Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.

of the latter tend also to ionise thus increasing the hydrogen ion concen-
tration, pH.
The connection between electrode potential (E
h
) and the hydrogen ion
concentration (pH) was investigated in Belgium by M. Pourbaix. He
derived diagrams which connect these values with the electrochemical reac-
tions between metals and water which was made either acid or alkaline.
Both electrode potential, Eh, and hydrogen ion concentration, pH, influ-
ence the transfer of electrons in the system so that hydrogen evolution and
oxygen absorption depend on these factors and these reactions are the
basis of electrolytic corrosion. The effects of E
h
and pH on the extent to
which water will liberate either hydrogen or oxygen during what we term
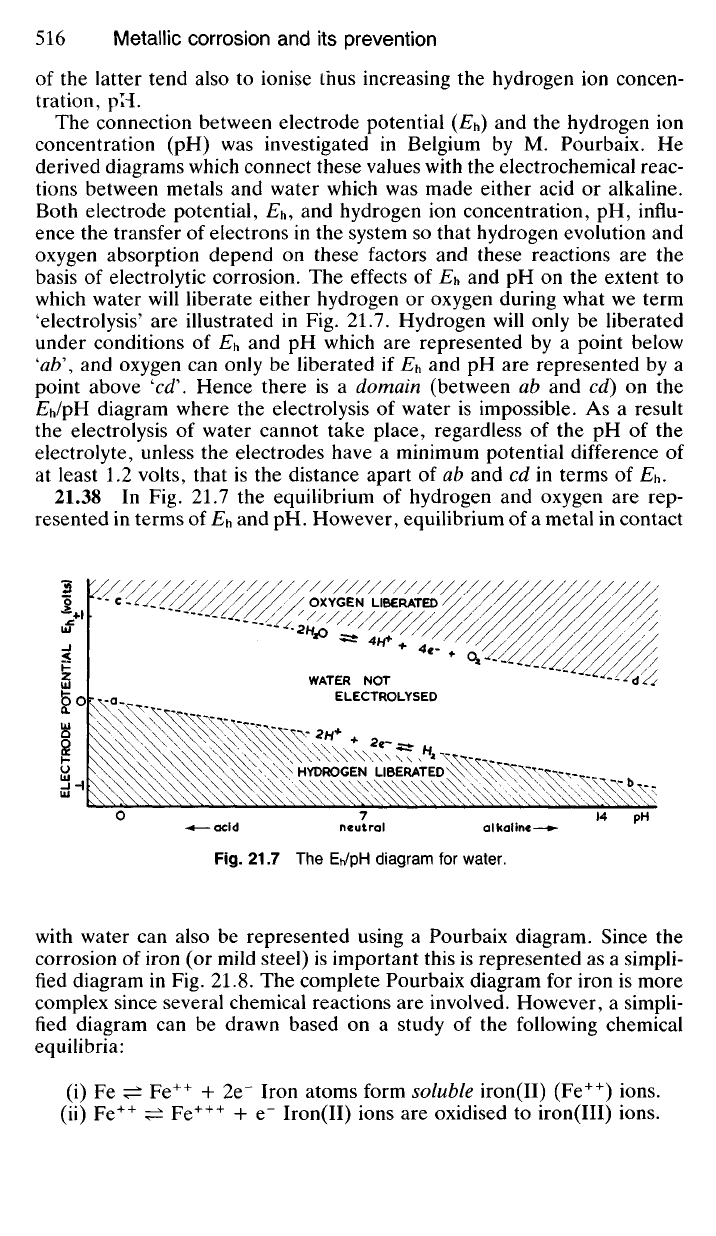
'electrolysis' are illustrated in Fig. 21.7. Hydrogen will only be liberated
under conditions of Eh and pH which are represented by a point below
'ab\
and oxygen can only be liberated if E
h
and pH are represented by a
point above 'cd\ Hence there is a domain (between ab and cd) on the
EJpH diagram where the electrolysis of water is impossible. As a result
the electrolysis of water cannot take place, regardless of the pH of the
electrolyte, unless the electrodes have a minimum potential difference of
at least 1.2 volts, that is the distance apart of ab and cd in terms of Eh.
21.38 In Fig. 21.7 the equilibrium of hydrogen and oxygen are rep-
resented in terms of E
h
and pH. However, equilibrium of a metal in contact
Fig.
21.7 The Eh/pH diagram for water.
with water can also be represented using a Pourbaix diagram. Since the
corrosion of iron (or mild steel) is important this is represented as a simpli-
fied diagram in Fig. 21.8. The complete Pourbaix diagram for iron is more
complex since several chemical reactions are involved. However, a simpli-
fied diagram can be drawn based on a study of the following chemical
equilibria:
(i) Fe ^ Fe
++
+ 2e~ Iron atoms form soluble iron(II) (Fe
++
) ions,
(ii) Fe
++
^± Fe
+++
+ e" Iron(II) ions are oxidised to iron(III) ions.
ELECTRODE
POTENTtAL
E
h
(volts)
OXYGEN LIBERATED
WATER NOT
ELECTROLYSED
HYDROGEN LIBERATED
acid
neutral
alkaline
PH
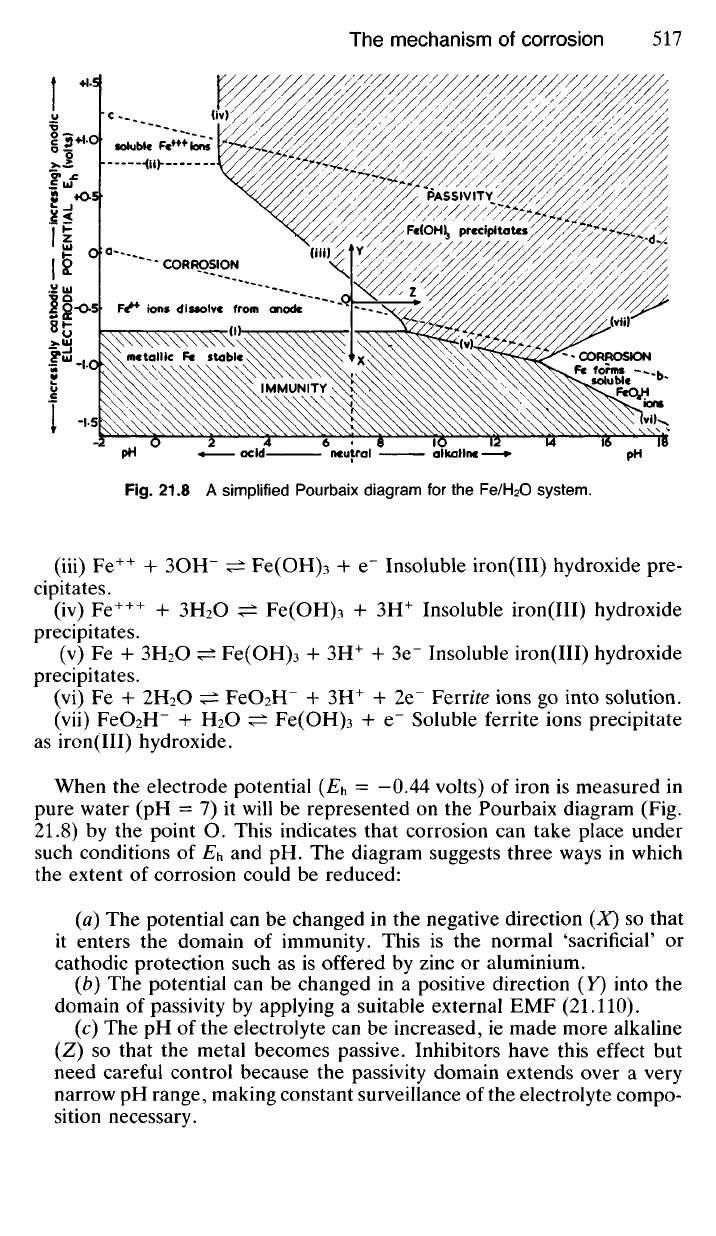
Fig.
21.8 A simplified Pourbaix diagram for the Fe/H
2
O system.
(iii) Fe
++
+ 3OH~ ^± Fe(OH)
3
+ e" Insoluble iron(III) hydroxide pre-
cipitates.
(iv) Fe
+++
H- 3H
2
O ^± Fe(OH)
3
+ 3H
+
Insoluble iron(III) hydroxide
precipitates.
(v) Fe + 3H
2
O ^± Fe(OH)
3
+ 3H
+
+ 3e~ Insoluble iron(III) hydroxide
precipitates.
(vi) Fe + 2H
2
O ^± FeO
2
H" + 3H
+
+ 2e~ Ferrite ions go into solution.
(vii) FeO
2
H" + H
2
O ^± Fe(OH)
3
+ e" Soluble ferrite ions precipitate
as iron(III) hydroxide.
When the electrode potential (E
h
= -0.44 volts) of iron is measured in
pure water (pH = 7) it will be represented on the Pourbaix diagram (Fig.
21.8) by the point O. This indicates that corrosion can take place under
such conditions of Eu and pH. The diagram suggests three ways in which
the extent of corrosion could be reduced:
(a) The potential can be changed in the negative direction (X) so that
it enters the domain of immunity. This is the normal 'sacrificial' or
cathodic protection such as is offered by zinc or aluminium.
(b) The potential can be changed in a positive direction (Y) into the
domain of passivity by applying a suitable external EMF (21.110).
(c) The pH of the electrolyte can be increased, ie made more alkaline
(Z) so that the metal becomes passive. Inhibitors have this effect but
need careful control because the passivity domain extends over a very
narrow pH range, making constant surveillance of the electrolyte compo-
sition necessary.
incrcsingly
cathodic
incrcsingly
anodic
ELECTRODE
POTENTIAL
E
h
(volts)
soluble Fe"* ions
CORROSION
Fc*
4
ions dissolve from anode
metallic Fc stable
IMMUNITY
PASSIVITY
Fe(OHl
3
precipitous
CX)RROSION
Fe forms
soluble
^FeCVH
ions
PH
acid
neutral
alkaline pH
Electrolytic Action or Wet Corrosion Involving
Mechanical Stress
21.40 It has already been shown (21.35 and 21.36) that electrolytic cor-
rosion can occur as a result of different parts of a microstructure being of
different compositions and, hence, different electrode potentials. Thus
intergranular corrosion can occur in a cast metal since impurities, segre-
gated at grain boundaries, have different electrode potentials to the rest of
the structure. Nevertheless a perfectly uniform wrought metal may corrode
more rapidly if it is in the cold-worked condition.
As we have seen cold-working causes a movement of dislocations
towards crystal boundaries (4.19). This 'pile-up' of dislocations results in
the formation of a region of elastic strain in the vicinity of the crystal
boundary and the iocked-up' stresses associated with this strain constitute
a region of higher energy level than is present in the remainder of the
crystal. If an electrolyte is present then energy can dissipate itself by the
grain-boundary atoms going into solution as ions. That is the high-energy
grain-boundary material is anodic to the rest of the crystal.
21.41 This type of intergranular corrosion manifests itself in many
instances. The 'season cracking' of 70-30 brass has been mentioned
(16.33),
but of greater significance in the modern world is the accelerated
corrosion which occurs in mild steel which has been cold formed. In many
instances it is possible to apply a stress-relieving annealing process to the
cold-worked material but in other cases this is inappropriate either because
mechanical distortion of the structure would result or because the results
of cold-work are necessary to give rigidity and strength. Adequate protec-
tion of the surface by some form of coating is then the only way to prevent
corrosion.
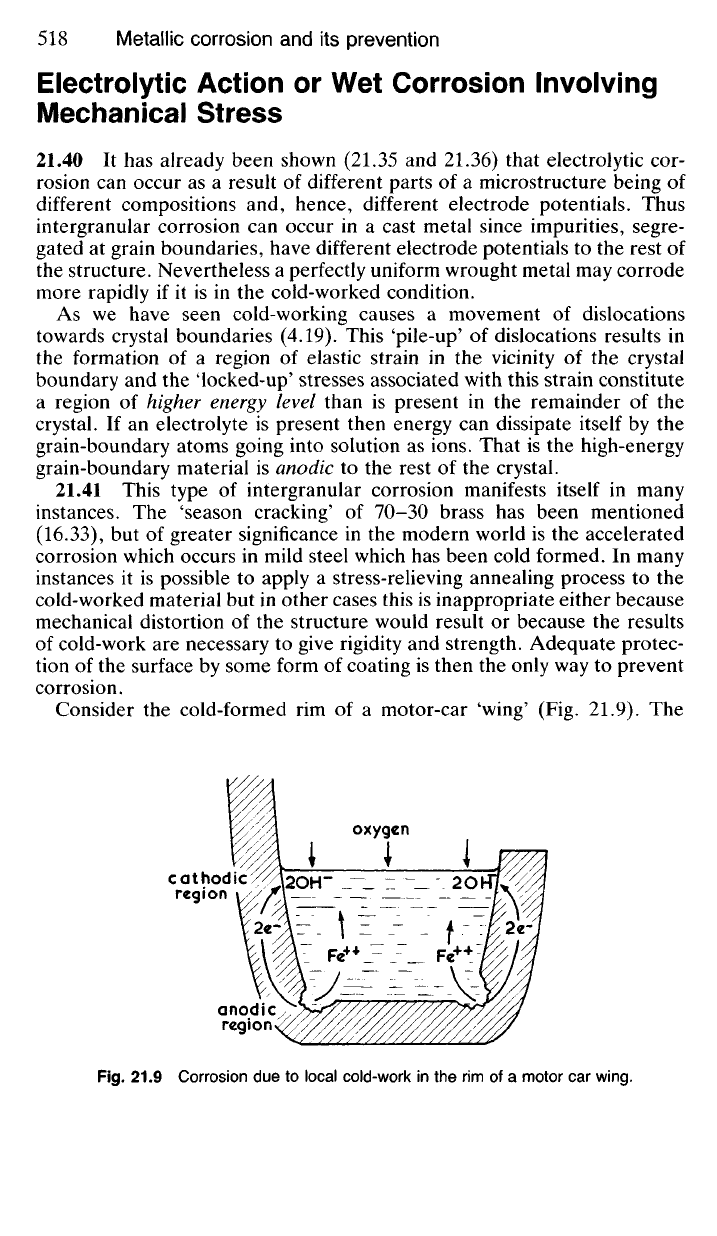
Consider the cold-formed rim of a motor-car 'wing' (Fig. 21.9). The
oxygen
cathodic
region
anodic
region
Fig.
21.9 Corrosion due to local cold-work in the rim of a motor car wing.
forming operation will have cold-worked the rim more severely than the
rest of the structure making it anodic to its surroundings so that, assuming
that the paint work is defective, corrosion will be accelerated there particu-
larly when salt-laden moisture collects during winter. This type of corrosion
in metals is generally termed stress corrosion and may not necessarily be
caused by cold-work. For example, locked-up stresses may be set up near
to welded joints as a result of non-uniform cooling. Similar situations may
occur during the cooling of a casting. It must be admitted, however, that
in both welded joints and castings galvanic corrosion is much more likely
to be the result of variations in composition rather than variations in strain
energy.
21.42 Corrosion assists in other forms of failure which plague the
engineer. Thus corrosion fatigue refers to the failure of a component in
which the propagation of a fatigue crack is helped by corrosion, or indeed
initiated by corrosion. A surface flaw may be accentuated by corrosion and
then act as a focal point for the initiation of a fatigue crack (5.65). Once
such a fatigue crack has started its progress will be accelerated by corrosion
due to the anodic effect of high stress concentrations always present at the
root of the crack.
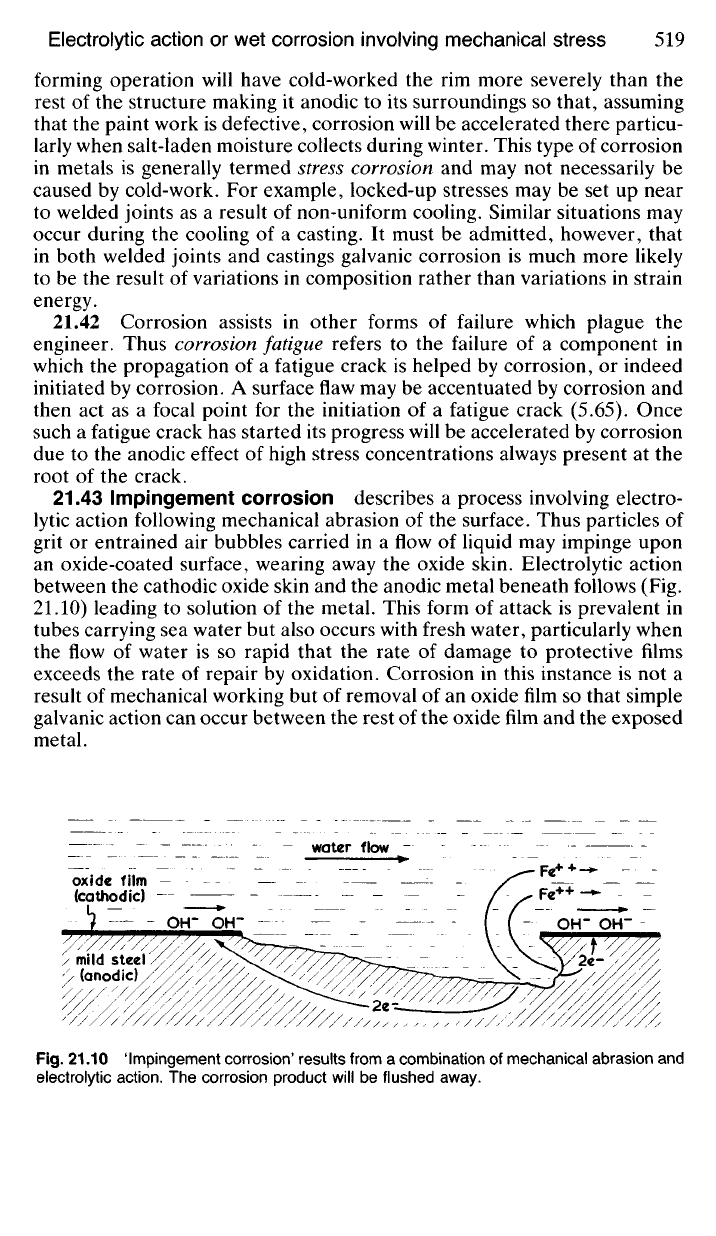
21.43 Impingement corrosion describes a process involving electro-
lytic action following mechanical abrasion of the surface. Thus particles of
grit or entrained air bubbles carried in a flow of liquid may impinge upon
an oxide-coated surface, wearing away the oxide skin. Electrolytic action
between the cathodic oxide skin and the anodic metal beneath follows (Fig.
21.10) leading to solution of the metal. This form of attack is prevalent in
tubes carrying sea water but also occurs with fresh water, particularly when
the flow of water is so rapid that the rate of damage to protective films
exceeds the rate of repair by oxidation. Corrosion in this instance is not a
result of mechanical working but of removal of an oxide film so that simple
galvanic action can occur between the rest of the oxide film and the exposed
metal.
Fig.
21.10
'Impingement corrosion' results from
a
combination
of
mechanical abrasion
and
electrolytic action.
The
corrosion product will
be
flushed away.
oxide film
(cathodic)
water flow
mild steel
(anodic)
Electrolytic Action or Wet Corrosion Involving
Electrolytes of Non-uniform Composition
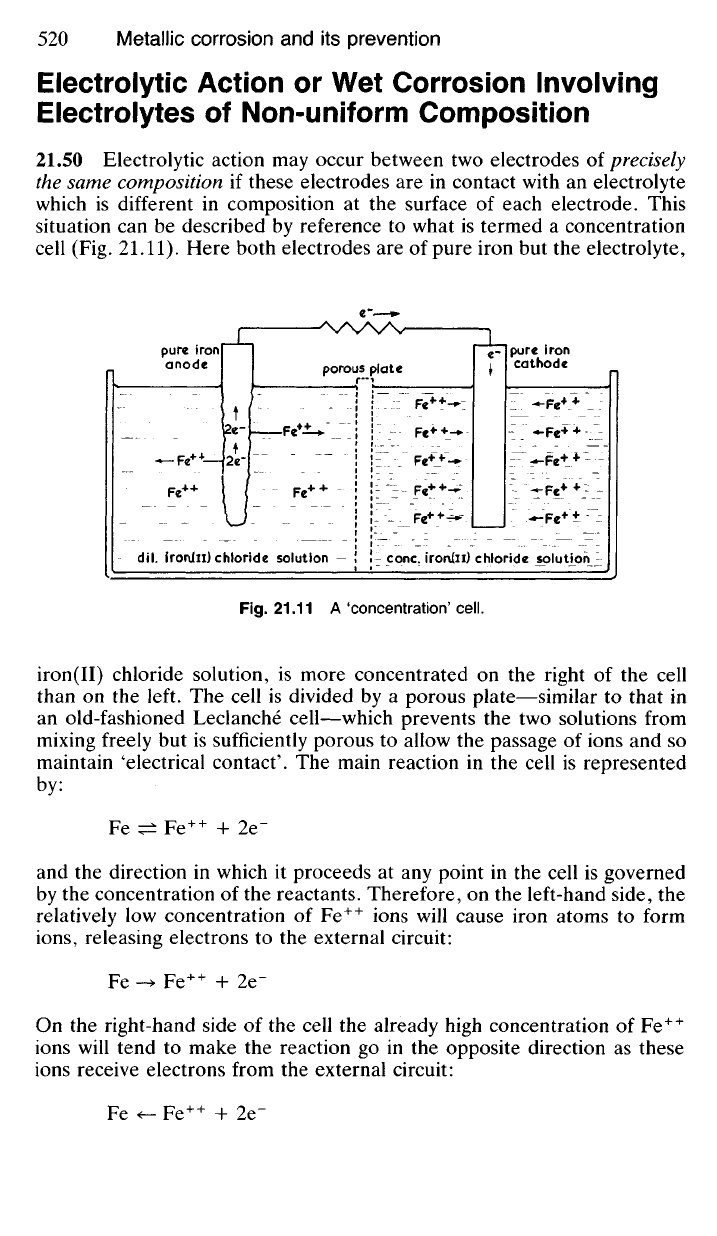
21.50 Electrolytic action may occur between two electrodes of precisely
the same composition if these electrodes are in contact with an electrolyte
which is different in composition at the surface of each electrode. This
situation can be described by reference to what is termed a concentration
cell (Fig. 21.11). Here both electrodes are of pure iron but the electrolyte,
Fig.
21.11 A'concentration'cell.
iron(II) chloride solution, is more concentrated on the right of the cell
than on the left. The cell is divided by a porous plate—similar to that in
an old-fashioned Leclanche cell—which prevents the two solutions from
mixing freely but is sufficiently porous to allow the passage of ions and so
maintain 'electrical contact'. The main reaction in the cell is represented
by:
Fe ^± Fe
++
4- 2e"
and the direction in which it proceeds at any point in the cell is governed
by the concentration of the reactants. Therefore, on the left-hand side, the
relatively low concentration of Fe
++
ions will cause iron atoms to form
ions,
releasing electrons to the external circuit:
Fe -» Fe
++
+ 2e"
On the right-hand side of the cell the already high concentration of Fe
++
ions will tend to make the reaction go in the opposite direction as these
ions receive electrons from the external circuit:
pure iron
anode
porous plate
pure iron
cathode
dil. ironin) chloride solution
cone, ironin) chloride solution
Since iron atoms are entering solution as Fe
++
ions at the left-hand elec-
trode this is obviously the anode, whilst the electrode on the right-hand
side becomes the cathode as is indicated by the movement of electrons in
the external circuit.
21.51 Electrolytic action of this type is the basis of corrosion which
occurs in a variety of different instances in engineering practice. For
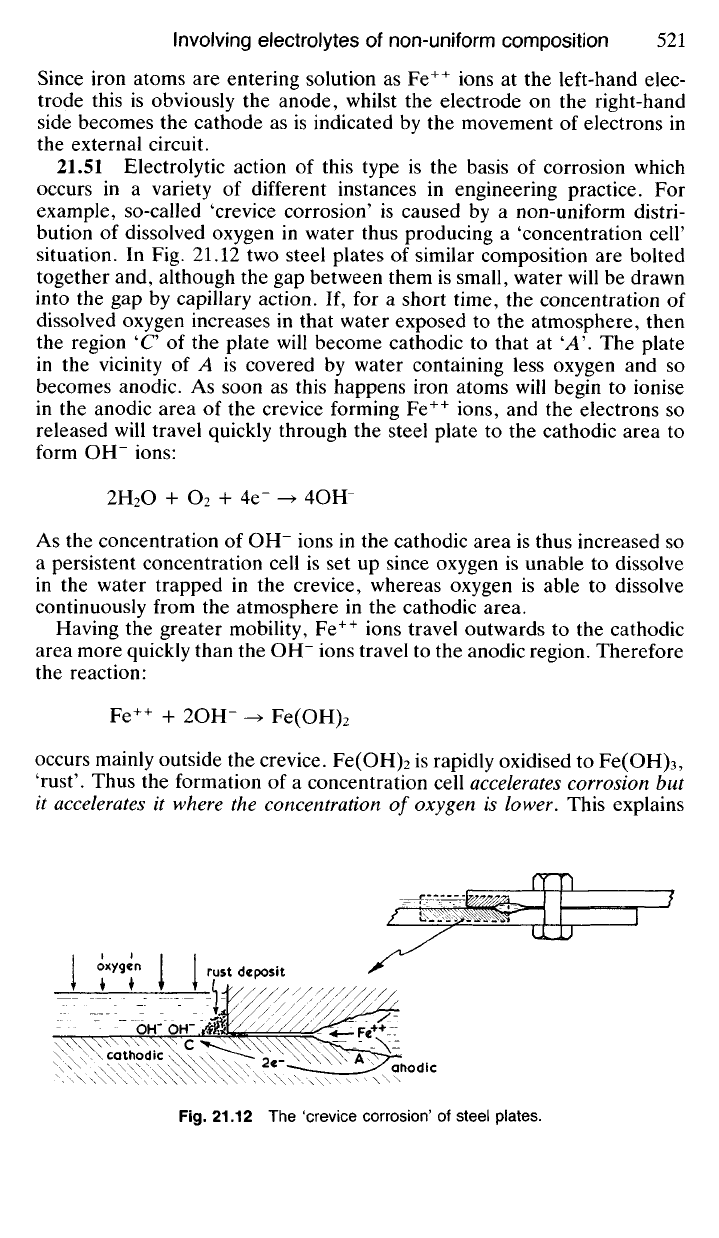
example, so-called 'crevice corrosion' is caused by a non-uniform distri-
bution of dissolved oxygen in water thus producing a 'concentration cell'
situation. In Fig. 21.12 two steel plates of similar composition are bolted
together and, although the gap between them is small, water will be drawn
into the gap by capillary action. If, for a short time, the concentration of
dissolved oxygen increases in that water exposed to the atmosphere, then
the region 'C of the plate will become cathodic to that at 'A\ The plate
in the vicinity of A is covered by water containing less oxygen and so
becomes anodic. As soon as this happens iron atoms will begin to ionise
in the anodic area of the crevice forming Fe
++
ions, and the electrons so
released will travel quickly through the steel plate to the cathodic area to
form OH" ions:
2H
2
O + O
2
+ 4e" -> 4OH
As the concentration of OH" ions in the cathodic area is thus increased so
a persistent concentration cell is set up since oxygen is unable to dissolve
in the water trapped in the crevice, whereas oxygen is able to dissolve
continuously from the atmosphere in the cathodic area.
Having the greater mobility, Fe
++
ions travel outwards to the cathodic
area more quickly than the OH" ions travel to the anodic region. Therefore
the reaction:
Fe
++
+ 2OH" -> Fe(OH)
2
occurs mainly outside the crevice. Fe(OH)
2
is rapidly oxidised to Fe(OH)
3
,
'rust'. Thus the formation of a concentration cell
accelerates
corrosion but
it
accelerates
it where the concentration of oxygen is lower. This explains
Fig.
21.12 The 'crevice corrosion' of steel plates.
ahodic
cathodic
rust deposit
oxygen
why steel corrodes most
in
places where
it
would seem
to be
better
pro-
tected from
the
atmosphere.
21.52
The
fairly rapid rusting which occurs
in the
vicinity
of
defective
paintwork
on a
motor
car is
explained
in Fig.
21.13.
Water begins
to
penetrate beneath
the
loose paint film
but
oxygen does
not and so the
metal beneath
the
paint becomes anodic
to
that which
is
exposed
to the
atmosphere. Crevice corrosion then proceeds
as
above.
Fig.
21.13
Sub-paint rusting
of a
motor
car
body. Another case
of
crevice corrosion.
One frequently hears
a
motor-car owner bemoaning
the
fact that 'rust
is eating
its way
beneath
the
painting work'.
In
fact
of
course
the
reverse
is true—Fe
++
ions
are
moving outwards from beneath
the
paint
to
form
rust
at the
cathodic surface. Some
of the
Fe
++
ions will meet
up
with
OH"
ions
as
these move slowly inwards beneath
the
paint.
As a
result some rust
forms beneath
the
paint producing blisters which lift
it off.
Accumulations
of
chemically-inert dirt
on the
surface
of
exposed metal
will also give rise
to
this form
of
corrosion. Such encrustations allow moist-
ure
to
penetrate
and
this moisture becomes increasingly deficient
in
oxygen
thus giving rise
to
anodic spots beneath
the
dirt. This situation
is
made
worse
by the
salt used
on
roads
in
winter since
Cl"
ions will tend
to
congregate along with
OH"
ions outside
the
dirt encrustations
and con-
siderably increase
the
concentration gradient within
the
electrolyte.
It is
far more important
to
wash away accumulated dirt from
the
hidden parts
of
a
motor-car—particularly during winter—than
it is to
waste time
pol-
ishing
the
areas which
are
visible. 'Wings' invariably rust from beneath
particularly where
'mud
traps' have been incorporated into
the
design.
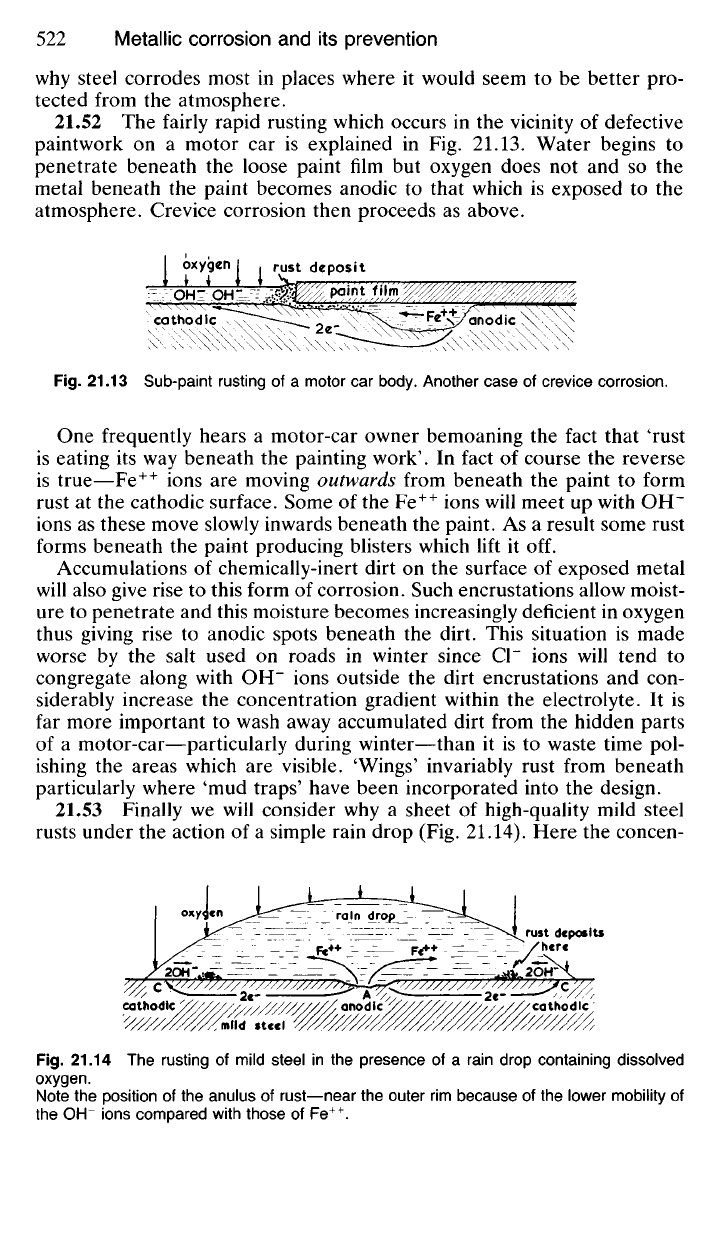
21.53 Finally
we
will consider
why a
sheet
of
high-quality mild steel
rusts under
the
action
of a
simple rain drop
(Fig.
21.14). Here
the
concen-
Fig.
21.14 The
rusting
of
mild steel
in the
presence
of a
rain drop containing dissolved
oxygen.
Note
the
position
of the
anulus
of
rust—near
the
outer
rim
because
of the
lower mobility
of
the
OH"
ions compared with those
of
Fe
++
.
oxydcn
rain drop
rust deposits
here
cathodic
anodic
mild steel
cathodic
oxygen
rust deposit
paint film
anodic
cathodic

tration of dissolved oxygen in the rain drop will be higher at 'C than at
'A'
where the depth of water is greater, so that dissolved oxygen reaches
the metal surface at A more slowly. A concentration cell is therefore set
up so that atoms of iron at A go into solution as Fe
++
ions releasing
electrons which pass through the 'external circuit' (the mild stbel sub-
surface) to C where OH" ions form. A deposit of Fe(OH)
2
is produced
mainly around the periphery of the rain drop and this oxidises immediately
to form Fe(OH)
3
or 'rust'.
This type of corrosion would appear to be important on the massive scale
with the many off-shore structures associated with oil and gas operations in
the North Sea, for as water depth increases so the oxygen content decreases
as in the case with the droplet of water described above. However the rate
of corrosion is probably influenced more directly by factors such as sea
water velocity (impingement corrosion—21.43), chlorine ion
(-Cl")
con-
centration, marine organisms, temperature and the rise and fall of the
tides.
The Prevention of Corrosion
21.60 There are two principal methods by which corrosion may be pre-
vented or minimised. First, the metallic surface can be insulated from the
corrosive medium by some form of protective coating. Such coatings
include various types of paints and varnishes, metallic films having good
corrosion-resistance and artificially thickened oxide films. All of these are
generally effective in protecting surfaces from atmospheric corrosion. Zinc
coatings are used to protect iron and steel from the rusting action of moist
atmospheres and though zinc offers its 'sacrificial protection' (21.33) ^s a
second line of defence, it should be clearly understood that the main objec-
tive is to produce a sound continuous film of zinc which will seal off the iron
completely from atmospheric action. Sacrificial protection is a temporary
phenomenon and is only effective for a limited time since the zinc dissolves
quickly once electrolytic action begins.
Tin coatings offer protection against most animal and vegetable juices
encountered in the canning industry but due to current very high costs of
tin extremely thin coatings of the metal are used on the mild-steel cans.
This tin coat is now generally covered by a film of some organic polymer
(plastics material) as the main protection, so that modern tin cans tend to
rust on the outside rather than the inside.
21.61 In circumstances where corrosive action is severe, or where
mechanical abrasion is likely to damage a surface coating, it may be neces-
sary to use a metal or alloy which has an inherent resistance to corrosion.
Such corrosion-resistant alloys are relatively expensive, so that their use is
limited generally to chemical-engineering plant, marine-engineering equip-
ment and other special applications.
The Use of a Metal or Alloy Which Is Inherently
Corrosion-resistant
21.70 The corrosion-resistance of a pure metal or a homogeneous solid
solution is generally superior to that of an alloy in which two or more
phases are present in the microstructure. As mentioned above, the exist-
ence of two phases leads to electrolytic action when the surface of the alloy
comes into contact with an electrolyte. The phase with the lower electrode
potential will behave anodically and dissolve, leading to pitting of the alloy
surface; and the greater the difference in electrode potentials between the
phases, the more rapid will be corrosion. Resistance to corrosion will
generally be at a minimum when the second phase is segregated at the
crystal boundaries, particularly if this phase is anodic to the matrix. In
such circumstances serious intercrystalline corrosion will occur.
21.71 Most of the alloys which are used because of their high corrosion-
resistance exhibit solid-solution structures. Aluminium-magnesium alloys
(17.30) containing up to 7.0 % magnesium fulfil these conditions and are
particularly resistant to marine atmospheres. 18-8 stainless steel is com-
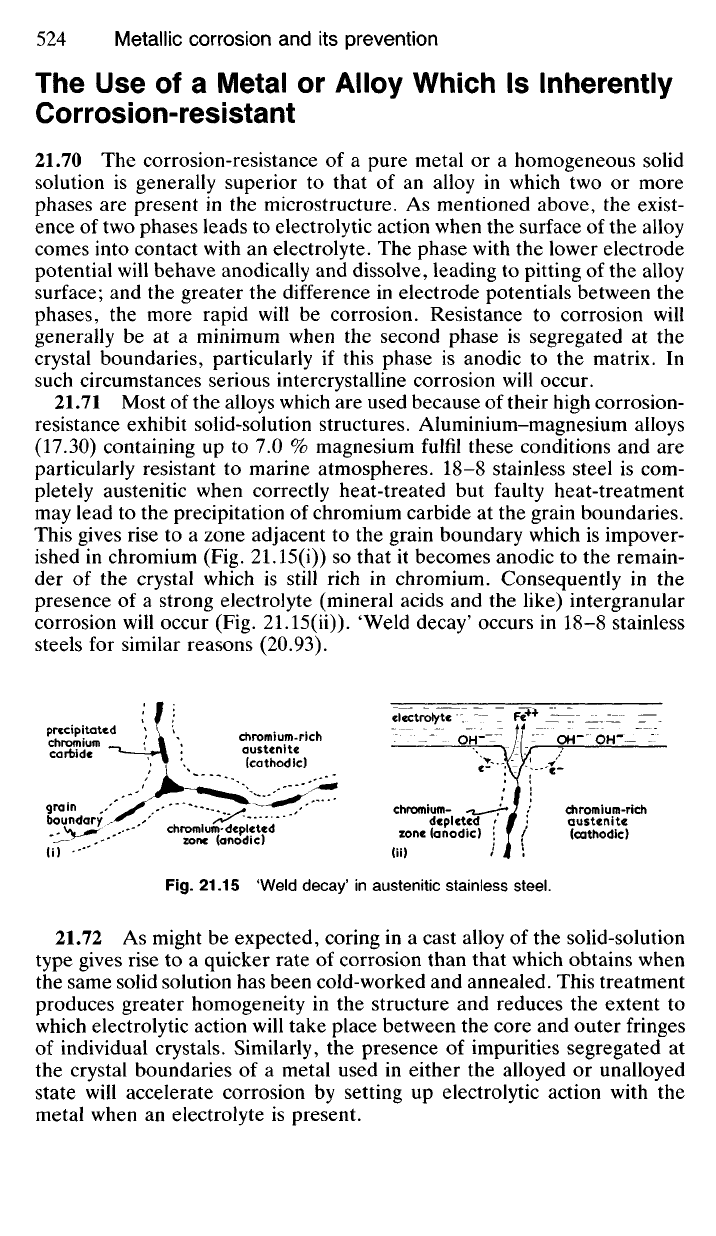
pletely austenitic when correctly heat-treated but faulty heat-treatment
may lead to the precipitation of chromium carbide at the grain boundaries.
This gives rise to a zone adjacent to the grain boundary which is impover-
ished in chromium (Fig. 21.15(i)) so that it becomes anodic to the remain-
der of the crystal which is still rich in chromium. Consequently in the
presence of a strong electrolyte (mineral acids and the like) intergranular
corrosion will occur (Fig. 21.15(ii)). 'Weld decay' occurs in 18-8 stainless
steels for similar reasons (20.93).
Fig.
21.15 'Weld decay
1
in austenitic stainless steel.
21.72 As might be expected, coring in a cast alloy of the solid-solution
type gives rise to a quicker rate of corrosion than that which obtains when
the same solid solution has been cold-worked and annealed. This treatment
produces greater homogeneity in the structure and reduces the extent to
which electrolytic action will take place between the core and outer fringes
of individual crystals. Similarly, the presence of impurities segregated at
the crystal boundaries of a metal used in either the alloyed or unalloyed
state will accelerate corrosion by setting up electrolytic action with the
metal when an electrolyte is present.
electrolyte
chromium-rich
austenite
(cathodic)
chromium-
depleted
zone (anodic)
chromium-rich
austenite
(cathodic)
precipitated
chromium
carbide
grain
boundary
chromium-depleted
zone (anodic)
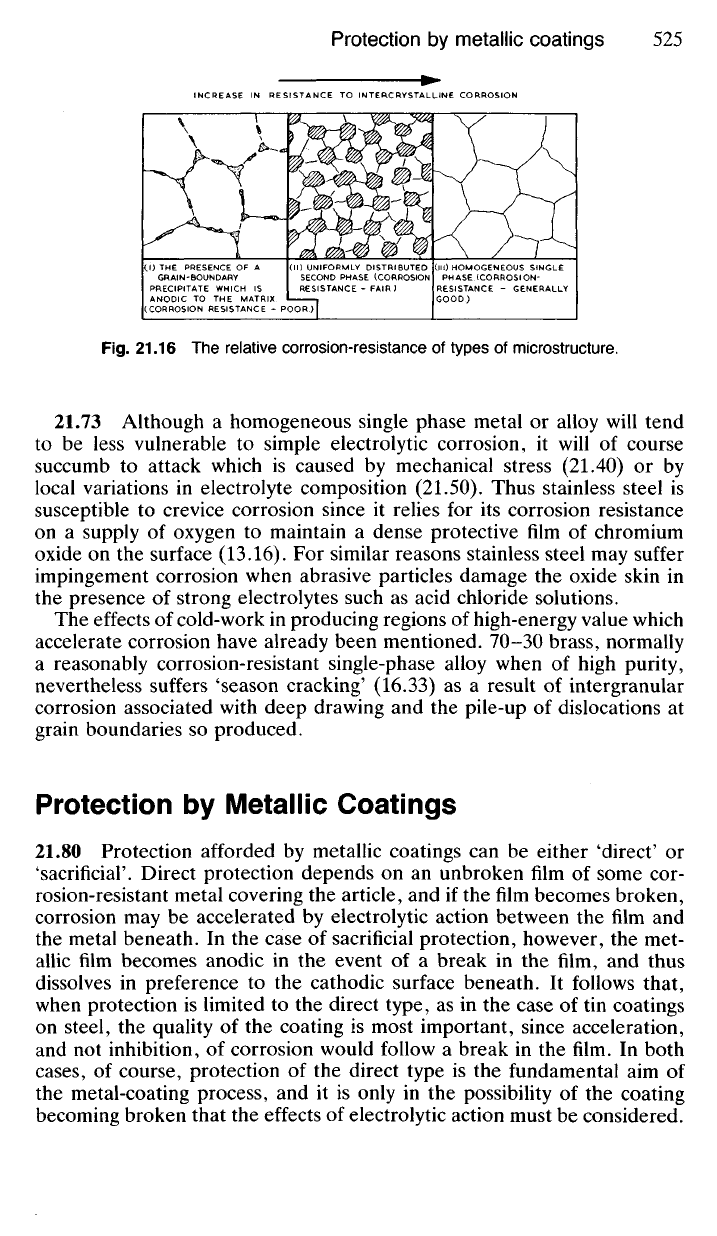
Fig.
21.16 The relative corrosion-resistance of types of microstructure.
21.73 Although a homogeneous single phase metal or alloy will tend
to be less vulnerable to simple electrolytic corrosion, it will of course
succumb to attack which is caused by mechanical stress (21.40) or by
local variations in electrolyte composition (21.50). Thus stainless steel is
susceptible to crevice corrosion since it relies for its corrosion resistance
on a supply of oxygen to maintain a dense protective film of chromium
oxide on the surface (13.16). For similar reasons stainless steel may suffer
impingement corrosion when abrasive particles damage the oxide skin in
the presence of strong electrolytes such as acid chloride solutions.
The effects of cold-work in producing regions of high-energy value which
accelerate corrosion have already been mentioned. 70-30 brass, normally
a reasonably corrosion-resistant single-phase alloy when of high purity,
nevertheless suffers 'season cracking' (16.33) as a result of intergranular
corrosion associated with deep drawing and the pile-up of dislocations at
grain boundaries so produced.
Protection by Metallic Coatings
21.80 Protection afforded by metallic coatings can be either 'direct' or
'sacrificial'. Direct protection depends on an unbroken film of some cor-
rosion-resistant metal covering the article, and if the film becomes broken,
corrosion may be accelerated by electrolytic action between the film and
the metal beneath. In the case of sacrificial protection, however, the met-
allic film becomes anodic in the event of a break in the film, and thus
dissolves in preference to the cathodic surface beneath. It follows that,
when protection is limited to the direct type, as in the case of tin coatings
on steel, the quality of the coating is most important, since acceleration,
and not inhibition, of corrosion would follow a break in the film. In both
cases,
of course, protection of the direct type is the fundamental aim of
the metal-coating process, and it is only in the possibility of the coating
becoming broken that the effects of electrolytic action must be considered.
INCREASE IN RESISTANCE TO INTERCRYSTALLINE CORROSION
THE PRESENCE OF A
GRAIN-BOUNDARY
PRECIPITATE WHICH IS
ANODIC TO THE MATRIX.
(CORROSION RESISTANCE - POOR.)
UNIFORMLY DISTRIBUTED
RESISTANCE - FAIR )
HOMOGENEOUS SINGLE
PHASE (CORROSION-
RESISTANCE - GENERALLY
GOOD)
