Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.


A number of methods are available for the production of metallic coat-
ings.
The most widely used are either electro-plating or dipping the articles
to be coated into a bath of the molten metal. Other methods involve
spraying or volatilising the protective metal on to the surface whilst mech-
anical 'cladding' is also used.
21.81 'Cladding' is used chiefly in the production of 'clad' sheet and
is employed prior to the final manufacturing stages of a component. The
base metal is sandwiched between pieces of the coating metal and the
sandwich is then rolled to the required thickness. In some cases the film
of protective metal can be sprayed on to the base surface and then rolled
on. 'Alclad' is duralumin coated with pure aluminium (up to 0.01 mm thick
depending upon the overall thickness of the stock) but other high-strength
alloys of aluminium, which have poor corrosion resistance because of their
multi-phase microstructures, are also clad. Mild steel clad with stainless
steel is also available but it is important to note that with all forms of
cladding damage to the protective skin can take place during fabrication.
21.82 Hot-dip Metal Coating Tin and zinc are the metals most often
used to produce metallic coatings in this manner, though use is also made
of aluminium. Ideally the coating should adhere to the base surface by
solid solution between the two. In some cases however, brittle intermetallic
compounds are formed at or near the interface such as FeSn2, FeSn, or
Fe
2
Sn in the case of hot-dip tinning.
(a) Hot-dip Tinning. Tinplate is principally used in the manufacture of
cans for packaging a wide variety of foodstuffs. The non-toxic properties
of tin, combined with its good corrosion-resistance and the ease with which
tin-coated articles can be soldered, make it a useful metal for coating
articles which come into contact with all types of food.
The mild-steel sheet used for the manufacture of tinplate is usually about
0.25 mm thick, and, after being annealed, it is freed from all traces of
oxide by pickling in either dilute sulphuric acid or dilute hydrochloric acid.
The tinning unit comprises a thermostatically controlled vessel of molten
tin in which is submerged a system of guides and rollers for conducting the
pickled sheets down through a layer of molten flux into the tin and then
upwards and out of the tin through a layer of palm oil. As they pass
through the palm oil, the sheets are subjected to a squeegeeing action by
tinned-steel rollers, which serve to control the final thickness of coating on
the sheets.
After leaving the tinning machine, the tinned sheets are cooled and then
cleaned, usually by washing them in an alkaline detergent. Finally, they
are polished in either bran or wood meal.
Other iron and steel items of kitchenware such as mincing machines and
mixers, are coated by hot-dipping after fabrication. After being cleaned,
degreased and pickled, the component is immersed in a pot of molten tin,
on the surface of which is a layer of flux. If the operations have been
correctly carried out a brilliant finish is obtained which requires little in
the way of after-work.
(b) Hot-dip Galvanising. The term galvanising is derived from the name
of Luigi Galvani, the Italian physiologist, whose classic observations of

dead frogs, in 1786, led to Volta's development of the electric cell. In 1829
Faraday observed that when zinc and iron, in contact with each other,
were exposed to the air in the presence of a salt solution the iron did not
rust; whereas if the zinc were removed the iron rusted rapidly. Faraday
attributed this to 'the effect of chemical action ... in exciting electricity'.
In 1836 a Frenchman, Sorel, took out a patent for a process of coatmg
steel with zinc by hot-dipping, and named the process galvanising. The
word was used to indicate the sacrificial electro-chemical protection
afforded iron by the zinc.
Before being galvanised, the surface of steel must be thoroughly cleaned,
either by shot blasting or, more generally, by pickling in dilute sulphuric
acid. In order to prevent contamination of the molten zinc by iron, the
work is then washed to remove all traces of iron salts formed by pickling.
It is necessary, however, to protect the surface of the work from fresh
oxidation, so it is immersed in a flux bath consisting of a solution of zine
chloride and ammonium chloride. This flux is usually dried on to the sur-
face in an oven which is provided with an adequate draught and working
at a low temperature, to prevent interaction between the flux and the iron
surface.
The work, with its protective flux coating, is then immersed in the molten
zinc bath. The flux coating peels away to form a layer on the surrounding
bath surface, which in turn is protected from oxidation; and the surface of
the work is left clean so that it is immediately wetted by the molten zinc.
The thickness of the coating produced depends largely upon the tempera-
ture of the bath and the'rate at which the work is withdrawn, but good-
quality galvanised articles should carry between 0.3 and 0.6 kg/m
2
of zinc
on the surface. Thinner and more flexible coatings are obtained by using
a zinc bath containing about 0.2% aluminium. This limits the formation of
intermetallic compounds of iron and zinc on the steel surface.
In addition to corrugated iron for roofing, the large number of galvanised
products includes buckets, barbed wire, water tanks, marine fittings, bolts,
and fencing fittings, agricultural equipment and window frames. Many
galvanised products such as dust bins have been replaced by plastics mould-
ings whilst aluminium is becoming increasingly popular for the manufac-
ture of window frames.
(c) Hot-dip Coating with Aluminium (Aluminising) is used in the protec-
tion of iron and steel surfaces under a wide variety of trade names, the
best-known of which is 'Aludip'. In all of these processes the surface must
be cleaned by grit blasting, de-greasing and/or pickling. It is then carefully
dried before immersion in a bath of molten aluminium at about 700
0
C,
generally through a layer of molten flux. In some processes steel sheet is
heated in an atmosphere of hydrogen at 1000
0
C prior to dipping in order
to reduce any oxide films present, but in addition to the extra cost of this
treatment the quality of the product is often less satisfactory.
In some methods, particularly in the USA, the aluminium-dipped mild-
steel sheet is then rolled to give a better surface finish. This product is used
to replace tinplate for some containers.
(d) Hot-dip Coating with ZinclAluminium Alloys. These relatively new

commercial processes are said to give a much more corrosion-resistant
coating on steel sheet, strip, wire and tube than is obtained by conventional
hot-dip galvanising. The high-aluminium coatings Galvalume and
Zincalume use an alloy containing 55Al-45Zn and small amounts of sili-
con; whilst Galfan coatings are based on 95Zn-5Al (with very small
additions of 'rare earth' metals). Though Galfan coating is less corrosion
resistant than those of the 55Al-45Zn alloys it has better formability and
retains paint more effectively.
21.83 Coating by Means of a Spray of Molten Metal Metal spraying
consists in projecting so-called 'atomised' particles of molten metal from
a special pistol on to a suitably prepared surface. Surface preparation
usually involves blasting the surface with an abrasive; steel grit having
replaced sharp silica sand for this purpose because of health hazards
involved when the latter is used. It is essential that the surface is rough-
ened, rather than peened or polished by the grit in order that good
adhesion shall take place. Hence attention to the condition of the grit is
important. The metals most commonly used for spraying are zinc and
aluminium, though coatings of tin, lead, cadmium, copper, silver and stain-
less steel can be so deposited.
Early metal spraying processes, originally invented by Dr. Schoop, used
an oxyacetylene flame to carry particles of metal on to the work surface.
The metal was injected into the flame as a powder. Later modifications of
the Schoop process used electrical melting of the metal. An arc was struck
between two zinc wires in the pistol and the molten zinc so produced
was 'atomised' and carried forward in a blast of compressed air. Flame
impingement methods are now the most common, using a specially
designed flame gun in conjunction with either a continuous rod or a hopper
feeding powder to the high-velocity flame. The temperature is generally
sufficient to melt the metal, but not necessarily so, and in either case the
small particles are projected with high energy at the work surface.
The most important factor is rigid control of the flame conditions. If
too high a temperature or too oxidising flame conditions prevail then the
particles may be oxidised before they reach the work surface. Nevertheless
it is a satisfactory method for structural steel provided that flame conditions
are controlled.
Metal spraying has wide application in view of its portability and flexi-
bility; thus, large structures, such as storage tanks, pylons and bridges, can
be sprayed on site. Notable recent examples include the Forth Road Bridge
and the Volta River Bridge (Ghana), both of which were zinc coated using
modern developments of the Schoop process.
21.84 Sherardising is a cementation process, similar in many respects
to carburising, in that zinc is made to combine with an iron or steel surface
by heating the work with zinc dust at a temperature below the melting
point of zinc. The first patent for this process was taken out in London in
1901 by Sherard Cowper-Coles, after whom the process is named. Much
thinner coatings can be obtained by sherardising than are possible with
hot-dip galvanising, and generally about 0.15 kg/m
2
of zinc is used. More-
over, a more even film is obtained, thus making possible the treatment

of such articles as nuts and bolts, the threaded portions of which would
undoubtedly become clogged by hot-dipping.
As in all other coating processes, the surface of the work must first
be properly prepared. This usually involves degreasing, followed by acid
pickling in cold 50% hydrochloric acid or in hot 10% sulphuric acid. Shot
blasting is also used, particularly in the case of iron castings, to remove
graphite and core sand.
The work is packed into mild-steel drums along with some zinc
powder. The drums are heated to 370
0
C and rotated slowly so that the
tumbling action brings all the components into contact with the powdered
zinc.
'Calorising' is a process in which steel components are coated with alu-
minium by a similar method. The components are treated in a heated
rotating cylinder containing a mixture of aluminium and aluminium oxide
powders. It is used principally to provide a protective coating for heat-
treatment pots, ladles and furnace components. Nickel alloys may be pro-
tected from attack by sulphur gases by aluminising them. The components
are packed into a heat-resistant box along with aluminium powder. Air
is then purged from the box by flushing it with argon or nitrogen. The
tightly-sealed box is then heated for several hours to promote aluminisation
of the surface. 'Chromising' is a similar process involving chromium coat-
ing of a mild steel surface in which corrosion resistance comparable with
that of a 12% chromium steel can be achieved.
21.85 Electro-plating The formation of metal coatings by electro-
deposition is well known, and a wide variety of metals can be thus used,
including copper, nickel, chromium, cadmium, gold and silver. Tin and
zinc can also be electro-deposited, and a coating thus formed has advan-
tages over one produced by hot-dipping in respect of flexibility, uniformity
and control of thickness of film.
The usual degreasing and pickling processes must be applied to the
surface before any attempt is made at electro-plating it. Most instances of
peeling and blistering in the case of chromium-plating are due to inad-
equate preparation of the surface. The author has seen specimens in which
the layer of chromium had been deposited on to oxide scale, with the
inevitable peeling of the chromium as a result.
In the actual process of electro-plating the article to be plated is made
the cathode in an electrolytic cell. Sometimes the metal to be deposited is
contained, as a soluble salt, in the electrolyte, in which case the anode is
a non-reactive conductor, such as stainless steel, lead or carbon. In most
cases,
however, the anode consists of a plate of the pure metal which is
being deposited, whilst the electrolyte will contain a salt or salts of the
same metal. Then, the anode gradually dissolves and maintains the concen-
tration of the metal in the electrolyte as it is deposited on to the articles
forming the cathode.
The conditions under which deposition takes place are very important,
so that the cell voltage, the current density (measured in amperes per
square metre of cathode surface), the ratio of anode area to cathode area
and the time of deposition, as well as the composition and temperature of
the electrolyte, must all be strictly controlled if a uniform adherent and
nonporous film is to be obtained.
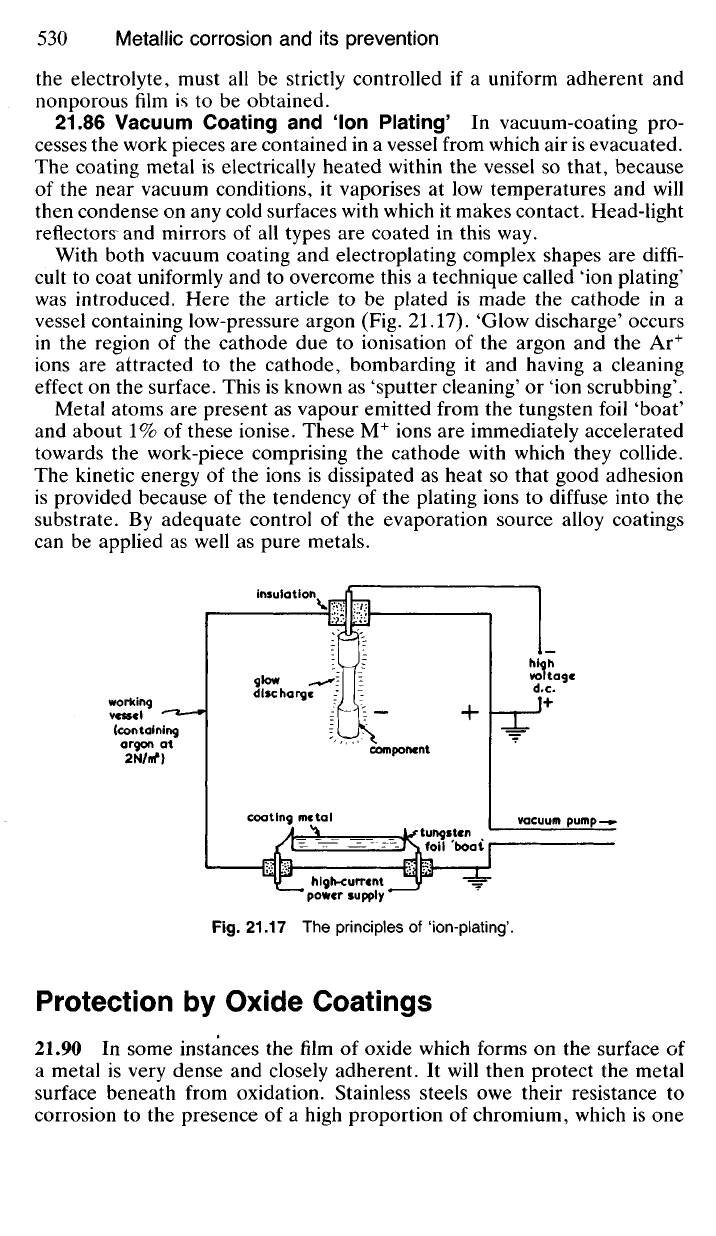
21.86 Vacuum Coating and 'Ion Plating' In vacuum-coating pro-
cesses the work pieces are contained in a vessel from which air is evacuated.
The coating metal is electrically heated within the vessel so that, because
of the near vacuum conditions, it vaporises at low temperatures and will
then condense on any cold surfaces with which it makes contact. Head-light
reflectors and mirrors of all types are coated in this way.
With both vacuum coating and electroplating complex shapes are diffi-
cult to coat uniformly and to overcome this a technique called ion plating'
was introduced. Here the article to be plated is made the cathode in a
vessel containing low-pressure argon (Fig. 21.17). 'Glow discharge' occurs
in the region of the cathode due to ionisation of the argon and the Ar
+
ions are attracted to the cathode, bombarding it and having a cleaning
effect on the surface. This is known as 'sputter cleaning' or 'ion scrubbing'.
Metal atoms are present as vapour emitted from the tungsten foil 'boat'
and about 1% of these ionise. These M
+
ions are immediately accelerated
towards the work-piece comprising the cathode with which they collide.
The kinetic energy of the ions is dissipated as heat so that good adhesion
is provided because of the tendency of the plating ions to diffuse into the
substrate. By adequate control of the evaporation source alloy coatings
can be applied as well as pure metals.
Fig.
21.17 The principles of 'ion-plating'.
Protection by Oxide Coatings
21.90 In some instances the film of oxide which forms on the surface of
a metal is very dense and closely adherent. It will then protect the metal
surface beneath from oxidation. Stainless steels owe their resistance to
corrosion to the presence of a high proportion of chromium, which is one
working
vessel
(containing
argon at
2N/m*)
insulation
high
voltage
d.c.
glow
discharge
component
vacuum pump
coating metal
tungsten
foil 'boat
high-current
power supply

of these elements that form oxide films impervious to oxygen. The blueing
of ordinary carbon steel by heating it in air produces an oxide film of such
a nature that it affords partial protection from corrosion.
21.91 Anodising Reference has already been made (21.21) to the
protection afforded aluminium by the natural film of oxide which forms
on its surface. Anodic oxidation, or anodising, is an electrolytic process
for thickening this oxide film. This process may be applied for several
reasons, such as to provide a key for painting, to provide an insulating
coating for an electrical conductor or to provide a surface which may be
dyed, as well as to increase the resistance of aluminium to corrosion.
Before being anodised the surface of the article must be chemically
clean. Preliminary treatment involves sand-blasting, scratch brushing or
barrel polishing, according to the nature of the component. This is followed
by degreasing in either the liquid or vapour of trichlorethylene; or by
electrolytic cleaning. The latter process is used mainly for highly polished
surfaces. The article is made the cathode in an electrolytic bath comprising
a 1% solution of sodium hydroxide, and treatment is continued for about
half a minute at a voltage of 14 and a current density of 550 A/m
2
. The
mechanism of this cleaning process is largely one of flotation of the film of
grease by hydrogen bubbles given off at the surface of the article which
comprises the cathode.
In the actual anodising operation which follows, the aluminium article
to be treated is made the anode in an electrolyte containing either chromic,
sulphuric or oxalic acid; the cathode being a plate of lead or stainless steel.
When an electric current is passed, oxygen is formed at the anode and
immediately combines with the aluminium surface of the article. The layer
of oxide thus formed grows outwards from the surface of the aluminium.
The normal thickness of a satisfactory anodic film produced commercially
varies between 0.007 and 0.015 mm, and a film having a thickness within
these limits would be formed by anodising a component in a 15% sulphuric
acid solution at 20
0
C for about thirty minutes, using a current density of
about 100 A/m
2
at a cell voltage of 15. A longer period of treatment
produces a thicker, but soft and spongy, film which would be unsatisfactory
in service. The thickness of the natural film produced on an aluminium
surface by exposure to air at normal temperatures is of the order of
0.000 013 mm.
The surface produced by anodising can be dyed by immersion in either
hot or cold baths of
dyestuff,
but whether or not the film is dyed, it should
always be sealed. The anodic coating is somewhat porous as it leaves
the electrolytic tank and is easily stained, but sealing renders the coating
impermeable. A simple, but quite effective, sealing process consists in
treatment with hot or boiling water, which renders the coating non-
absorptive without any visible change in appearance. Other sealing pro-
cesses include treatment in solutions of 1% nickel or cobalt acetates at
100
0
C,
followed by boiling in water, the application of linseed oil or lanolin
to the surface or the application, while the article is warm, of a mixture
containing equal parts of turpentine, oleic acid and stearic acid.
21.92 Beryllium Oxide Coatings The addition of small amounts of

beryllium to aluminium-base alloys causes a coating of beryllium oxide,
BeO,
to form on the surface. This is even more protective than the natural
skin of aluminium oxide, Al
2
Ch, described above. Unfortunately beryllium
is an extremely expensive metal. Nevertheless 0.1-0.3% beryllium is added
to some high-quality aluminium alloy castings for aerospace purposes.
In addition to providing the protective BeO skin beryllium acts as a
'scavenger' of oxygen and nitrogen in the melt. It also refines the structure
of any iron-based intermetallic compounds which may be present, replac-
ing the coarse needles with small equi-axed crystals.
Protection
by
Other Non-metallic Coatings
21.100 Coatings of this type usually offer only a limited protection against
corrosion and are, more often than not, used only as a base for painting.
21.101 Phosphating A number of commercial processes fall under
this heading, but in all of them a coating of phosphate is produced on the
surface of steel or zinc-base alloys by treating them in or with a solution
containing phosphoric acid and, generally, a metallic phosphate. The phos-
phate film which forms on the surface is usually grey in colour, about
0.0005 mm thick and offers only limited protection against corrosion but
since its surface is rough it provides an excellent 'key' for paint, varnish or
lacquer.
In automobile body work it also limits any filiform or scab corrosion
resulting from stone chipping by thus preventing any 'crevice corrosion'
(21.51) beneath the paint film.
In the automobile industry the spot-welded car body (termed the 'body
in white' at this stage) is usually given a preliminary degreasing treatment.
The phosphate coating is then achieved by using a phosphoric acid spray
in order that the hydrogen so liberated is flushed away:
Fe 4- 2H
3
PO
4
-> Fe(H
2
PO
4
)
2
+ H
2
1
The reaction will then proceed to completion:
3Fe(H
2
PO
4
)
2
-> Fe
3
(PO
4
)
2
+ 4H
3
PO
4
Iron phosphate, Fe
3
(PO
4
)
2
, forms as an insoluble grey adherent crystalline
coating on the surface being treated.
A number of proprietary processes based on phosphating have been
introduced since 1903. Some readers may have used the preparation Kurust
which is available for the small-scale treatment of rusted steelwork, princi-
pally the bodywork of decrepit—though not necessarily ancient—motor
cars.
21.102
Chromating Chromate coatings are produced on magnesium-
base alloys, and on zinc and its alloys, by immersing the articles in a bath
containing potassium bichromate along with various other additions. The

colour of the films varies with the bath and alloy, from yellow to grey and
black. With steel a chromate film has little permanence but it is sometimes
used following phosphating or zinc-coating of steel and as an additive to
zinc-based primer paints.
21.103 Electrophoresis This process—also known as 'electro-
painting'—is used mainly to apply undercoat paint to steel which has
already been phosphated. The chemistry of the process is fairly involved,
but briefly, the paint bath consists of colloidal particles of the appropriate
'resin' suspended in a water solution which contains an electrolyte. DC
electrodes one of which is the article being painted, are immersed in the
bath.
The resin particles, constituting the paint, acquire a charge by adsorbing
ions from the electrolyte so that they are then attracted towards the appro-
priate electrode. Sometimes the ions are provided by one of the reagents
in the paint bath. For example, if a colloid is kept in suspension by soap
solution the negatively-charged ion of the soap (the 'fatty acid' radical)
may be adsorbed by the colloid particles giving them a negative charge so
that the combined particle is attracted to the anode which will be the article
being painted.
This method produces a very uniform paint film on both flat surfaces
and sharp edges. As soon as the colloidal particle is deposited it insulates
that portion of the surface and so other charged particles then 'seek out'
bare areas which are still charged. For this reason a very complete and
uniform film can be deposited and if an ammeter is included in the circuit
it will read zero when coating is complete. The fact that the paint is carried
in a water solution reduces fire risks but at the same time the resultant film
is dense because a phenomenon known as electro-osmosis causes molecules
of the liquid to migrate away from the electrode so that the paint film
becomes denser. The process is of obvious value in the motor industry
where complete bodies can be coated by dipping into an electrophoretic
bath.
21.104 The 'Elphal' Process is similar in principle but is used to coat
steel strip with aluminium. Here finely-divided aluminium is carried in a
water/alcohol bath which also contains an electrolyte. The aluminium par-
ticles are not ions and neither this process nor electrophoresis generally
should be confused in any way with electroplating. In this case aluminium
particles become charged by attachment to ions from the electrolyte and
consequently migrate to the steel surface (electrode) where they adhere
loosely. The solvent is removed by heating and the coating then compacted
by cold rolling. Finally the aluminium film is sintered on to the surface of
the steel by heat treatment.
21.105 Surface Protection at High Temperatures The drive towards
higher efficiency and performance means that engineering environments
are becoming increasingly hostile. The first line of defence against deterio-
ration of any component is its surface. Unfortunately many materials which
are designed for strength do not offer a high resistance to corrosion and
wear, especially at high temperatures. This is a major problem in the design
of gas turbine engines, particularly in respect of the blades.
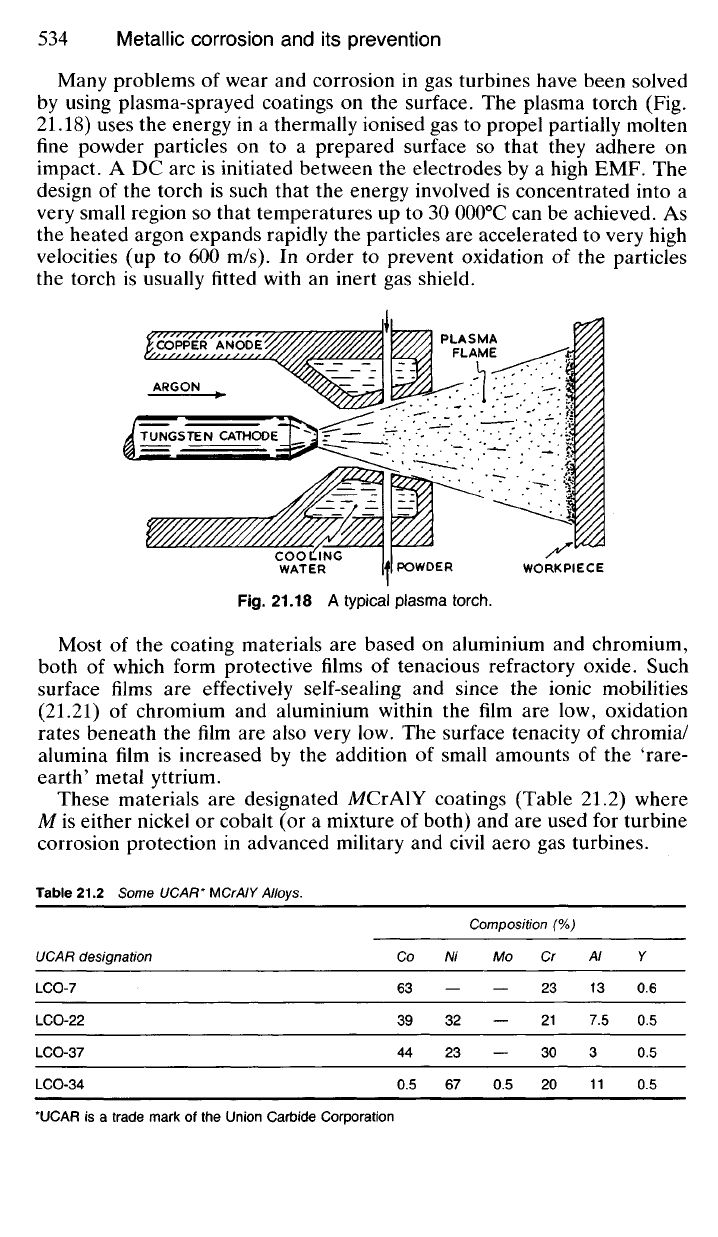
Many problems
of
wear
and
corrosion
in gas
turbines have been solved
by using plasma-sprayed coatings
on the
surface.
The
plasma torch
(Fig.
21.18) uses
the
energy
in a
thermally ionised
gas to
propel partially molten
fine powder particles
on to a
prepared surface
so
that they adhere
on
impact.
A DC arc is
initiated between
the
electrodes
by a
high
EMF. The
design
of the
torch
is
such that
the
energy involved
is
concentrated into
a
very small region
so
that temperatures
up to 30
000
0
C
can be
achieved.
As
the heated argon expands rapidly
the
particles
are
accelerated
to
very high
velocities
(up to 600 m/s). In
order
to
prevent oxidation
of the
particles
the torch
is
usually fitted with
an
inert
gas
shield.
Fig.
21.18 A
typical plasma torch.
Most
of the
coating materials
are
based
on
aluminium
and
chromium,
both
of
which form protective films
of
tenacious refractory oxide. Such
surface films
are
effectively self-sealing
and
since
the
ionic mobilities
(21.21)
of
chromium
and
aluminium within
the
film
are low,
oxidation
rates beneath
the
film
are
also very
low. The
surface tenacity
of
chromia/
alumina film
is
increased
by the
addition
of
small amounts
of the
'rare-
earth' metal yttrium.
These materials
are
designated MCrAlY coatings (Table
21.2)
where
M
is
either nickel
or
cobalt
(or a
mixture
of
both)
and are
used
for
turbine
corrosion protection
in
advanced military
and
civil aero
gas
turbines.
Table
21.2
Some UCAR* UCrAIY Alloys.
Composition
(%)
UCAR designation
Co Ni Mo Cr Al Y
LCO-7
63 — — 23 13 0.6
LCO-22
39 32 — 21 7.5 0.5
LCO-37
44 23 — 30 3 0.5
LCO-34
0.5 67 0.5 20 11 0.5
*UCAR
is a
trade mark
of the
Union Carbide Corporation
PLASMA
FLAME
COPPER ANODE
ARGON
TUNGSTEN CATHODE
COOLING
WATER
POWDER
WORKPIECE
Cathodic Protection
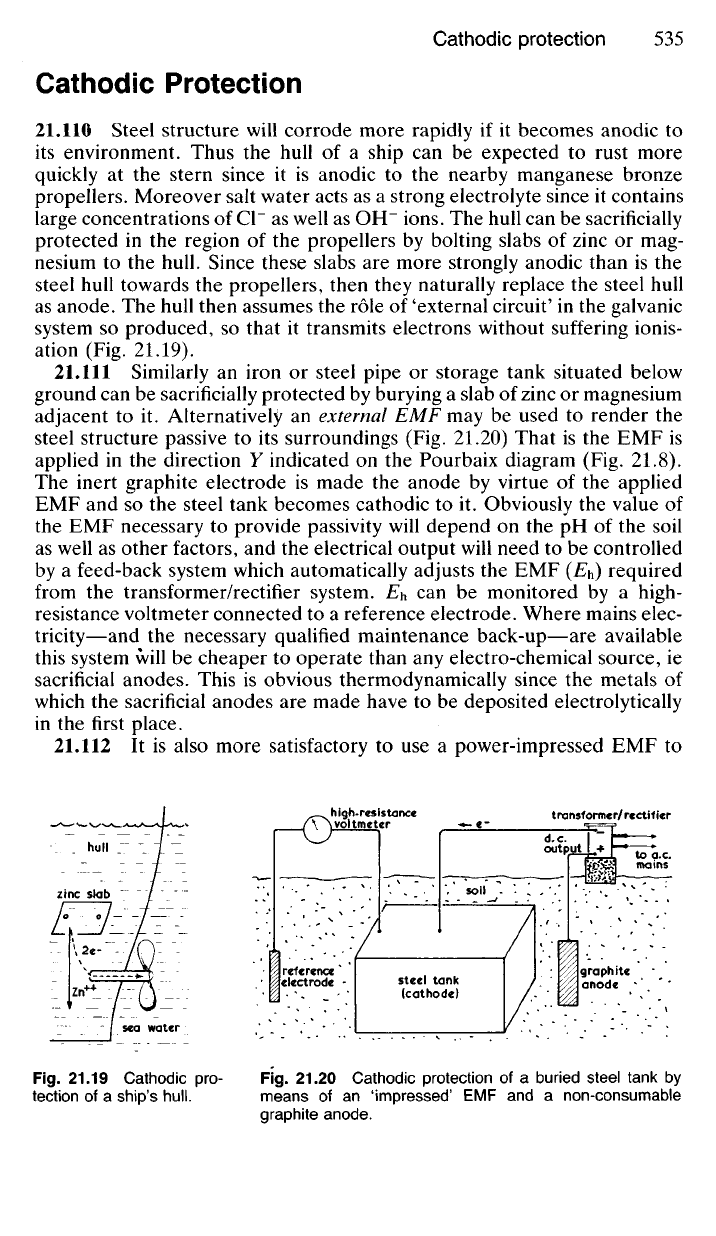
21.110 Steel structure will corrode more rapidly if it becomes anodic to
its environment. Thus the hull of a ship can be expected to rust more
quickly at the stern since it is anodic to the nearby manganese bronze
propellers. Moreover salt water acts as a strong electrolyte since it contains
large concentrations of Cl" as well as OH" ions. The hull can be sacrificially
protected in the region of the propellers by bolting slabs of zinc or mag-
nesium to the hull. Since these slabs are more strongly anodic than is the
steel hull towards the propellers, then they naturally replace the steel hull
as anode. The hull then assumes the role of 'external circuit' in the galvanic
system so produced, so that it transmits electrons without suffering ionis-
ation (Fig. 21.19).
21.111 Similarly an iron or steel pipe or storage tank situated below
ground can be sacrificially protected by burying a slab of zinc or magnesium
adjacent to it. Alternatively an external EMF may be used to render the
steel structure passive to its surroundings (Fig. 21.20) That is the EMF is
applied in the direction Y indicated on the Pourbaix diagram (Fig. 21.8).
The inert graphite electrode is made the anode by virtue of the applied
EMF and so the steel tank becomes cathodic to it. Obviously the value of
the EMF necessary to provide passivity will depend on the pH of the soil
as well as other factors, and the electrical output will need to be controlled
by a feed-back system which automatically adjusts the EMF (E
h
) required
from the transformer/rectifier system. E
h
can be monitored by a high-
resistance voltmeter connected to a reference electrode. Where mains elec-
tricity—and the necessary qualified maintenance back-up—are available
this system will be cheaper to operate than any electro-chemical source, ie
sacrificial anodes. This is obvious thermodynamically since the metals of
which the sacrificial anodes are made have to be deposited electrolytically
in the first place.
21.112
It is also more satisfactory to use a power-impressed EMF to
Fig.
21.19
Cathodic
pro-
tection
of a
ship's
hull.
Fig.
21.20
Cathodic protection
of a
buried steel tank
by
means
of an
'impressed'
EMF and a
non-consumable
graphite anode.
transformer/rectifier
d.c.
output
to
a c.
mains
graphite
anode
steel tank
(cathode)
soil
high-resistance
voltmeter
reference
electrode
hull
zinc slab
sea water
