Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.


Metallic Corrosion and
its Prevention
21.10 Of all metallurgical problems which confront the engineer, few can
be economically more important than the prevention of metallic corrosion.
In Great Britain alone it is estimated that the cost of metallic corrosion is
of the order of 3% of the annual gross national product which is equivalent
to £10 billion or £150 per capita. This is perhaps less surprising when one
considers, as an example, the team of painters permanently employed in
protecting the steel of the Forth Railway Bridge from the ravages of air,
rain and sea water. Nevertheless even more significant is the amount of
steel which is allowed to rust away for lack of adequate protection. This
amounts to some 1000 tonnes every single day—surely an expensive way
of 'saving jobs'. But enough of this cynicism.
Other metals, in addition to iron and steel, corrode when exposed to
the atmosphere. The green corrosion-product which covers a copper
roof,
or the white, powdery film formed on some unprotected aluminium alloys
is clear evidence of this.
Metals do not corrode where there is no atmosphere. The expensive
Hasselblad camera left behind on the Moon by the American astronauts
will remain in perfect condition as far as its metal parts are concerned,
though radiation may damage some of its non-metallic components. More-
over little corrosion of most engineering alloys, including carbon steels,
occurs in a dry atmosphere at ambient temperatures; but when the atmos-
phere is also moisture laden it is a very different matter and 'rusting' of
steel is rapid. Fossil fuels, and in particular coal, contain varying quantities
of sulphur and nitrogen compounds. On combustion these compounds
yield sulphur dioxide and some oxides of nitrogen which, if not extracted
from the flue gases, will escape to the atmosphere where they will combine
with moisture to form the corrosive agents sulphurous, nitrous and nitric
acids.
Over much of Europe, both West and East, this accelerates the
corrosion of metals, destroys ancient and modern buildings and kills large
tracts of our forests. To extract the acidic oxides from flue gases is an
21

expensive process which, in turn, will create some of its own environmental
problems but it must be done. The only alternative seems to be the use of
nuclear-derived power in our current state of technology.
The Mechanism of Corrosion
21.20 Oxidation or 'Dry' Corrosion The metals of Group I and II of
the Periodic Classification react readily with oxygen so that, apart from
beryllium and magnesium which are useful because of their very low spe-
cific gravities combined with good strength, they are of little use as
materials in constructional engineering. Most of the engineering metals are
to be found in the 'transition' groups where affinity for atmospheric oxygen
is rather less. Oxidation of many of these metals is extremely slow at
ambient temperatures but occurs much more rapidly as the temperature
rises as is demonstrated by the scaling of steel at red heat. When iron is
heated in an atmosphere containing available oxygen it becomes coated
with a layer of black oxide scale, FeO:
2Fe 4- O
2
-> 2FeO
The above equation is only a simplified way of expressing the reaction. In
fact atoms of iron have been oxidised whilst atoms of oxygen have been
reduced. These processes are related to a transfer of electrons from atoms
of iron to atoms of oxygen:
Fe
—>
Fe
+4
" + 2 electrons (e~) (Oxidation)
O + 2 electrons (e") -> O~~ (Reduction)
The terms 'oxidation' and 'reduction' have a wider meaning in chemistry
and involve reactions in which oxygen takes no part. Thus, sulphur gases
will attack nickel alloys at high temperatures giving rise to intercrystalline
corrosion by the formation of nickel sulphide films. In this instance nickel
has been 'oxidised' even though the element oxygen is not involved:
Ni -> Ni
++
+ 2e" (Oxidation)
S + 2e~ -> S~~ (Reduction)
Consequently, in its wider sense, oxidation can be described as a process
where the atom involved loses electrons; whilst reduction can be described
as a process where the atom gains electrons.
21.21 Affinity for oxygen is not the sole criterion affecting the rate at
which a metal oxidises. Although aluminium has a very high affinity for
oxygen it is nevertheless corrosion resistant. This is due largely to the
dense and impervious nature of the oxide film which forms on the surface
and so protects it from further attack. The extent to which an oxide film
will protect the metal beneath depends upon two factors:
(i) the continuity of the film and how effectively it bonds to the metallic
surface. Some films are porous and offer poor protection whilst others
crack or peel away with repeated heating and cooling of the metal. Such
metals and alloys oxidise progressively.
(ii) the mobility of metal and non-metal ions within the oxide film.
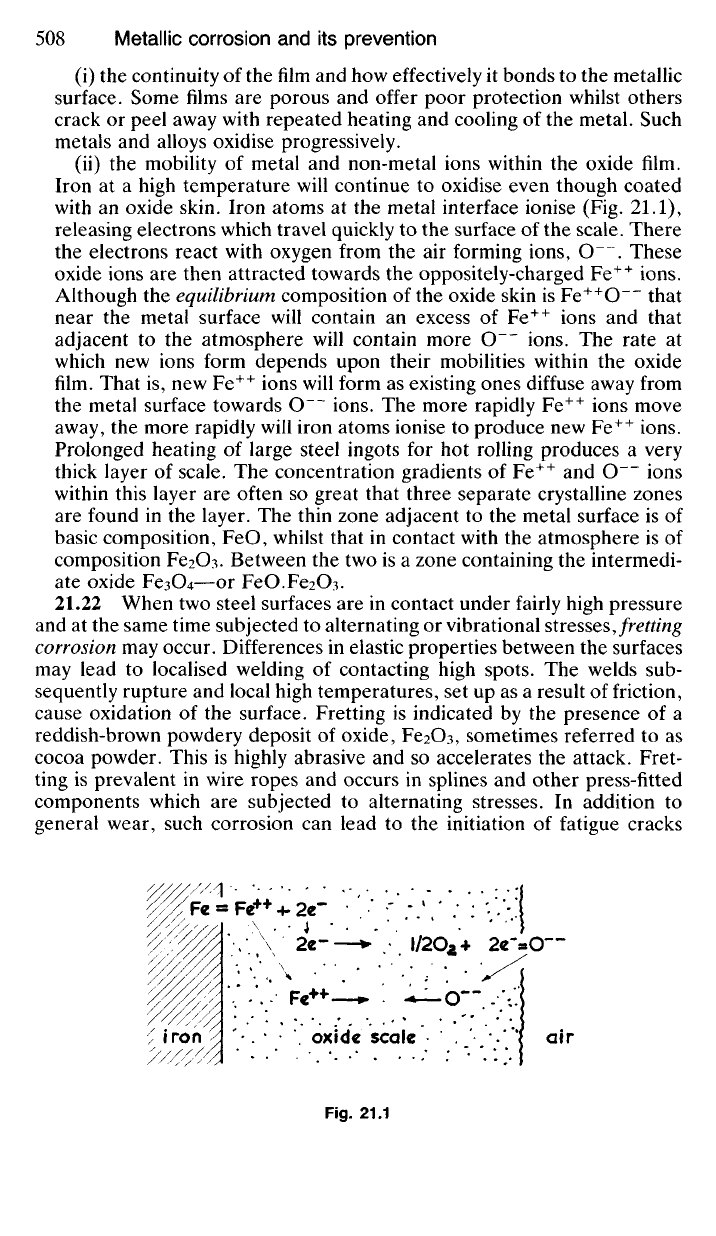
Iron at a high temperature will continue to oxidise even though coated
with an oxide skin. Iron atoms at the metal interface ionise (Fig. 21.1),
releasing electrons which travel quickly to the surface of the scale. There
the electrons react with oxygen from the air forming ions, O . These
oxide ions are then attracted towards the oppositely-charged Fe
++
ions.
Although the equilibrium composition of the oxide skin is Fe
++
O~~ that
near the metal surface will contain an excess of Fe
++
ions and that
adjacent to the atmosphere will contain more O ions. The rate at
which new ions form depends upon their mobilities within the oxide
film. That is, new Fe
++
ions will form as existing ones diffuse away from
the metal surface towards O ions. The more rapidly Fe
++
ions move
away, the more rapidly will iron atoms ionise to produce new Fe
++
ions.
Prolonged heating of large steel ingots for hot rolling produces a very
thick layer of scale. The concentration gradients of Fe
++
and O ions
within this layer are often so great that three separate crystalline zones
are found in the layer. The thin zone adjacent to the metal surface is of
basic composition, FeO, whilst that in contact with the atmosphere is of
composition Fe
2
O
3
. Between the two is a zone containing the intermedi-
ate oxide Fe
3
O
4
—or FeO-Fe
2
O
3
.
21.22 When two steel surfaces are in contact under fairly high pressure
and at the same time subjected to alternating or vibrational stresses, fretting
corrosion may occur. Differences in elastic properties between the surfaces
may lead to localised welding of contacting high spots. The welds sub-
sequently rupture and local high temperatures, set up as a result of friction,
cause oxidation of the surface. Fretting is indicated by the presence of a
reddish-brown powdery deposit of oxide, Fe
2
O
3
, sometimes referred to as
cocoa powder. This is highly abrasive and so accelerates the attack. Fret-
ting is prevalent in wire ropes and occurs in splines and other press-fitted
components which are subjected to alternating stresses. In addition to
general wear, such corrosion can lead to the initiation of fatigue cracks
iron
air
Rg.
21.1
oxide scale

particularly since the affected components are subjected to vibrational
stresses. Though difficult to eliminate completely it can be minimised by
excluding air by the use of a high-pressure grease or by the use of a solid
high-pressure lubricant such as molybdenum disulphide.
21.23 As indicated above oxidation in industrial atmospheres is rarely
a simple process involving oxygen only. Contaminants such as carbon diox-
ide,
carbon monoxide, sulphur dioxide, oxides of nitrogen and water vap-
our are frequently responsible for the very rapid deterioration of metals
at high temperatures. Under varying conditions carbon dioxide can pro-
mote both oxidation and carburisation in alloys. This phenomenon leads
to green rot in Nimonic Alloys—precipitation of &23C6 leaves the matrix
so depleted in protective chromium that extensive oxidation of the nickel
to green NiO occurs rapidly.
Nimonic and other nickel-base superalloys are rapidly attacked in the
presence of sulphur-rich combustion gases. This leads to the formation of
black NiS so that the effect is aptly described as black plague. Solid ash
from burning fuel, carried in the combustion gas stream, can also react
chemically with surface oxidation products. As a result a fluxing action
may take place resulting in the formation of fluid products at temperatures
above 650
0
C. Fluid glassy slags so formed dissolve oxides rapidly so that a
metallic surface is exposed to further oxidation. The very rapid corrosion
of Fe-Ni-Cr alloys which occurs under such conditions is known as
catastrophic oxidation.
21.30 Electrolytic Action or Wet Corrosion Involving Two Dissimi-
lar Elements Electrolytic action in one form or another is responsible
for the bulk of corrosion which occurs in metals at ambient temperatures.
In this particular instance it will occur when two dissimilar metals of differ-
ent 'electrode potential' are in electrical contact with each other and with
an 'electrolyte'. The term 'electrolyte' describes some substance which
contains both positively- and negatively-charged ions, able to move about
freely within it.
Much of this chemical action is similar to that which occurs in a simple
Galvanic cell (Fig. 21.2), consisting of a copper plate and a zinc plate,
immersed in dilute sulphuric acid (the electrolyte). When the external
circuit is closed a current begins to flow through the ammeter. This current
is composed of electrons which are released in the zinc plate and, as their
concentration builds up there, are forced to flow to the copper plate. As
a result of the loss of electrons zinc atoms become zinc ions (Zn
++
) and
pass into solution in the electrolyte:
Zn -> Zn
++
+ 2e"
As the electrons, which have been forced round the external circuit by
pressure of numbers, collect on the copper plate it becomes negatively
charged so that hydrogen ions (H
+
), present in the electrolyte from ionised
sulphuric acid, are attracted to the copper plate where they combine with
the available electrons to form ordinary atoms and, hence, molecules of
hydrogen so that bubbles of the gas form on the copper plate:
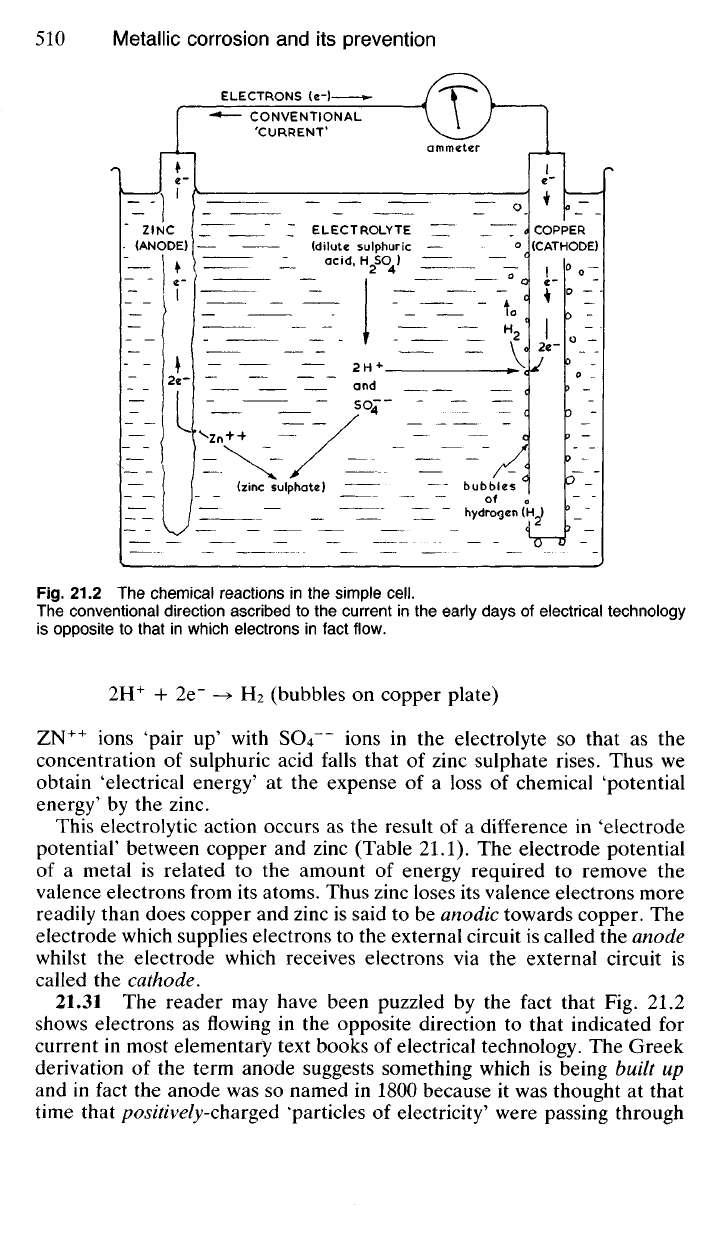
Fig.
21.2 The
chemical reactions
in the
simple
cell.
The conventional direction ascribed
to the
current
in the
early days
of
electrical technology
is opposite
to
that
in
which electrons
in
fact flow.
2H
+
+ 2e~
—>
H
2
(bubbles
on
copper plate)
ZN
++
ions 'pair
up'
with
SO
4
ions
in the
electrolyte
so
that
as the
concentration
of
sulphuric acid falls that
of
zinc sulphate rises. Thus
we
obtain 'electrical energy'
at the
expense
of a
loss
of
chemical 'potential
energy'
by the
zinc.
This electrolytic action occurs
as the
result
of a
difference
in
'electrode
potential' between copper
and
zinc (Table 21.1).
The
electrode potential
of
a
metal
is
related
to the
amount
of
energy required
to
remove
the
valence electrons from
its
atoms. Thus zinc loses
its
valence electrons more
readily than does copper
and
zinc
is
said
to be
anodic towards copper.
The
electrode which supplies electrons
to the
external circuit
is
called
the
anode
whilst
the
electrode which receives electrons
via the
external circuit
is
called
the
cathode.
21.31
The
reader
may
have been puzzled
by the
fact that
Fig. 21.2
shows electrons
as
flowing
in the
opposite direction
to
that indicated
for
current
in
most elementary text books
of
electrical technology.
The
Greek
derivation
of the
term anode suggests something which
is
being built
up
and
in
fact
the
anode
was so
named
in 1800
because
it was
thought
at
that
time that positively-charged 'particles
of
electricity' were passing through
ELECTRONS
CONVENTIONAL
'CURRENT'
ammeter
ZINC
(ANODE)
ELECTROLYTE
(dilute
sulphuric
acid,
H
2
SO
4
)
COPPER
(CATHODE)
(zinc
sulphate)
bubbles
of
hydrogen
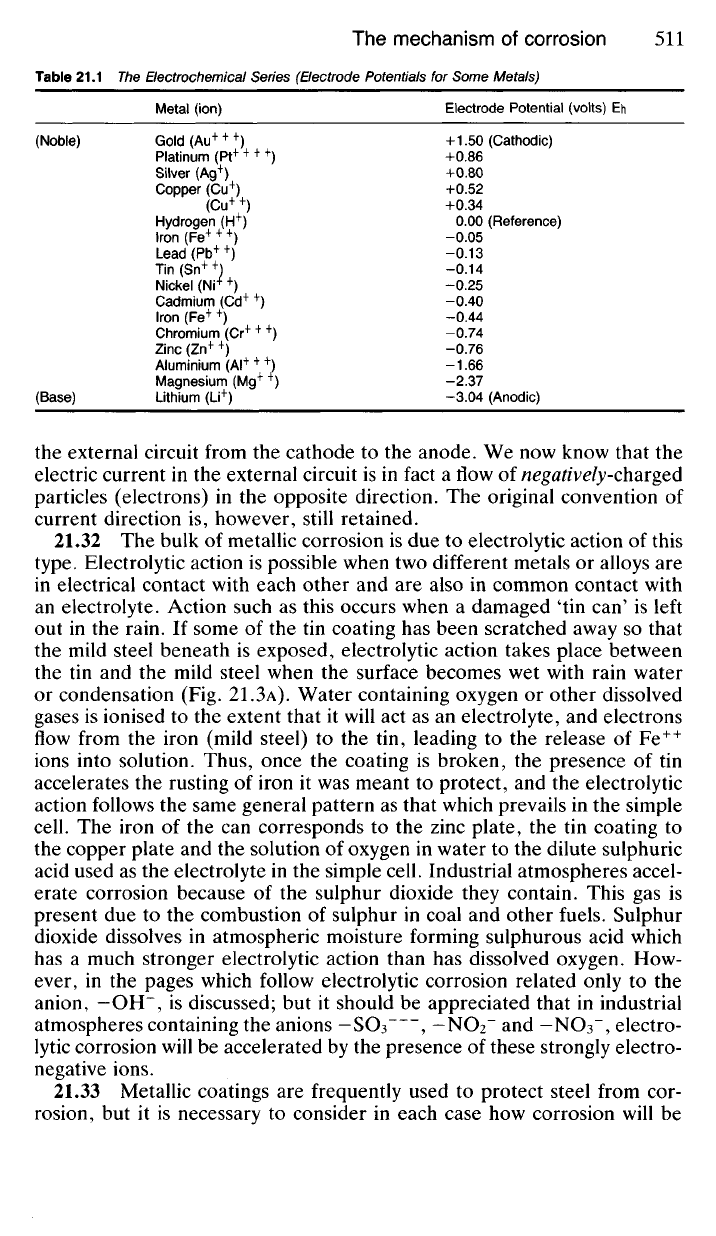
Table 21.1 The Electrochemical Series (Electrode Potentials for Some Metals)
Metal (ion) Electrode Potential (volts) Eh
(Noble) Gold (Au
+ + +
) +1.50 (Cathodic)
Platinum (Pt
+ + + +
) +0.86
Silver (Ag
+
) +0.80
Copper (Cu
+
) +0.52
(Cu
+
+
) +0.34
Hydrogen (H
+
) 0.00 (Reference)
Iron (Fe
+ + +
) -0.05
Lead (Pb
++
) -0.13
Tin (Sn
++
) -0.14
Nickel (Nr
+
) -0.25
Cadmium (Cd
+ +
) -0.40
Iron (Fe
+ +
) -0.44
Chromium (Cr
+ + +
) -0.74
Zinc (Zn
+ +
) -0.76
Aluminium (Al
+++
) -1.66
Magnesium (Mg
+ +
) -2.37
(Base) Lithium (Li
+
) -3.04 (Anodic)
the external circuit from the cathode to the anode. We now know that the
electric current in the external circuit is in fact a flow of
negatively-charged
particles (electrons) in the opposite direction. The original convention of
current direction is, however, still retained.
21.32 The bulk of metallic corrosion is due to electrolytic action of this
type.
Electrolytic action is possible when two different metals or alloys are
in electrical contact with each other and are also in common contact with
an electrolyte. Action such as this occurs when a damaged 'tin can' is left
out in the rain. If some of the tin coating has been scratched away so that
the mild steel beneath is exposed, electrolytic action takes place between
the tin and the mild steel when the surface becomes wet with rain water
or condensation (Fig. 21.3A). Water containing oxygen or other dissolved
gases is ionised to the extent that it will act as an electrolyte, and electrons
flow from the iron (mild steel) to the tin, leading to the release of Fe
++
ions into solution. Thus, once the coating is broken, the presence of tin
accelerates the rusting of iron it was meant to protect, and the electrolytic
action follows the same general pattern as that which prevails in the simple
cell. The iron of the can corresponds to the zinc plate, the tin coating to
the copper plate and the solution of oxygen in water to the dilute sulphuric
acid used as the electrolyte in the simple cell. Industrial atmospheres accel-
erate corrosion because of the sulphur dioxide they contain. This gas is
present due to the combustion of sulphur in coal and other fuels. Sulphur
dioxide dissolves in atmospheric moisture forming sulphurous acid which
has a much stronger electrolytic action than has dissolved oxygen. How-
ever, in the pages which follow electrolytic corrosion related only to the
anion, -OH", is discussed; but it should be appreciated that in industrial
atmospheres containing the anions -SO
3
, —NO2~ and -NO
3
", electro-
lytic corrosion will be accelerated by the presence of these strongly electro-
negative ions.
21.33 Metallic coatings are frequently used to protect steel from cor-
rosion, but it is necessary to consider in each case how corrosion will be
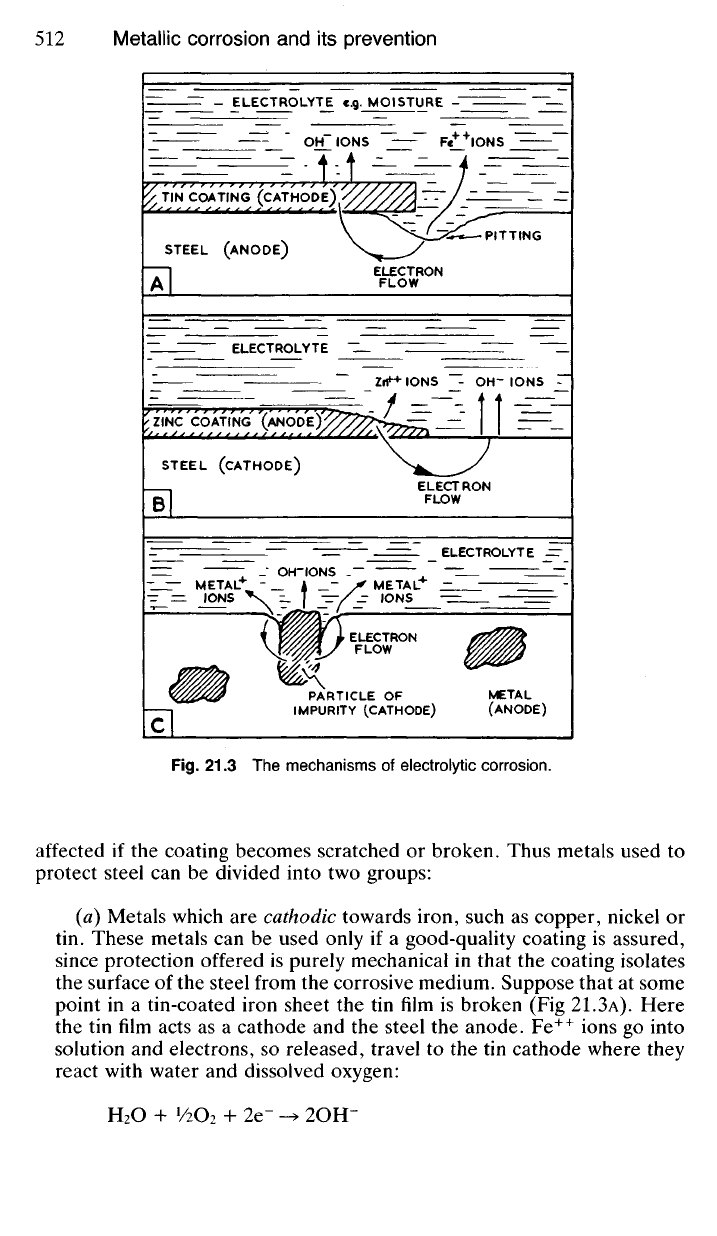
Fig.
21.3 The
mechanisms
of
electrolytic
corrosion.
affected
if the
coating becomes scratched
or
broken. Thus metals used
to
protect steel
can be
divided into
two
groups:
(a) Metals which
are
cathodic towards iron, such
as
copper, nickel
or
tin. These metals
can be
used only
if a
good-quality coating
is
assured,
since protection offered
is
purely mechanical
in
that
the
coating isolates
the surface
of
the steel from
the
corrosive medium. Suppose that
at
some
point
in a
tin-coated iron sheet
the tin
film
is
broken
(Fig
21.3A). Here
the
tin
film acts
as a
cathode
and the
steel
the
anode. Fe
++
ions
go
into
solution
and
electrons,
so
released, travel
to the tin
cathode where they
react with water
and
dissolved oxygen:
ELECTROLYTE e.g. MOISTURE
OH IONS Fe
+4
IONS
TIN COATING (CATHODE)
PITTING
STEEL (ANODE)
ELECTRON
FLOW
A
ELECTROLYTE
B
ZrI
1
+ IONS
OH-
IONS
ZINC COATING (ANODE)
STEEL (CATHODE)
ELECTRON
FLOW
Thus hydroxide ions (OH ) are released at the cathode and, away from
the region of the cell, these combine with Fe
++
ions:
Fe
+
+ + 2OH- -> Fe(OH)
2
Iron(II) hydroxide, Fe(OH)
2
, so formed quickly oxidises to iron(III)
hydroxide, Fe(OH)
3
, the basis of the reddish-brown substance we call
rust. Thus corrosion of the steel is actually accelerated when a coating
of some substance which is cathodic to steel becomes damaged. Fortu-
nately, in the case of both tin and nickel, metals commonly used to coat
iron and steel, the difference in electrode potential in the cell so formed
is small, so that the acceleration of attack is not great. The further apart
metals are in the electro-chemical series (Table 21.1) the greater the rate
of corrosion of the anode.
(b) Metals which are anodic towards iron, such as zinc and aluminium.
These metals will go into solution in preference to steel (Fig
21.3B)
to
which they will therefore offer some chemical, as well as mechanical,
protection. Since they protect the steel by going into solution themselves,
such protection is generally referred to as 'sacrificial'. Zinc is used in
this way for galvanising steel products, whilst aluminium is effective as
a protective paint partly for the same reason. It must be emphasised
however that the object of galvanising should be to produce a sound
continuous coating of zinc on the surface because sacrificial protection
will only be temporary should the coating be porous.
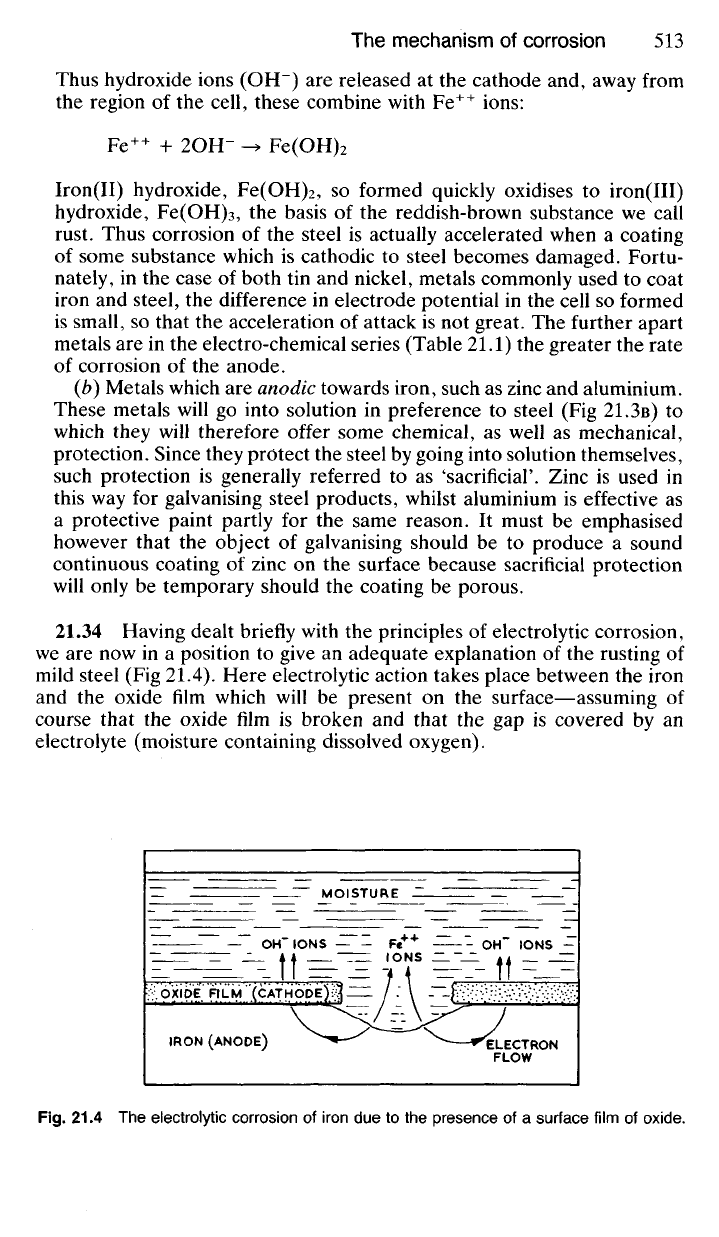
21.34 Having dealt briefly with the principles of electrolytic corrosion,
we are now in a position to give an adequate explanation of the rusting of
mild steel (Fig 21.4). Here electrolytic action takes place between the iron
and the oxide film which will be present on the surface—assuming of
course that the oxide film is broken and that the gap is covered by an
electrolyte (moisture containing dissolved oxygen).
Fig.
21.4 The electrolytic corrosion of iron due to the presence of a surface film of
oxide.
IRON
(ANODE)
ELECTRON
FLOW
MOISTURE
OH
IONS
IONS
OH"
IONS
OXIDE
FILM
(CATHODE)
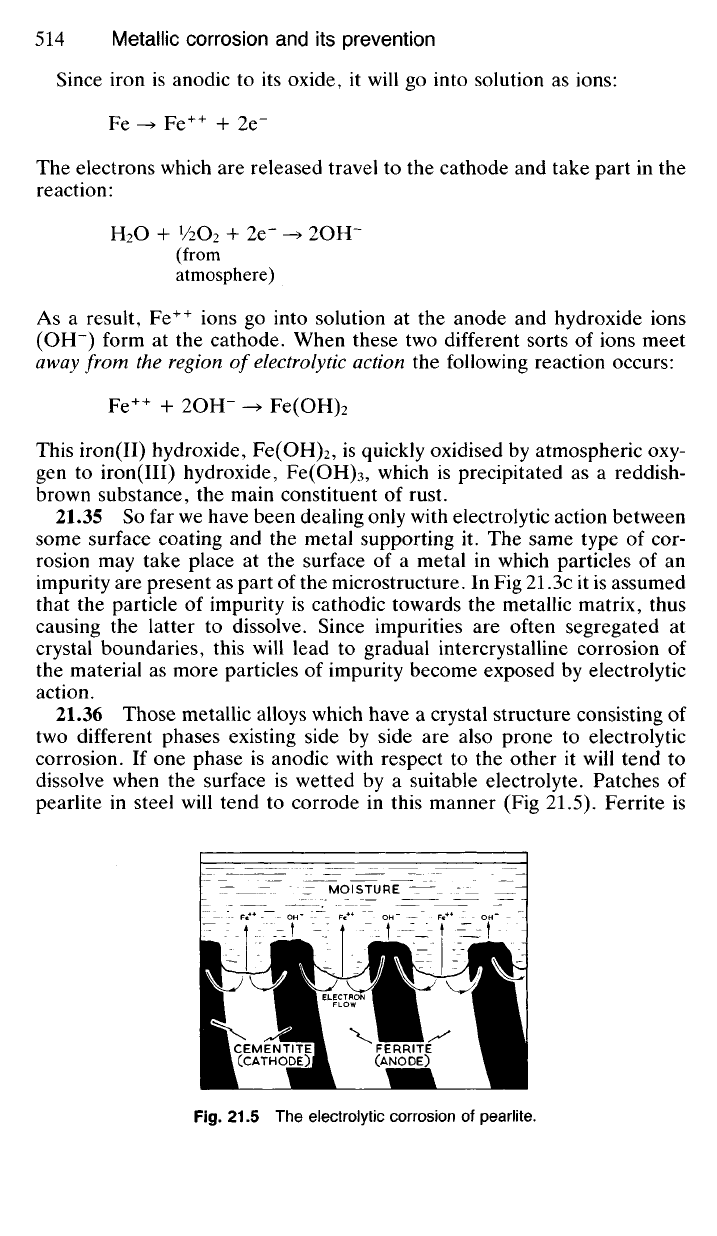
Since iron is anodic to its oxide, it will go into solution as ions:
Fe -> Fe
++
+ 2e"
The electrons which are released travel to the cathode and take part in the
reaction:
H
2
O + '/2O
2
+ 2e" -> 2OH-
(from
atmosphere)
As a result, Fe
++
ions go into solution at the anode and hydroxide ions
(OH~) form at the cathode. When these two different sorts of ions meet
away from the region of
electrolytic
action the following reaction occurs:
Fe
++
+ 2OH- -> Fe(OH)
2
This iron(II) hydroxide, Fe(OH)
2
, is quickly oxidised by atmospheric oxy-
gen to iron(III) hydroxide, Fe(OH)
3
, which is precipitated as a reddish-
brown substance, the main constituent of rust.
21.35 So far we have been dealing only with electrolytic action between
some surface coating and the metal supporting it. The same type of cor-
rosion may take place at the surface of a metal in which particles of an
impurity are present as part of the microstructure. In Fig 21.3c it is assumed
that the particle of impurity is cathodic towards the metallic matrix, thus
causing the latter to dissolve. Since impurities are often segregated at
crystal boundaries, this will lead to gradual intercrystalline corrosion of
the material as more particles of impurity become exposed by electrolytic
action.
21.36 Those metallic alloys which have a crystal structure consisting of
two different phases existing side by side are also prone to electrolytic
corrosion. If one phase is anodic with respect to the other it will tend to
dissolve when the surface is wetted by a suitable electrolyte. Patches of
pearlite in steel will tend to corrode in this manner (Fig 21.5). Ferrite is
Fig.
21.5 The electrolytic corrosion of pearlite.
MOISTURE
ELECTRON
FLOW
CEMENTITE
(CATHODE)
FERRITE
(ANODE)
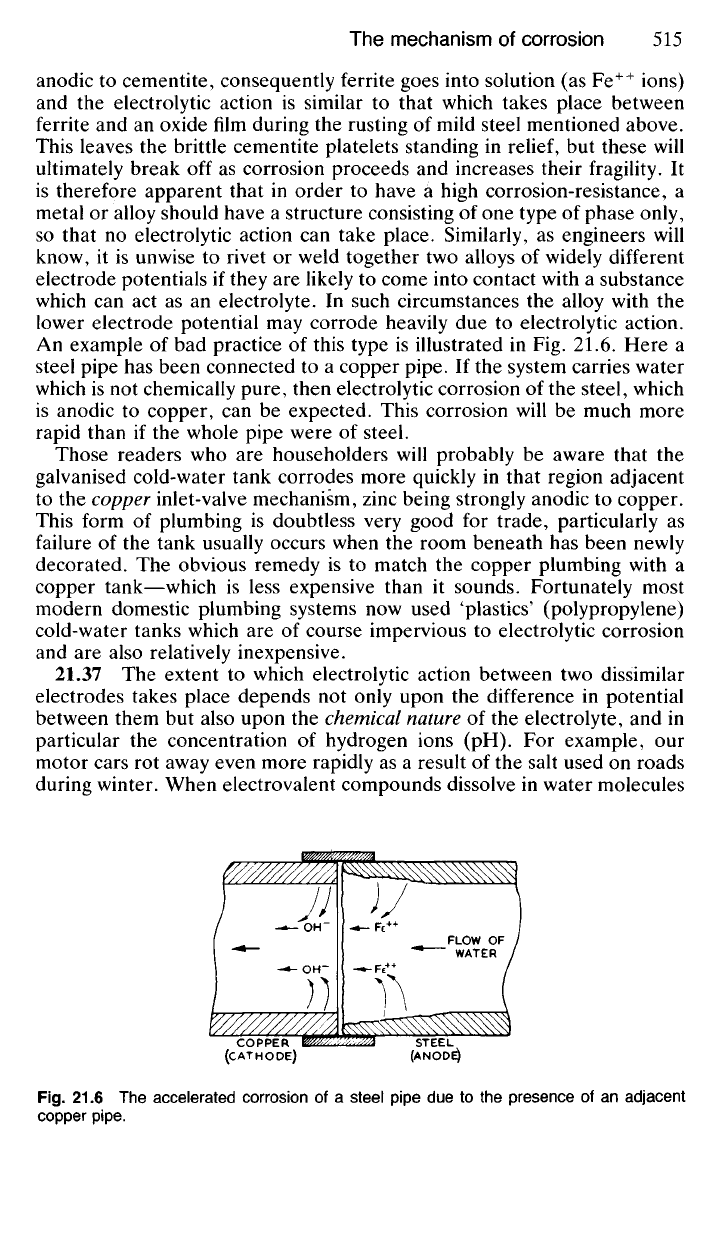
anodic to cementite, consequently ferrite goes into solution (as Fe
++
ions)
and the electrolytic action is similar to that which takes place between
ferrite and an oxide film during the rusting of mild steel mentioned above.
This leaves the brittle cementite platelets standing in
relief,
but these will
ultimately break off as corrosion proceeds and increases their fragility. It
is therefore apparent that in order to have a high corrosion-resistance, a
metal or alloy should have a structure consisting of one type of phase only,
so that no electrolytic action can take place. Similarly, as engineers will
know, it is unwise to rivet or weld together two alloys of widely different
electrode potentials if they are likely to come into contact with a substance
which can act as an electrolyte. In such circumstances the alloy with the
lower electrode potential may corrode heavily due to electrolytic action.
An example of bad practice of this type is illustrated in Fig. 21.6. Here a
steel pipe has been connected to a copper pipe. If the system carries water
which is not chemically pure, then electrolytic corrosion of the steel, which
is anodic to copper, can be expected. This corrosion will be much more
rapid than if the whole pipe were of steel.
Those readers who are householders will probably be aware that the
galvanised cold-water tank corrodes more quickly in that region adjacent
to the copper inlet-valve mechanism, zinc being strongly anodic to copper.
This form of plumbing is doubtless very good for trade, particularly as
failure of the tank usually occurs when the room beneath has been newly
decorated. The obvious remedy is to match the copper plumbing with a
copper tank—which is less expensive than it sounds. Fortunately most
modern domestic plumbing systems now used 'plastics' (polypropylene)
cold-water tanks which are of course impervious to electrolytic corrosion
and are also relatively inexpensive.
21.37 The extent to which electrolytic action between two dissimilar
electrodes takes place depends not only upon the difference in potential
between them but also upon the chemical nature of the electrolyte, and in
particular the concentration of hydrogen ions (pH). For example, our
motor cars rot away even more rapidly as a result of the salt used on roads
during winter. When electrovalent compounds dissolve in water molecules
Fig. 21.6 The
accelerated corrosion
of a
steel
pipe
due to the
presence
of an
adjacent
copper
pipe.
COPPER
(CATHODE)
STEEL
(ANODE)
FLOW
OF
WATER
