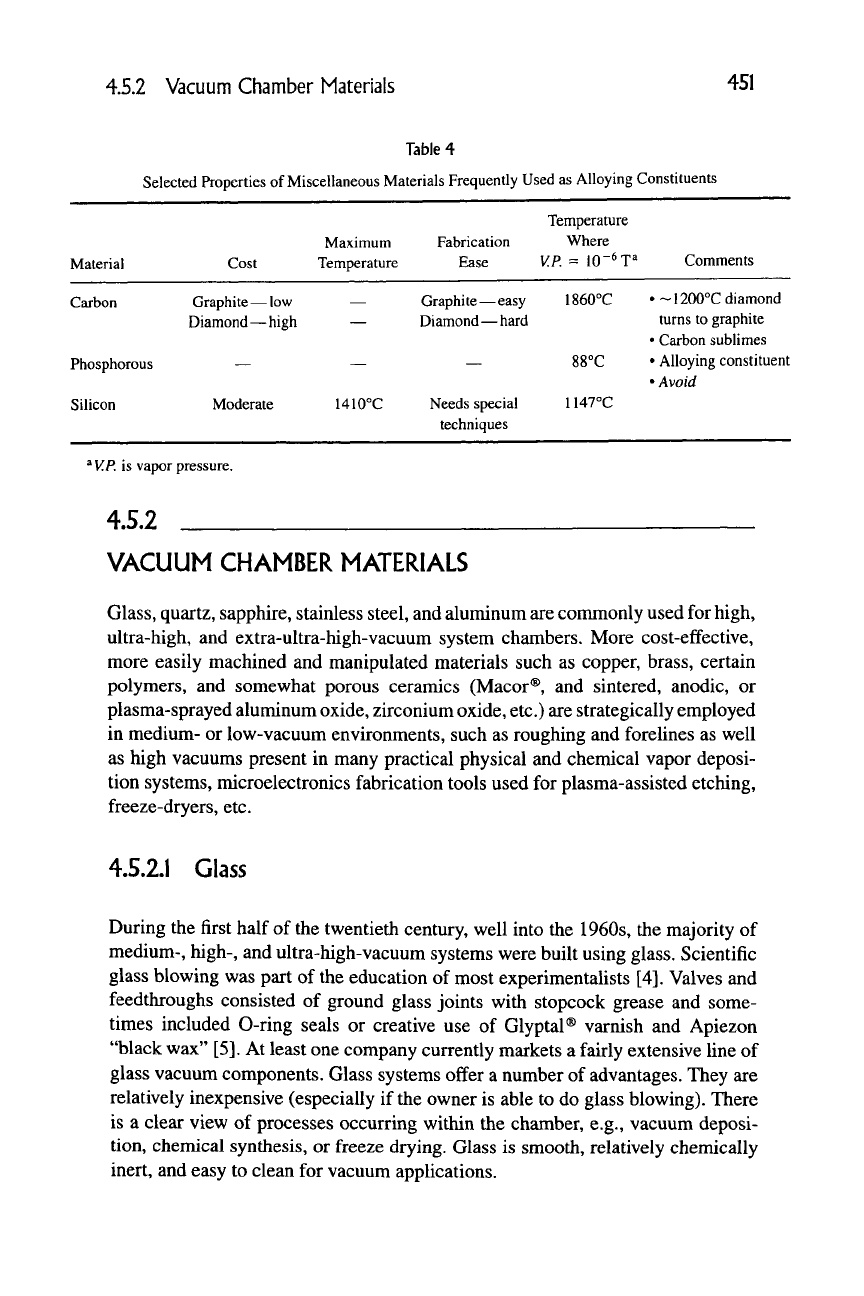
Table 4
Selected Properties of Miscellaneous Materials Frequently Used as Alloying Constituents
Material
Carbon
Phosphorous
Silicon
Cost
Graphite — low
Diamond—high
—
Moderate
^
V.P.
is vapor pressure.
4.5.2
Maximum
Temperature
—
—
1410°C
Fabrication
Ease
Graphite—easy
Diamond—hard
—
Needs special
techniques
Temperature
Where
VR = 10'^ T^
1860°C
88°C
1147°C
Comments
•~1200°C diamond
turns to graphite
• Carbon sublimes
• Alloying constituent
• Avoid
VACUUM CHAMBER MATERIALS
Glass,
quartz, sapphire, stainless steel, and aluminum are commonly used for high,
ultra-high, and extra-ultra-high-vacuum system chambers. More cost-effective,
more easily machined and manipulated materials such as copper, brass, certain
polymers, and somewhat porous ceramics (Macor®, and sintered, anodic, or
plasma-sprayed aluminum
oxide,
zirconium
oxide,
etc.) are strategically employed
in medium- or low-vacuum environments, such as roughing and forelines as well
as high vacuums present in many practical physical and chemical vapor deposi-
tion systems, microelectronics fabrication tools used for plasma-assisted etching,
freeze-dryers, etc.
4.5.2.1 Glass
During the first half of the twentieth century, well into the 1960s, the majority of
medium-, high-, and ultra-high-vacuum systems were built using glass. Scientific
glass blowing was part of the education of most experimentalists [4]. Valves and
feedthroughs consisted of ground glass joints with stopcock grease and some-
times included
0-ring
seals or creative use of Glyptal® varnish and Apiezon
"black wax"
[5].
At least one company currently markets a fairly extensive line of
glass vacuum components. Glass systems offer a number of advantages. They are
relatively inexpensive (especially if the owner is able to do glass blowing). There
is a clear view of processes occurring within the chamber, e.g., vacuum deposi-
tion, chemical synthesis, or freeze drying. Glass is smooth, relatively chemically
inert, and easy to clean for vacuum applications.
