Hoffman D.M., Singh B., Thomas J.H. (Eds). Handbook of Vacuum Science and Technology
Подождите немного. Документ загружается.

2.5.2 Cryopump Basics
157
An aspect of molecular flow that is important for all high-vacuum pumps is
that the pump does not draw or "suck" molecules toward the entrance. The pump
must wait for the arrival of a molecule in its quasi-random motion, and then trap
it. Because the cryopump traps the molecule by adsorption and condensation onto
a cold surface, it can be considered a piece of cryogenic "flypaper." A molecule
lands on the cold surface and never takes off again. Other molecules land on the
surface, to form progressively thicker layers of condensate.
2.5.2.2 Vapor Pressure
Cryopumps are omnivorous: They condense all gases at very high speeds. The
ability of a surface to trap a particular gas depends on the vapor pressure charac-
teristic of the gas. Nearly all gases follow the basic Clausius-Clapeyron relation
[4] of the vapor pressure P and the temperature T in Kelvins.
log P = -A{l/T) + constant
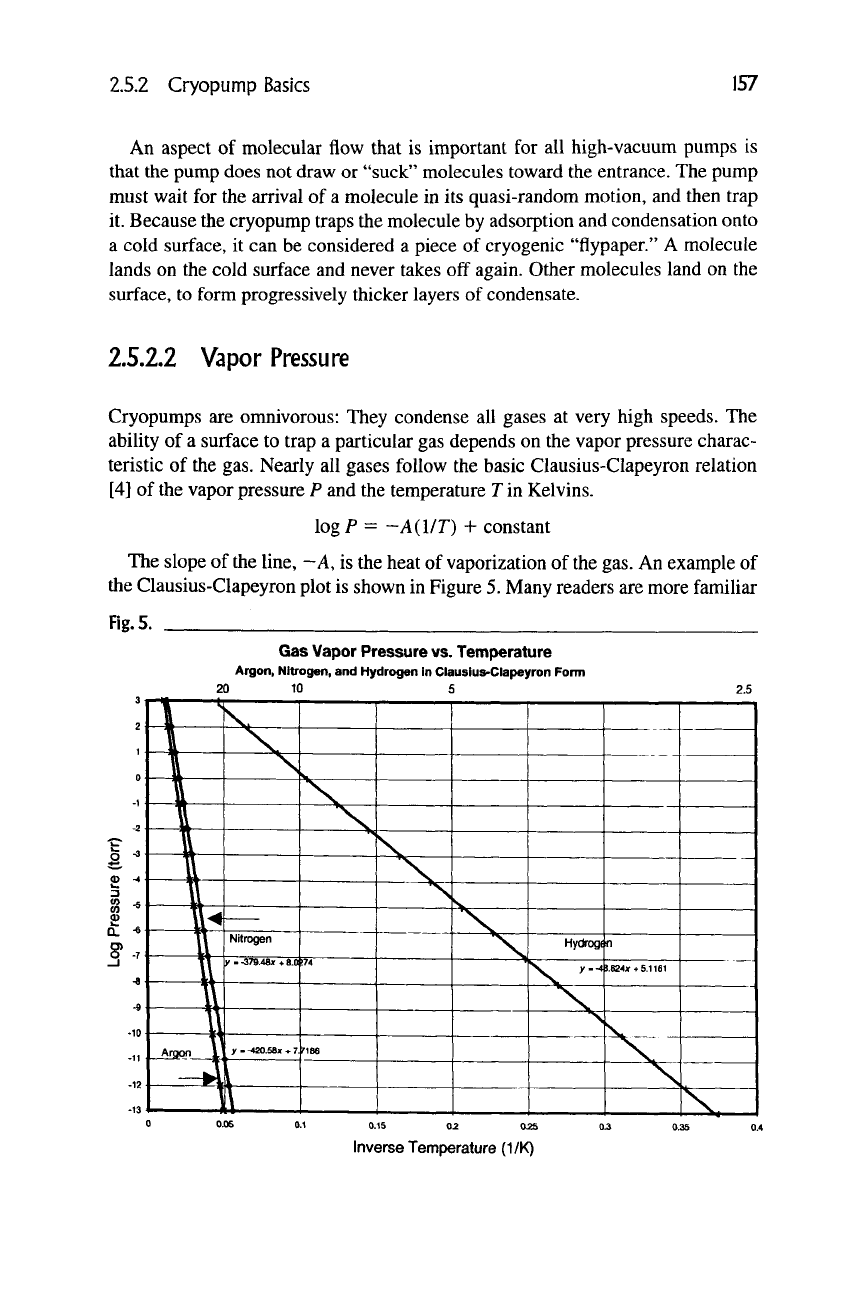
The slope of the line, -A, is the heat of vaporization of the gas. An example of
the Clausius-Clapeyron plot is shown in Figure 5. Many readers are more familiar
Fig.
5.
o
(D
v_
D
(O
CO
CL
Gas Vapor Pressure vs. Temperature
Argon,
Nitrogen, and Hydrogen in Clausius-Clapeyron Form
20 10 5
2.5
1
lA.^
1
Ll^
1 Argon L'
Nitrogen
y
m
-379.48X • 8.0
y - -420.58X • 7
S74
ri86
Hydrogt
\. y-<
fi
J.624X •5.1161
^< 1
Inverse Temperature (1/K)
158
Chapter 2.5: Cryogenic Pumps
Fig.
6.
Gas Vapor Pressure vs. Temperature
Hydrogen, Nitrogen, Argon, and Water in Honig & Hoolc Form
3
3
1 -'
(0
>
5 ^
-13
-15
Hydr
age
I
\7]
K
Nitrog
7^-
jn i
Ar
-
jon
1
/
Watelr
10 100
Temperature (K)
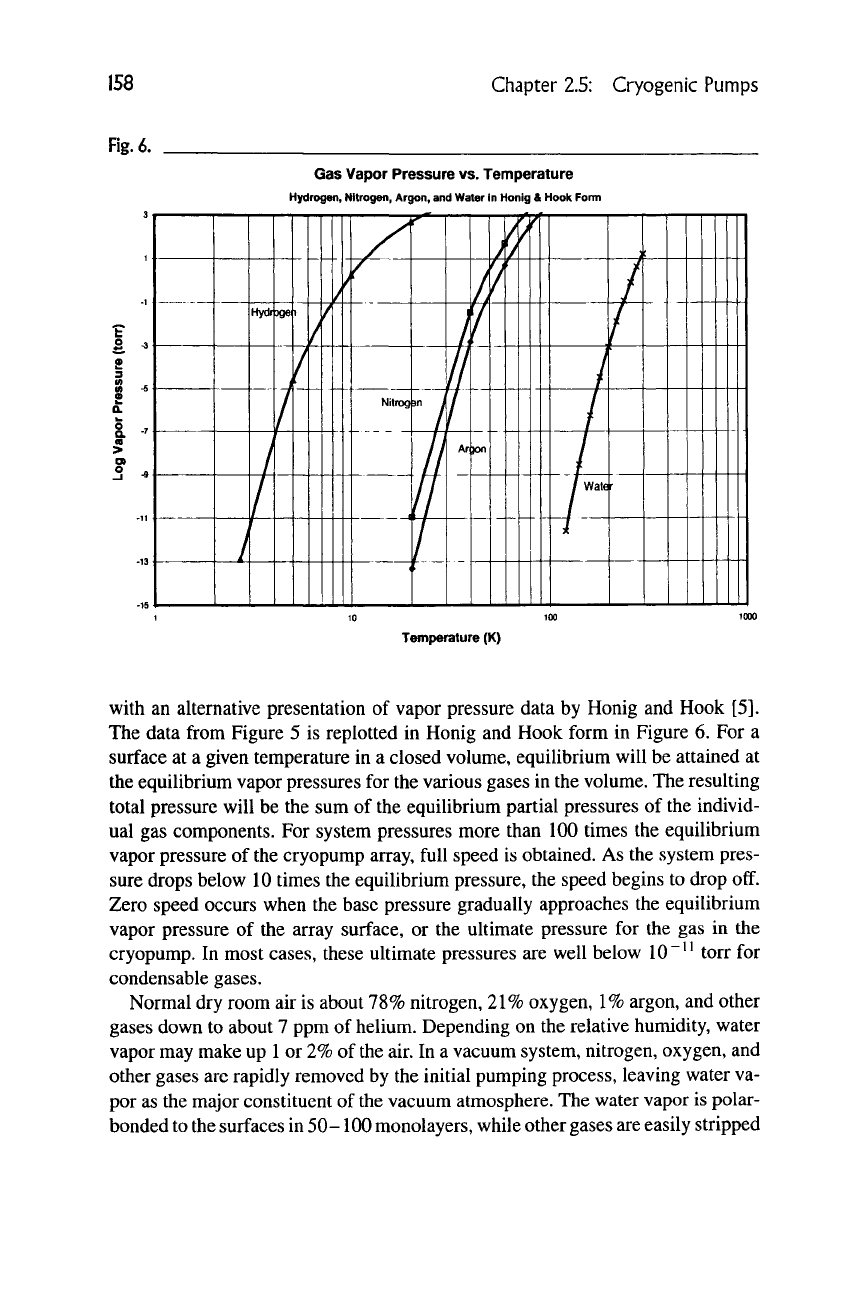
with an alternative presentation of vapor pressure data by Honig and Hook [5].
The data from Figure 5 is replotted in Honig and Hook form in Figure 6. For a
surface at a given temperature in a closed volume, equilibrium will be attained at
the equilibrium vapor pressures for the various gases in the volume. The resulting
total pressure will be the sum of the equilibrium partial pressures of the individ-
ual gas components. For system pressures more than 100 times the equilibrium
vapor pressure of the cryopump array, full speed is obtained. As the system pres-
sure drops below 10 times the equilibrium pressure, the speed begins to drop off.
Zero speed occurs when the base pressure gradually approaches the equilibrium
vapor pressure of the array surface, or the ultimate pressure for the gas in the
cryopump. In most cases, these ultimate pressures are well below 10"^^ torr for
condensable gases.
Normal dry room air is about 78% nitrogen,
21%
oxygen, 1% argon, and other
gases down to about 7 ppm of helium. Depending on the relative humidity, water
vapor may make up
1
or 2% of the air. In a vacuum system, nitrogen, oxygen, and
other gases are rapidly removed by the initial pumping process, leaving water va-
por as the major constituent of the vacuum atmosphere. The water vapor is polar-
bonded to the surfaces in 50-100 monolayers, while other gases are easily stripped
2.5.2 Cryopump Basics '59
away. Water may make up 98% of the gas load of a vacuum system at 10""^ torr.
Consequently, high pumping speeds for water vapor are crucial in all vacuum
systems.
2.5-2.3 Condensation
Water is relatively easy to pump onto cryogenic surfaces. At 130 K, an equi-
librium vapor pressure of 10"^^ torr is obtained, ensuring nearly 100% pump-
ing efficiency to pressures below 10"^ torr. A surface below 113 K will provide
greater than 99% efficiency to below 10'^^ torr. Consequently, operation of a
cryopump surface in the 65-90 K range is 100% efficient for pumping water and
high-molecular-weight gases and vapors in any practical vacuum system. Tem-
peratures below 20 K are necessary for condensing nitrogen, oxygen, argon, and
most other condensable gases. At these temperatures, the condensates are dense
solids, like ice. It should be noted that it is not practical to condense hydrogen, he-
lium, and neon, even at temperatures in the 6-20 K
range.
These three gases must
be pumped by special adsorption surfaces within the cryopump.
For all other gases, however, the condensation surfaces in the 10-20 K range
provide 100% efficiency pumping at constant speed over the entire pressure range
from below
10 ~ ^^
torr to above
10 ""^
torr. As the pressure rises into the low
10
"^
torr range, the pumping speeds increase by about 20-40% due to the onset of
transitional flow. In most applications, the speed of the cryopump can be treated
as essentially constant at all pressures.
The process of condensation requires removal of heat from the gas molecules
through contact with the cold surfaces. Remarkably, the quantity of heat removed
from gases near room temperature is quite low. For nitrogen, the conversion of
gas at 300 K to a solid at 20 K is a complex though instantaneous process.
Nitrogen Condensation Budget
Cooling Process
Gas cooled 300 K to 77 K
Liquefaction at 77 K
Cool liquid 77 to 63 K
Transition liquid to solid I
Cool solid I 63 K to 36 K
Transition solid I to solid II
Cool solid II to 20 K
Total
Enthalpy
(calories/gram-mole)
1600
1300
189
172
267
54
123
3700

160 Chapter 2.5: Cryogenic Pumps
This translates to about
1
watt of cooling required for each 100 std. cmVminute
of nitrogen gas flow into the system, about the flow in a sputtering system at 2 X
10 ~^
torr. For the same pump operating in the
10
"^ range or below, the total con-
densation load is less than 1 milliwatt. It is for this reason that even very large
cryopumps can be constructed with a relatively small refrigeration capacity at
20 K and below. For comparison, the mechanical work involved in the transport
of 100 std. cmVminute (0.2 torr-L/sec) of gas in a vacuum system requires only
about 30 milliwatts of power.
It is frequently advisable to control the operating temperature of the inlet array
and thermal radiation shield at a preset value. Generally, the temperature is set in
the range of
65
to 90 K to prevent the partial adsorption of
Ar,
N2, CO, and other
gases on the first-stage array parts. For example, the first-stage temperature can
drop to 40-45 K in a cryopump with very low thermal loads. If the pump is then
used for sputtering with Ar at
10 ~^
torr, some argon is adsorbed in a very thin
layer on inlet array surfaces. When the gas flow is shut off at the end of the pro-
cess,
the chamber pressure may take a long time to recover to the 10"^ to 10"^
range as the lightly adsorbed gas is evolved from the inlet array. The presence of
a thick water ice deposit on the inlet array can make the problem worse due to the
larger effective surface area of the microcrystalline ice deposits. Raising the first-
stage array temperature to the 65-90 K range reduces the adsorption of Ar and
speeds the recovery to base pressures.
In principle, a first-stage array temperature in the range of 90-105 K might be
desirable for some processes requiring repetitive cycling over a large dynamic
pressure range. However, since the first and second stages of the refrigerator are
thermodynamically linked, it is not normally possible to drive up the first-stage
temperature to these high values without also dragging the second-stage tempera-
tures well above the optimum 10-14 K range.
2.5.2.4 Adsorption
The three light gases H2, He, and Ne cannot be pumped by condensation and
must be adsorbed. Activated carbon or charcoal from coconut shells, wood, or
other organic materials is used for this purpose. Treatment of the charcoal with
steam at high temperatures produces huge effective areas of 1000 m^/gram or
more. The carbon then has an interconnected network of minute channels or
fissures with tightly controlled pore size in the range of 10-30 angstroms. A gas
molecule landing on the outer surface of the carbon loses kinetic energy as it
cools to 10-12 K. Such loosely held molecules then diffuse rapidly into the inner
volume of the charcoal. At these temperatures, hydrogen molecules can be re-
tained at very low equilibrium vapor pressures as up to a monolayer of gas is ad-

2.S.2 Cryopump Basics
1^1
sorbed onto the carbon. Although this may not seem impressive, at approximately
10
^^
atoms/cm^ a gram of charcoal (about a teaspoon) can hold 200 std. cm^^ of
hydrogen. A handful of charcoal can hold 10-20 liters. A layer of charcoal gran-
ules or pellets is attached to copper sheets with vacuum-compatible epoxy or other
adhesives.
The remarkable ability of cold, activated carbon to adsorb gas is demonstrated
by the reduction in vapor pressure. A flat copper surface at 12 K would hold only
a small fraction of
a
monolayer of hydrogen while coming to an equilibrium pres-
sure of about 20 torr. By adding 50-100 grams of charcoal to the copper surfaces,
many liters of gas can be adsorbed before reaching an equilibrium pressure of
about 10"^^ to 10"^ torr. This is a reduction in pressure by 12 orders of magni-
tude with a simple technique.
Carbons are used in preference to zeolites because carbons are hydrophobic.
That is, when the cryopump is eventually regenerated, any adsorbed water vapor
can be removed from the charcoal by heating at 30-40°C, instead of the 200-
250°C required of zeolites. The activated carbons used in cryopumps have been
processed at very high temperatures to obtain nearly pure carbon with few impu-
rities.
Since both the carbon and the adhesive are at about 12 K whenever the
high-vacuum valve is open to the rest of the vacuum system, they are always
"sinks"
for gases and never sources of outgassing.
The operating temperature of the charcoal affects how much gas can be re-
tained at a given equilibrium pressure. At 10-15 K, a gram of charcoal will hold
about 200 std. cm^ of hydrogen at 5 X
10 ~^
torr or about 100 std. cm^ at 5 X
10 ~^
torr. These are the normal conditions of use of a cryopump at maximum
rated capacity. Temperatures below 10 K do not significantly improve effective
capacity, although lower ultimate pressures may be obtained in clean, baked UHV
systems with light hydrogen loads. At high hydrogen gas loads that produce par-
tial pressures of 10"^ torr and above, very low carbon temperatures are harmful.
The hydrogen may not be able to diffuse to the inside of the carbon granules
rapidly enough to maintain low pressures. Instead, operation of the charcoal in
the 12-14 K range is optimum to balance good capacity with maintainable, con-
stant pumping speed [6]. Above 25 K, though, the capacity of carbon to adsorb
hydrogen falls rapidly. It has the ability to adsorb (pump) many other gases, such
as nitrogen or argon, at temperatures well above 90 K.
Helium is more difficult to adsorb than hydrogen. A pump that can adsorb up
to 20 liters of hydrogen while holding pressures below 5 X 10"^ torr, can adsorb
only 5 std. cm^ of helium at the same maximum pressure level. It is fortunate that
helium is only a minor constituent in our atmosphere. The very low capacity for
helium precludes the use of cryopumps for any process that bleeds helium into
the vacuum chamber. It also requires that helium remain as only a trace impurity
in any other process gas, a condition not always obtained. In particular, conmier-

162 Chapter 2.5: Cryogenic Pumps
cial bottled gas suppliers and on-site air separation facilities may not count he-
lium or neon as impurities when they certify argon and nitrogen gas purity. The
presence of 10-100 ppm of helium in a process gas stream can quickly cause
base pressure problems in cryopumped systems.
2.5.2.5 Thermal Considerations
Thermal radiation loads also play a role in the design and operation of cryogenic
high-vacuum pumps. Pumps are usually mounted on a port of the vacuum cham-
ber with a thin gate valve at the opening. The vacuum chamber acts as a large
optical integrating cavity or "black body" at the wall temperature if there are
no other heat loads in the chamber. With a room temperature (295 K) wall, the
thermal load from infrared radiation on the inlet of the pump is approximately
0.05 watt/cm^, or about 16 watts across a standard
8-inch
diameter pump. With
hotter chamber surfaces, the thermal load goes up as
T"^
(in Kelvins). This limits
most cryopumps to operating on chambers with wall temperatures in the range of
50-100°C.
Most of the thermal load is absorbed by the first stage of the refrigerator as the
radiation strikes the inlet array of the pump. The inlet array of the pump is typi-
cally nickel plated to reduce the absorbed thermal load on the pump during the
cooldown process when the pump is empty of all gases. Once the pump has oper-
ated for a few hours or days, a thin layer of infrared-absorbing water ice forms on
the inlet array. This effect raises the thermal load on the inlet array. Some thermal
radiation passes through or around the inlet array to fall on a black thermal radia-
tion shield, which is also cooled to about 65-80 K by the first stage of the refrig-
erator. Because the refrigerator typically has three times the cooling capacity on
the
65
K
first
stage as on the
12
K second
stage,
the inlet array also serves as a shield
for the second-stage array to prevent thermal overloading of the low-temperature
surfaces.
Thermal sources in the vacuum chamber, such as heat lamps and deposition
sources, add other heat loads to the cryopump. Stainless steel vacuum chamber
walls are 90% reflective in the infrared, whereas aluminum surfaces may approach
98%
reflectivity at long optical wavelengths. This allows many bounces of the
radiation around the chamber with little attenuation. Infrared radiation, unseen by
the human eye, winds up in the "black hole" of the cryopump. Consequently,
attention to thermal shielding of the heat sources in the chamber or at the pump-
ing port may be necessary. That this shielding process can be effective is demon-
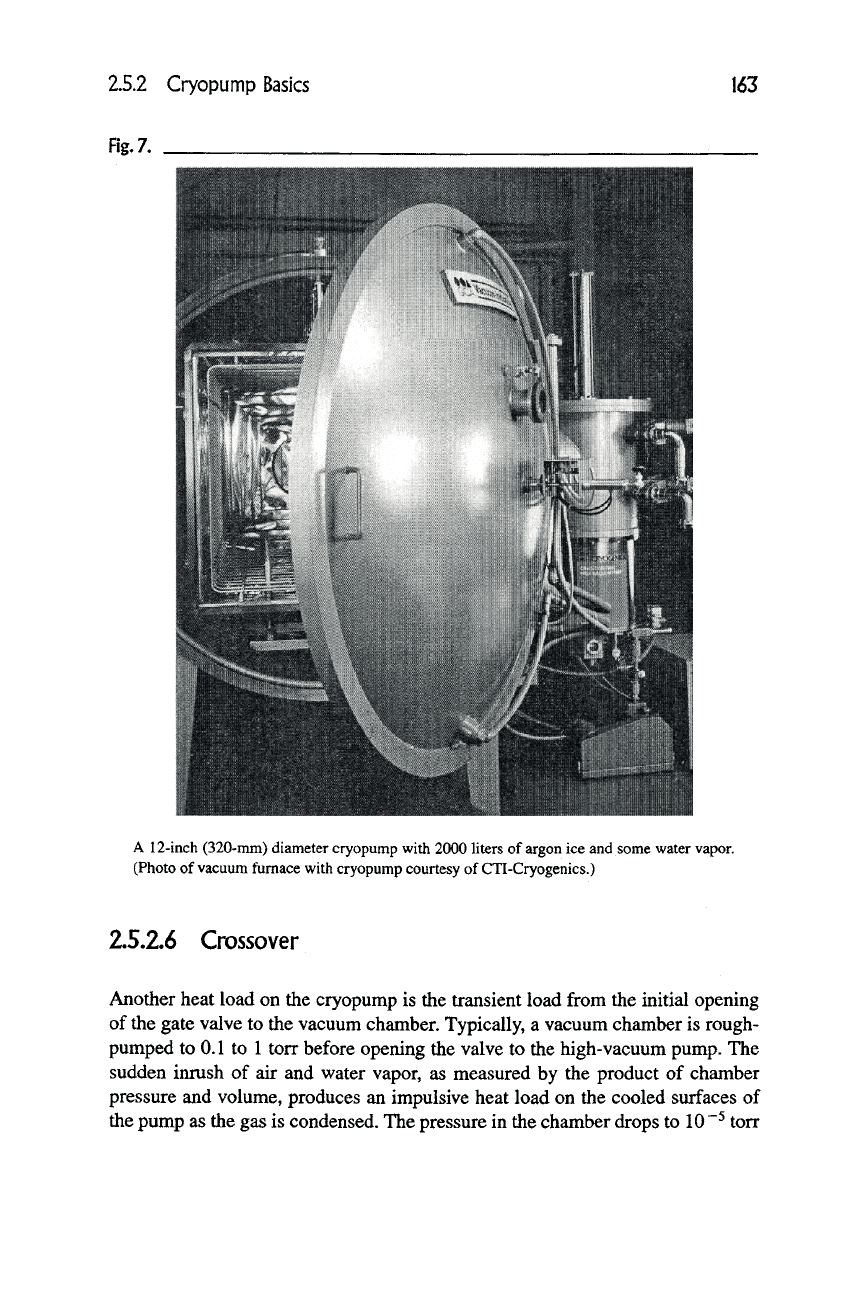
strated by the use of cryopumps on vacuum furnaces, such as the one shown in
Figure 7, having hot zones operating at 2000°C and above.
2.5.2 Cryopump Basics
\63
Fig.7.
A 12-inch (320-mm) diameter cryopump with 2000 liters of argon ice and some water vapor.
(Photo of vacuum furnace with cryopump courtesy of CTI-Cryogenics.)
2.5.2.6 Crossover
Another heat load on the cryopump is the transient load from the initial opening
of the gate valve to the vacuum chamber. Typically, a vacuum chamber is rough-
pumped to 0.1 to 1 torr before opening the valve to the high-vacuum pump. The
sudden inrush of air and water vapor, as measured by the product of chamber
pressure and volume, produces an impulsive heat load on the cooled surfaces of
the pump as the gas is condensed. The pressure in the chamber drops to
10
"^
torr

164 Chapter 2.5: Cryogenic Pumps
or less in a few seconds. The joules of heat extracted
from
the gas during cooling
momentarily exceed the ability of the refrigerator to remove heat and maintain
temperature. Now, the thermal mass of
the
cooled parts determines how high the
temperature will rise. The
crossover rating
of the pump, usually given in torr-
liters,
is that product of chamber pressure and volume that can be acconmiodated
without raising the second-stage array parts above 20
K.
This ensures that any ad-
sorbed hydrogen will not
be
released back
into
the system, causing
the
pressure to
go
up instead of
down.
Interestingly, continued use of
the
cryopump improves the
crossover rating because the condensed gas loads have significant thermal capac-
ity of their own. For example, 300 standard liters of argon gas condensed into
about
375
cm^ of argon ice weighing about
535
grams.
This ice at
12 K
has
a
heat
capacity approaching that of the mass of copper and stainless steel of the cryo-
pump parts at
12
K, effectively doubling the crossover rating.
2.5,2.7
Capacity
The capacity of
a
cryopump to store condensed gases depends on the dimensions
of the
pump.
Water vapor—and other
Type
I gases that
have
low vapor pressures
at
65
K—condense on the top surfaces of
the
inlet array and on the inside of the
thermal radiation shield. For
a
200-mm (8-inch) cryopump, nearly a liter of water
can be stored as ice, spread out over these surfaces in layers up to a few centime-
ters
thick.
The
ultimate limit
on
water capacity
is
that
the
ice will eventually choke
off
the
ability of other gases to reach the interior of
the
pump,
thus reducing their
pumping speed. In most applications, a few tens of cubic centimeters of water
will
be
collected over
a
few weeks of operation. Under
UHV
conditions,
the
pump
will not collect a thimble full of water or other gases in a
year!
A cryopump with
only a little condensed water
is
shown in Figure 8.
Argon, nitrogen, oxygen, and other Type II gases condensable at 20 K, collect
on the second-stage surfaces at 10-20 K. As they form ice layers of several cen-
timeters thickness, several things begin to happen. The ice grows upward until it
reaches the
65 K
inlet
array.
Since
the
Type
II gases
will
not condense at
65
K, the
ice continues to grow up through the inlet array without actually touching the
warmer surfaces. The Type II ice ball also begins to block access of
the
Type III
gases,
which do not condense at 20 K (hydrogen, helium, neon) to the carbon-
coated surfaces. Furthermore, the Type n ice is no longer thermally shielded by
the inlet, so the thermal loads increase on the second stage of
the
refrigerator. Be-
tween the increased thermal load and the increasing thermal gradient through the
ice,
the equilibrium pressure obtainable begins
to
rise.
This sets the practical limit
on capacity for Type II gases at about 1000 standard liters of gas for a 200-mm
cryopump before the equilibrium pressure rises into the
10
~^
torr
range.
For sys-
tems that must cycle rapidly and frequently from above 10 "^ for sputtering
to
be-

ZS.2 Cryopump Basics
165
Rg.8.
A 12-inch cryopump showing argon ice thickness of 2-3 cm as regeneration continues and ice
begins to melt. (Photo of cryopump inlet array with ice courtesy of CTI-Cryogenics.)
low 10"^ torr for chamber
cleanup,
reduced efifective capacities may be observed
as the time to recover to base pressure increases.
In practical
terms,
a cryopump can be used for a long time before reaching ca-
pacity. For static, high-vacuum pumping at 10"^ torr, an
8-inch
pump could run
for one to three years before requiring regeneration. On load-lock or batch ap-
plications without additional process gas loads, 2000-5000 pumpdown cycles
may be
obtained between
regenerations.
Sputtering
systems have
typical argon gas
loads of 50-150 std. cmVminute coupled with requirements for rapid recovery,
limiting time between regenerations to
1
-2
weeks.
Similarly, the heavy hydrogen
gas loads of ion implanters require regeneration at intervals of 5-15 days in full-
production situations.
2.5.2.8
Regeneration
As the effective capacity is reached, it
is
necessary to regenerate the cryopump to
restore full pumping speed and the ability to recover rapidly to
base
pressure.
Be-
cause three types of adsorption processes have been used to collect the gases, a
166
Chapter 2.5: Cryogenic Pumps
number of effects take place during regeneration. The simplest way to regenerate
a pump is simply to close the high-vacuum valve and turn off the refrigerator.
Since the pump will initially be under high vacuum, with all the surfaces cold, the
arrays and condensed gases will warm slowly as heat is conducted through the
cylinder of the refrigerator to the second-stage array. As the second-stage array
temperature (and the temperature of the charcoal attached to the array) climbs
above 25 K, hydrogen and other Type III gases are released into the pump vessel,
raising the pressure. As the pump pressure rises above
10 "-^
torr, the thermal con-
ductivity of the gas increases, providing more heat load on the arrays. Above 30 K,
the equilibrium vapor pressures of the Type II gases condensed on the second
stage begin to rise, and nitrogen and argon add to the rising pressure in the pump.
For a few minutes, the still-cold charcoal adsorbs the Type II gases, suppressing
the pressure rise. Eventually, the second stage warms sufficiently to liquefy the
Type II gases, which then flow to the warmed thermal radiation shield and outer
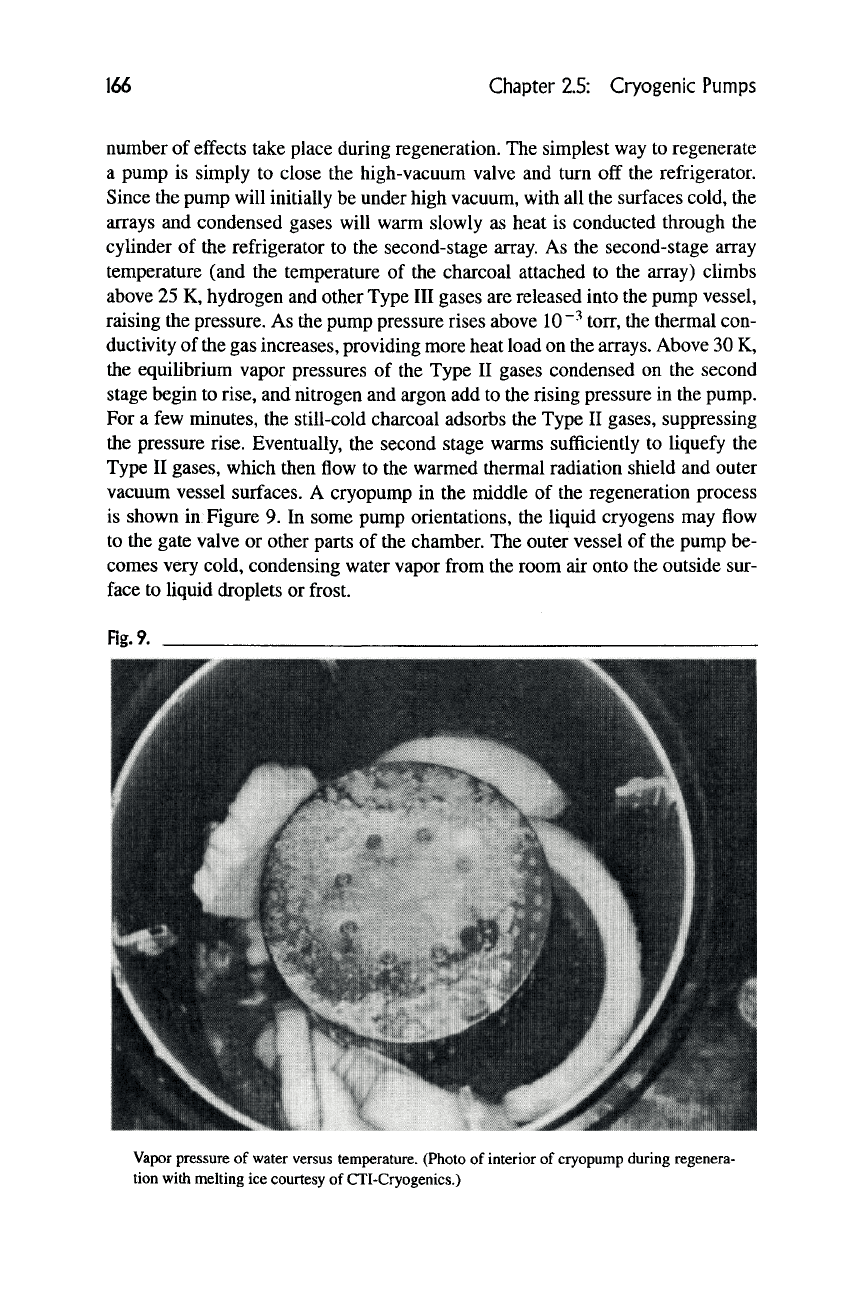
vacuum vessel surfaces. A cryopump in the middle of the regeneration process
is shown in Figure 9. In some pump orientations, the liquid cryogens may flow
to the gate valve or other parts of the chamber. The outer vessel of the pump be-
comes very cold, condensing water vapor from the room air onto the outside sur-
face to liquid droplets or frost.
Fig.
9.
Vapor pressure of water versus temperature. (Photo of interior of cryopump during regenera-
tion with melting ice courtesy of CTI-Cryogenics.)
