Hoffman D.M., Singh B., Thomas J.H. (Eds). Handbook of Vacuum Science and Technology
Подождите немного. Документ загружается.


2.4.3
Throughput
127
2A3
THROUGHPUT
2.4.3.1 Maximum Throughput
It may be observed that maximum throughput is often the important aspect of va-
por jet pump performance rather than pumping speed. The value of the maximum
throughput determines the amount of power required to operate a given pump. Di-
mensionally, throughput and power are equivalent. For pumps of current designs
using modern pumping fluids, approximately
1
kw of power is required to obtain
a maximum throughput of 1.2 torr L/sec.
For systems that remain under high vacuum for long periods of
time,
the maxi-
mum throughput is of little value. In such cases, provisions can be made to reduce
the power after the initial evacuation or to use lower-power heaters. Operation of
the pump at half power is possible without changes in pump design. Special de-
signs can be made for low-power (low-throughput) operation with appropriate at-
tention to corresponding reduction of forepressure tolerance.
For rapid frequent evacuation and for high gas load application, the value of
maximum throughput determines the choice of pump size.
If the graph in Figure 8 is replotted, interchanging the axis, a more customary
arrangement of dependent and independent variables is obtained, Figure
11.
Then
Fig.
11.
10''
10'
10"
or
O
r2
10
(?) 10
10
10^
10^
rTr~T 1 T--|
1 V >. Ny
r "^ ^v \
1 ^ N. \
I N )
r
^ ^
r ^"^
LIMITS OF
\- DIFFUSION PUMP
OPERATION
I 1 1 1
T ••"• I
1
UJ
H I
< H
>-
to
>-
< 1
UJ
to J
Q 1
< J
o 1
_J
^
UJ
s
1 1 .
10*2 10'
10
I02 10^ 10"*
THROUGHPUT, TORR LIT/SEC
Input pressure vs. throughput.

128 Chapter 2.4: Vapor Jet Pumps [Diffusion Pumps)
it is simpler to see the maximum throughput and the pressure stability limits and
the concept of overload when those limits are exceeded. The more convenient
way of looking at this is to keep in mind that for a given system gas load presented
to the pump there is a resulting inlet pressure. This will help in selecting the re-
quired pump size and in distinguishing between the requirements of evacuating a
chamber and maintaining a desired operation pressure at a given process gas load.
2A4
TOLERABLE FOREPRESSURE
2.4.4.1 Discharge Pressure
A vapor jet pump is designed for high-vacuum application. Thus, its boiler pres-
sure usually is 1-1.5 torr. This implies that the maximum pressure difference the
pump can be expected to produce is 1.5 torr. In addition, vapor jet pump working
fluids cannot be boiled at high pressures because the resulting high temperatures
will decompose the fluid at a unacceptable
rate.
Thus, such pumps must always be
backed by another pump that produces a pressure of generally less than 0.5 torr at
the discharge of the vapor jet pump.
Tolerable forepressure of a vapor jet pump is the maximum permissible pres-
sure at the foreline.
The pumping action of the vapor jet pump collapses completely when the tol-
erable forepressure is exceeded [2]. Essentially, the vapor of the discharge stage
of the pump does not have sufficient energy and density to provide a barrier for the
air in the foreline when its pressure exceeds a certain value (usually near 0.5 torr).
Then this air will flow across the pump in the wrong direction, carrying with it the
pumping fluid vapor.
Approximately half of the initial pressure is recovered in the form of tolerable
forepressure. When the tolerable forepressure is measured, a conventional non-
ultrahigh vacuum system usually does not reveal the dependence between the
discharge and the inlet pressures. This is because the maximum pressure ratio for
air is usually not exceeded unless the experiment is conducted under ultrahigh
vacuum inlet pressures.
2.4.4.2 Backing Pump Requirements
To select an appropriate backing pump for a given vapor jet pump, several ques-
tions must be considered. First, what is the size of the initial roughing pump, and

2.4.4 Tolerable Forepressure 129
is it to be used for both roughing and backing? Second, is the backing pump ex-
pected to perform at the maximum throughput of the vapor jet pump? Third, what
is the tolerable forepressure of the diffusion pump? Also, what is the volume of
the foreline ducts (or a special reservoir or ballast chamber that could be used in
the line as a buffer during roughing)? The nominal pumping speed of
the
required
backing pump for full load condition is obtained as follows:
S = QurJiTFP),
where gmax is the maximum throughput of the vapor jet pump and TFP stands for
tolerable forepressure. Setting aside consideration of safety factors and conduc-
tances of forelines, it should be noted that mechanical pumps often have reduced
speed when their inlet pressure is equal to the tolerable forepressure of the va-
por jet pump. A safety factor of 2 may be required to make sure that the toler-
able forepressure value is never exceeded even if mechanical pump and vapor
jet pump are not operating at their best. A numerical example follows. Assume
a pump with a maximum throughput of 4 torr L/sec and tolerable forepressure
(maximum permissible discharge pressure) of 0.5 torr at full load (at maximum
throughput). The required backing pump speed then becomes
4 torr L/sec
s = —— = 8 L/sec « 17 CFM
0.5 torr
A good choice for the backing pump would be a nominal 14 liters/sec (30 CFM)
pump, provided that the conductance between the two pumps are not severely
limited.
2.4.4.3 Safety Factors
The most important rule of vapor jet pump operation is: do not exceed tolerable
forepressure! In other words, in an operating pump, the maximum permissible
discharge pressure should not be exceeded under any circumstance. Observance
of this most basic requirement will eliminate most of the difficulties encountered
with vapor jet pumps, especially problems with noticeable backstreaming of the
pumping vapor into the vacuum system. High-vacuum systems should be designed
with interlocks, fail-safe valve arrangements, or clearly marked instructions to
preclude the possibility of exceeding the tolerable forepressure. As in most en-
gineering considerations, a safety factor should be included in establishing the
maximum permissible discharge pressure. A factor of 2 is a good general recom-
mendation. As much as 25% reduction of tolerable forepressure can be expected
near maximum throughput operation (full load), and some reduction can be ex-
pected from low heater power. The selection of the mechanical backing pump
must be made with these points in mind. Also, it should be noted that the desired
130
Chapter 2.4: Vapor Jet Pumps [Diffusion Pumps)
discharge pressure must be maintained near the vapor jet pump to avoid errors
caused by limited conductance of forelines and the reduction of mechanical pump
speed at lower pressures.
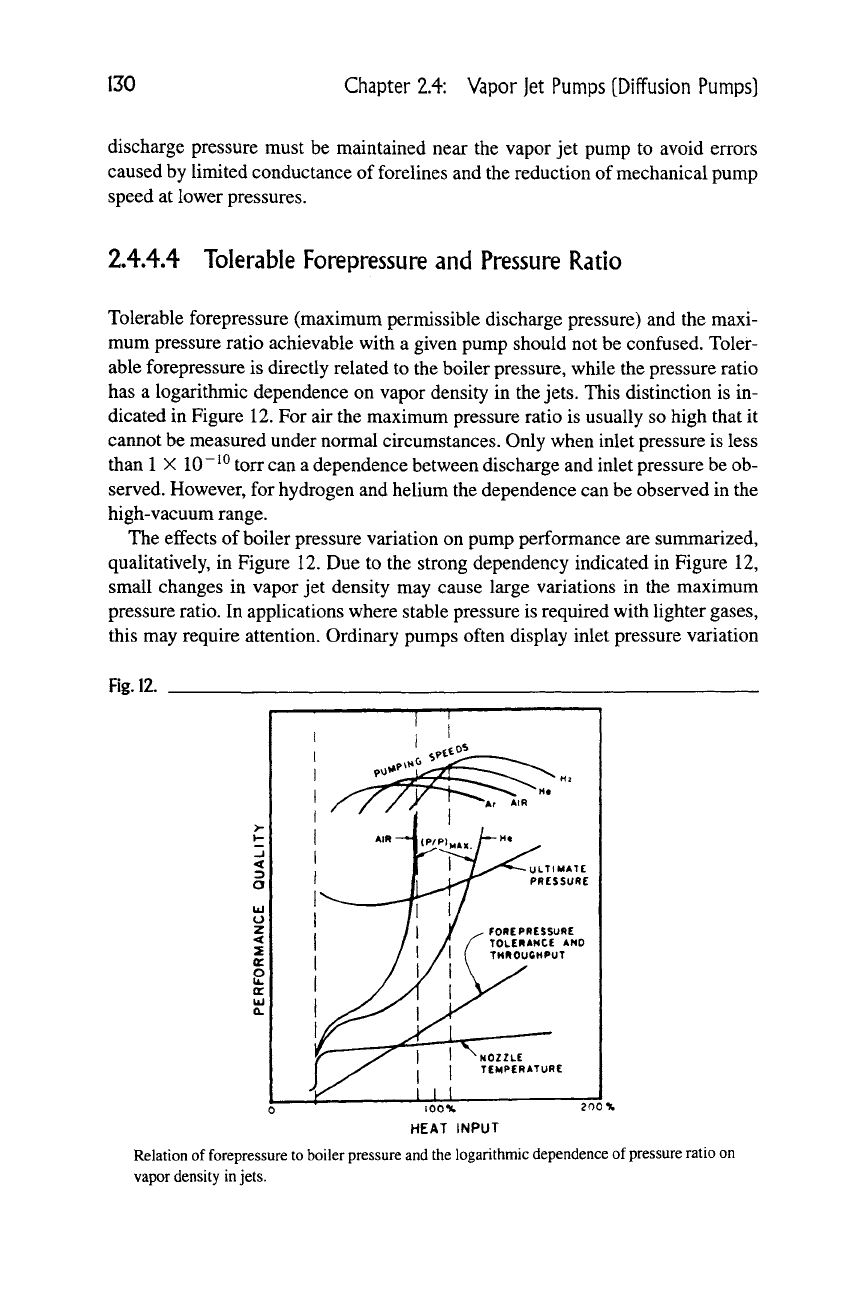
2A4.4 Tolerable Forepressure and Pressure Ratio
Tolerable forepressure (maximum permissible discharge pressure) and the maxi-
mum pressure ratio achievable with a given pump should not be confused. Toler-
able forepressure is directly related to the boiler pressure, while the pressure ratio
has a logarithmic dependence on vapor density in the
jets.
This distinction is in-
dicated in Figure 12. For air the maximum pressure ratio is usually so high that it
cannot be measured under normal circumstances. Only when inlet pressure is less
than
1
X
10 ~ ^^
torr can a dependence between discharge and inlet pressure be ob-
served. However, for hydrogen and helium the dependence can be observed in the
high-vacuum range.
The effects of boiler pressure variation on pump performance are summarized,
qualitatively, in Figure 12. Due to the strong dependency indicated in Figure 12,
small changes in vapor jet density may cause large variations in the maximum
pressure ratio. In applications where stable pressure is required with lighter gases,
this may require attention. Ordinary pumps often display inlet pressure variation
Fig.
12.
NOZZLE
TEMPERATURE
lOOX
HEAT INPUT
Relation of forepressure to boiler pressure and the logarithmic dependence of pressure ratio on
vapor density
in
jets.
2.4.4 Tolerable Forepressure
131
exceeding 5% for helium. This is unacceptable for some applications, such as
highly sensitive mass spectrometer leak detectors and analytical mass spectrome-
ters where helium is used either as a tracer or a carrier gas. Thus, special pump
designs having very stable boiler pressure with power control and stable pumping
action may be required.
In regard to pumping speed, it can be observed that the curves for helium and
hydrogen can have a rather steep slope near the nominal heat input value (100%).
Therefore, if variations of vapor density occur, they may result in noticeable pump-
ing speed variations for these gases.
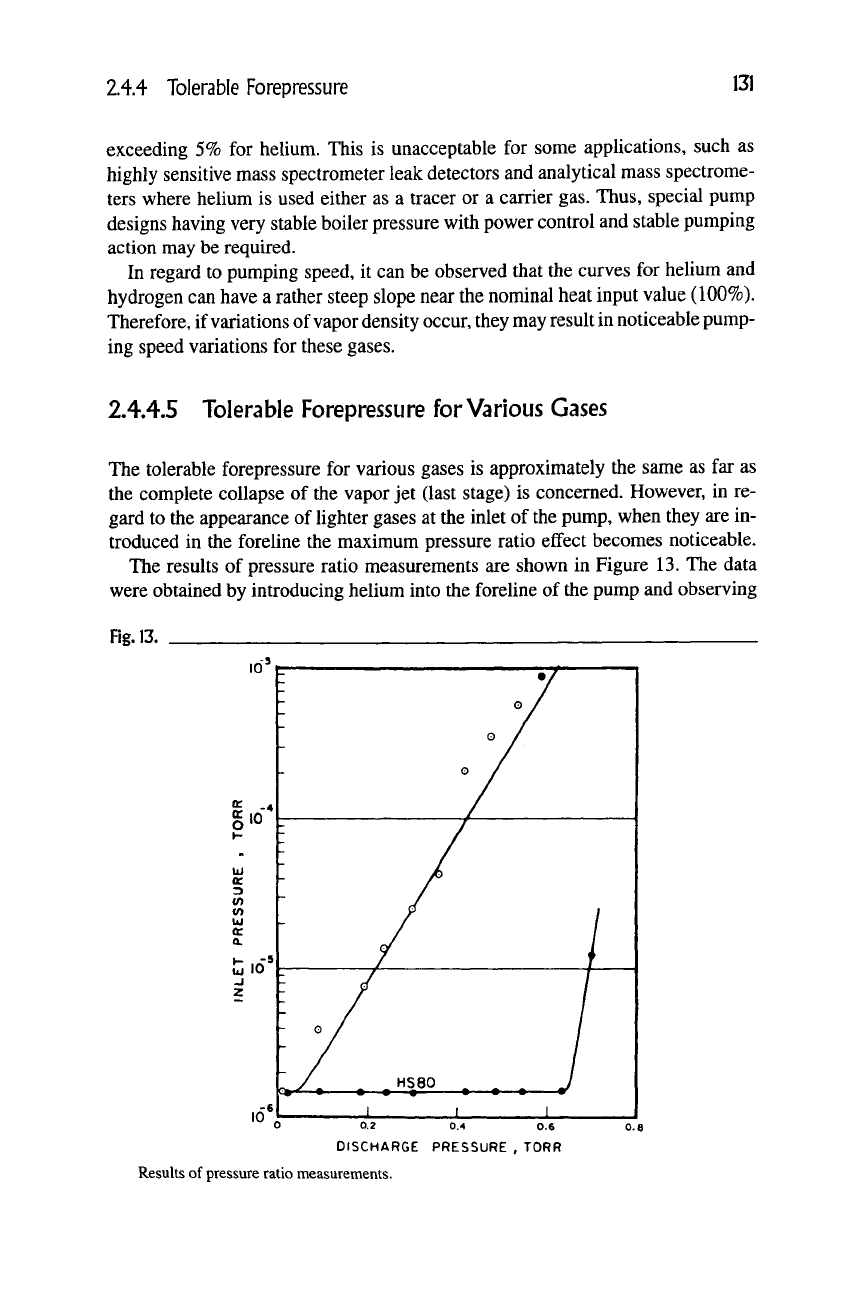
2.4.4.5 Tolerable Forepressure for Various Gases
The tolerable forepressure for various gases is approximately the same as far as
the complete collapse of the vapor jet (last stage) is concerned. However, in re-
gard to the appearance of lighter gases at the inlet of the pump, when they are in-
troduced in the foreline the maximum pressure ratio effect becomes noticeable.
The results of pressure ratio measurements are shown in Figure 13. The data
were obtained by introducing helium into the foreline of the pump and observing
Fig. 13.
0.2 0.4 0.6
DISCHARGE PRESSURE , TORR
Results of pressure ratio measurements.

132 Chapter 2.4: Vapor Jet Pumps [Diffusion Pumps]
the resulting effect on the inlet pressure. The upper curve represents an older 5-cm
pump. The comparison between the two pumps indicates a thousandfold improve-
ment in maximum pressure ratio, which can be sustained across the pump.
For the older pump in Figure 13, it is difficult to establish a concept of fore-
pressure tolerance for helium, at inlet pressures below
10
"^ torr. The newer pump
shows a behavior that can be interpreted as if a forepressure tolerance of 0.6 torr
for helium is present. This pump had a forepressure tolerance for air of about the
same magnitude.
2.4.5
ULTIMATE PRESSURE
2.4.5.1 Pressure Ratio Concept
Two distinct observations can be made regarding the ultimate pressure of
a
pump.
Ultimate pressure may be considered to be a gas load limit or a pressure ratio
limit. Both are of significance in practice, the latter usually only with light gases.
The pumping action of vapor jets does not cease at any pressure, however low.
The ultimate pressure of the pump depends on the ratio of pumped versus back-
diffused molecules, plus the ratio of gas load to the pumping speed. In addition,
the pump itself can contribute a gas load through backstreaming of pump fluid
vapor and its cracked fractions and the outgassing from its parts. Thus, in prac-
tice,
the observed total ultimate pressure is a composite consisting of several ele-
ments. In practice, the conmionly observed first limit is due to the pumping fluid,
although with the best fluid some system degassing (baking) may be necessary to
observe this limit when it is below 1 X
10 ~^
torr.
When liquid nitrogen traps are employed, the limiting pressure is usually given
by various gas loads in the system, even if the system consists only of the mea-
suring dome and a pressure gauge. Below 5 X 10"^ torr thorough baking is usu-
ally required.
For helium and hydrogen, a true pressure ratio limitation can be observed in
most pumps, although special designs can be made to improve the limit beyond
observable level.
2.4.5.2 Baffle and Trap Effects
Room temperature baffles at the inlet to the pump do not affect the ultimate pres-
sure except by shielding a gauge from a direct entry of pumping fluid vapor into
the gauge. Water-cooled baffles suppress the rate of reevaporation of condensed

2.4.5 Ultimate Pressure 133
or intercepted fluid, thereby reducing the density of vapor in the space between
the baffle and the trap. For substances such as vapor jet pump fluids, each 20°C
temperature change near room temperature will result in about an order of magni-
tude change in vapor pressure and hence the rate of evaporation. The lowered
vapor density will reduce the possibility of intercollisions and consequent bypass
through the trap without touching a refrigerated surface.
Cryogenic or refrigerated traps have basically two effects. They act as barriers
for the flow of condensable vapors from pump to system, but they also act as cryo-
pumps for condensable vapors emanating from the system. The latter may be the
primary effect on the ultimate pressure in many cases. In the high-vacuum region
and for unbaked systems using modern low-vapor-pressure pumping fluids, the
reduction of pressure (when traps are cooled) is primarily due to water vapor
pumping. In unbaked systems after the initial evacuation, water may constitute
90%
of the remaining gases and cooling of the trap simply increases the pumping
speed for water vapor (usually by a factor of 2 or 3).
2.4.5.3 Pressure Ratio for Lighter Gases
As noted previously, the pressure ratio can be sufficiently small for the light gases
to reveal the dependence of inlet pressure on the discharge pressure. Measure-
ments of pressure ratio for various gases have been reported as follows: hydrogen
3 X 10^-2 X 10^ helium 10-^-2 X 10^ neon 1 or 2 X 10^ CO and argon 10^,
oxygen and krypton 3-5 X 10^, and hydrocarbons
(C„H2n+2)
7 X 10^. In
modern pumps the helium pressure ratio is closer to 10^, and it can be increased
even as high as
10 ^^
by doubling the heat input. In practice, even an ion gauge op-
erated in the foreline can produce sufficient hydrogen to cause an increase of the
inlet pressure (the same occurrence has been observed in a turbomolecular pump
system).
As far as ultimate pressure is concerned, hydrogen can be a substantial part of
the residual gas composition due to its presence in metals, in the pumping fluid,
and in water vapor. This can be an important consideration for ultrahigh vacuum
work where some diffusion pumps may need a second pump in series. The same
consideration may apply to helium in leak detectors, mass spectrometers, molec-
ular beam experiments, etc.
2.4.5.4 Pumping Fluid Selection
A variety of
"organic"
liquids have been used as motive fluids in vapor jet pumps.
Criteria for the selection of the fluid are low vapor pressure at room temperature,
good thermal stability, chemical inertness, nontoxicity, high surface tension to
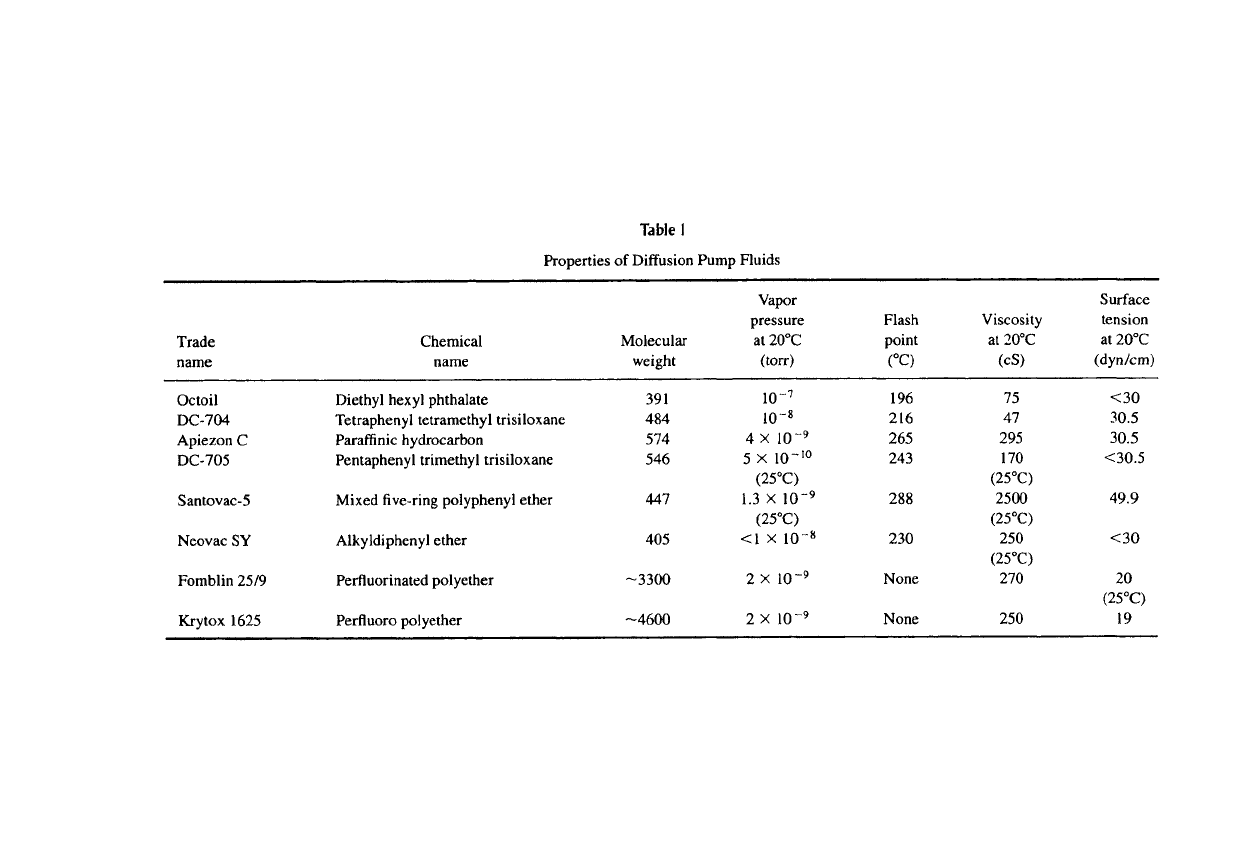
*3
E
a*
E
^
a
1
Si U
«3 O
'fc
o
S
I
3 53 w >»
C/3 ^3 cd 73
C/3
.^
u
•^ §
s
2 i
4^ w
o <o in
V ^
^ ^ '^'
O O rn
ON
OS
O
m
V
O O ON
r\| »o —
«Ot^--Ni^o Oo iTso
vo
NO
«o m
ON —< vO rf
-H CN (N (N
O o
Z,
I
ao
^ in
o ;3 u 2 u 2
- '^ °^ X °^ X
^^ m '^
—<
- V
X
X
-^ Tf rj- NO
ON 00 r^ Tt
C<^
Tt lO »0
? ?
8 8
I
.—4 >-•
•S E a <u
•as §.i
^ § .y S
J2 Si **^ -
g.
>
a
U w H C
•/^ 1^ ,? ,5^
u H di PM
CO U
S
o "^
o fr S t^
O Q < Q
X5
E

2.4.5 Ultimate Pressure 135
minimize creep, high flash and fire points, reasonable viscosity at ambient tem-
perature, low heat of vaporization, and, of
course,
low cost. A list of the presently
popular fluids and their properties is given in Table 1. The selection of the fluid
should be made giving due consideration to its operational stability in the pump
boiler. A breakthrough in the selection of fluids was made with the use of five-
ring polyphenyl ethers consisting of chains of carbon atoms interbonded by oxy-
gen
[3].
This fluid offered exceptional thermal and chemical stability and enabled
reaching ultimate pressures of 10"^ torr (approaching its vapor pressure at ambi-
ent temperatures) with only water-cooled baffles.
Operational characteristics of another low-vapor-pressure silicone fluid (DC-
705) were discussed by Crawley et al. [4]. Ultimate pressures of 10"^ torr with
water-cooled baffles and
10 ~^^
torr with a baffle at -20°C were reported [5].
Whatever fluid is used, its vapor may pervade the pumped system, dependent
on its vapor pressure, the pump design, and the type of trapping used. The vapor
can be broken down by the presence of hot filaments and bombardment by charged
particles. The polymerization of silicone fluids resulting from bombardment by
charged particles may cause an insulating film to be produced on electrode sur-
faces,
changing the characteristics of the electronic instrumentation. Octoil or
polyphenyl ethers are usually reconmiended to eliminate this problem in applica-
tions where mass spectrometers and other electron optical devices are used.
2.4.5.5 Residual Gas Analysis
With condensable gases of high molecular weight, it is extremely difficult to cor-
relate the ion currents indicated by the mass spectrometer with the rate of back-
streaming through the baffle. Generally, the residual gas analysis of a trapped
dif-
fusion pump system cannot be reliably performed with spectrometers having
poorer detectability than
10 ~^
torr. When the spectrometer tube and other parts of
the system are baked, the results can be very misleading. It may take weeks be-
fore equilibrium conditions are established.
Qualitative measurement can be made, however, with no more difficulty than
the ultra-high pressure measurements made with total pressure gauges such as an
ionization gauge.
A typical mass spectrum from a baffled vapor jet pump system, unbaked, oper-
ating in the ultra-high-vacuum region, with DC-705 motive fluid, is shown in Fig-
ure 14. The mass numbers 16, 19, and 35 are unusually high owing to the charac-
teristic properties of the particular spectrometer tube and the hydrogen peak is
not shown. The peaks 50, 51, 52, 77, and 78 are characteristic for the pumping
fluid. Crawley [4] also gives the residual gas analysis of a vapor jet pump system
using silicone DC-705 fluid under both unbaked and baked conditions. Wood and
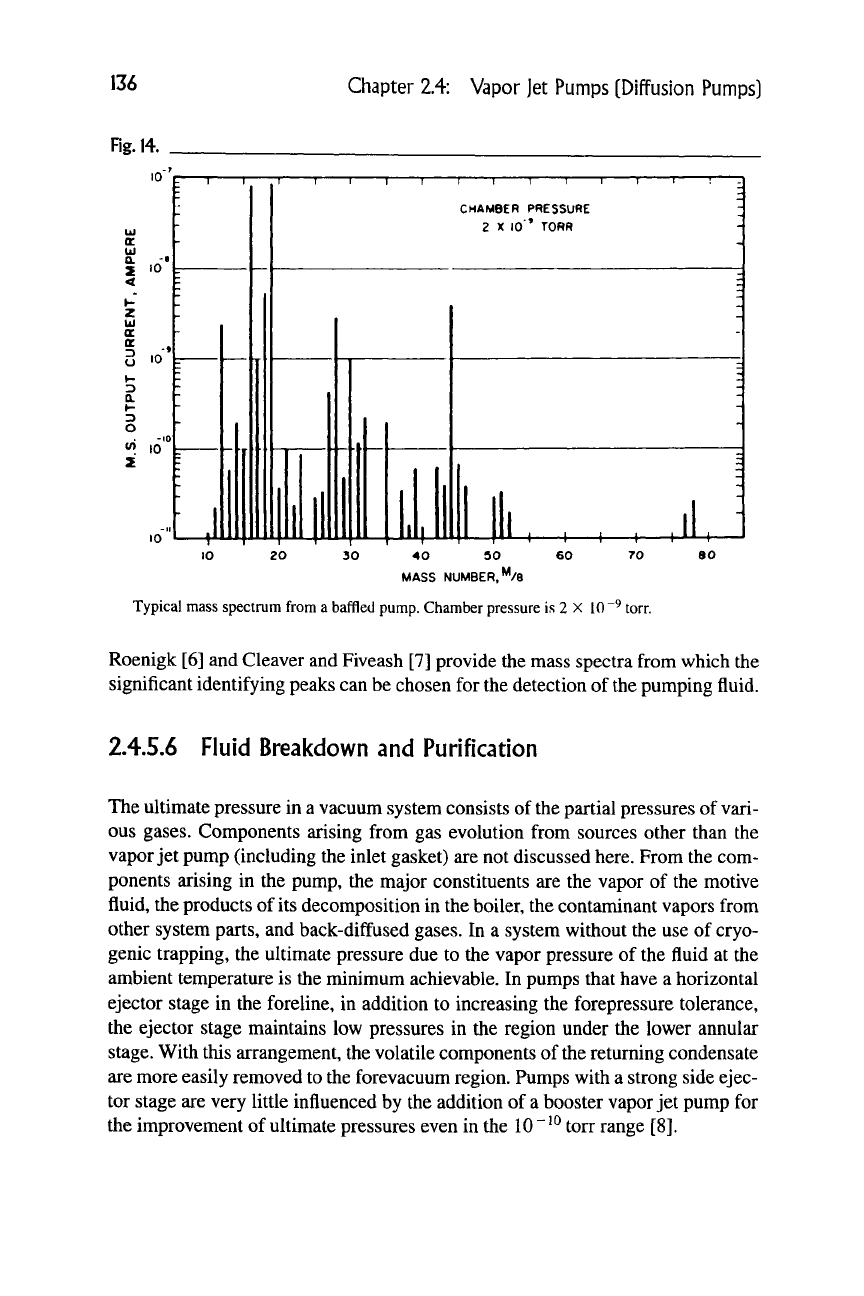
136
Chapter 2.4: Vapor Jet Pumps [Diffusion Pumps]
Fig.
14.
10
2
10
I-
z
UJ
3
«o
»-
CL
O
<^
10
10
F
T r
P
r
III
Li
i
I \ 1 T I •
••
T
T T I I 1 ' d
CHAMBER PRESSURE
1
2
X 10 '
TORR
H
111
J
Jl
4JI
H
1
i
III il,
1
10
20
30 40
50 60
MASS NUMBER, "^/e
Typical mass spectrum from a baffled pump. Chamber pressure is 2 X 10"^ torr.
Roenigk [6] and Cleaver and Fiveash [7] provide the mass spectra from which the
significant identifying peaks can be chosen for the detection of the pumping fluid.
2.4.5.6 Fluid Breakdown and Purification
The ultimate pressure in a vacuum system consists of the partial pressures of vari-
ous gases. Components arising from gas evolution from sources other than
the
vapor jet pump (including the inlet gasket) are not discussed here. From the com-
ponents arising
in
the pump, the major constituents are the vapor
of
the motive
fluid, the products of its decomposition in the boiler, the contaminant vapors from
other system parts, and back-diffused gases. In
a
system without the use of cryo-
genic trapping, the ultimate pressure due to the vapor pressure of the fluid at the
ambient temperature is the minimum achievable. In pumps that have a horizontal
ejector stage in the foreline,
in
addition to increasing the forepressure tolerance,
the ejector stage maintains low pressures
in
the region under the lower annular
stage. With this arrangement, the volatile components of the returning condensate
are more easily removed to the forevacuum region. Pumps with a strong side ejec-
tor stage are very little influenced by the addition of a booster vapor jet pump for
the improvement of ultimate pressures even in the
10 ~ ^^
torr range [8].
