Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.


tion are unlikely and components can be machine-finished before
treatment.
(ii) A very high surface hardness of 1150 H
v
is obtained with 'Nitral-
loy' steels containing aluminium.
(iii) Resistance to corrosion is good, particularly if the nitrided surface
is left unpolished.
(iv) Resistance to fatigue failure is good.
(v) Hardness is retained to 500
0
C whereas in a carburised component
hardness begins to fall at about 200
0
C.
(vi) The process is economical when large numbers of components are
to be treated.
(vii) It is a very 'clean' process compared with cyanide-bath treatment
where work must be rinsed to remove toxic salts. Such rinsing water,
which must be disposed of, constitutes a threat to the environment.
19.45 The Disadvantages of Nitriding as compared with case-
hardening are:
(a) The initial outlay for plant is higher than with case-hardening, so
that the process is economical only when large numbers of components
are to be treated.
(b) If the nitrided component is accidentally overheated the surface
hardness will be lost completely, and the component must be nitrided
again. A case-hardened component would only need to be heat-treated
again (unless heating had been so excessive as to cause decarburisation).
19.46 Carbonitriding is a surface-hardening process in which both
carbon and nitrogen are absorbed into the surface of the steel, nitrogen
further increasing the hardness of the carburised layer. Although salt-bath
carburising (19.22) also achieves this result, the term 'carbonitriding' is
used to describe a process in which gaseous media are used. Treatment
takes place at 800-875
0
C in a carbon monoxide/hydrocarbon atmosphere
to which has been added 3% to 8% ammonia. The relative proportions
of carbon and nitrogen dissolved may be controlled by varying both the
concentration of ammonia and the temperature.
Since the steel must be in its austenitic condition to permit rapid solution
of carbon this is in turn relatively unfavourable to the solution of nitrogen
which dissolves fifty times more quickly in ferrite than it does in austenite.
Nevertheless, solution of nitrogen in austenite is still considerable provided
that the treatment temperature is kept below 900
0
C since the solubility of
nitrogen in austenite falls as the temperature rises for a given concentration
of ammonia.
The presence of more than 0.2% nitrogen, along with 0.8% carbon
in the case, slows down transformation rates so that oil-quenching may
sometimes be used to harden the case. However, the M
s
temperature is
also considerably reduced by the presence of more than 0.4% nitrogen so
that large quantities of retained austenite may be present in the case after
quenching. Nevertheless carbonitriding imparts increased hardenability
and wear resistance compared with ordinary case-hardening.

Surface Hardening
by
Localised Heat-treatment
19.50 In these processes both core and case are of the same composition
and it is the heat-treatment which is localised. Since the case must contain
sufficient carbon to render it hardenable these treatments are applied to
medium carbon steels containing 0.35-0.5% carbon. Low-alloy steels con-
taining up to 1.0% chromium with, sometimes, 0.25% molybdenum and
0.5%
nickel are also used and the processes are particularly suited to the
treatment of components such as gear wheels, splined shafts and spindles
where only limited areas need be hardened. The compressive stresses
induced in the hardened surface layers improve the fatigue strength of the
component.
The component as a whole is first heat-treated by quenching and temper-
ing (or sometimes simply normalising) in order to achieve the necessary
core properties. Its surface is then austenitised by being heated locally and
immediately quenched to produce a hard martensitic structure. Thus the
carbon content of the component is constant at about 0.4% throughout,
but whilst the core structure will be tempered martensite (or ferrite/pearl-
ite) that of the case will be martensite. Core and case will generally be
separated by a 'cushion layer' of bainite which considerably reduces the
risk of spalling.
Two methods of surface heating are available:
19.51 Flame Hardening In this process the surface is heated by a gas
flame derived from ethine (acetylene), propane or natural gas. Manually-
operated torches are useful in treating small areas or localised surfaces
such as the cutting edge of a blanking tool or the tip of a screw. As soon
as the area has become austenitic the whole component is water-quenched.
For the progressive hardening of larger areas a gas torch with built-in water
jets can be used. The torch passes over the surface slowly enough to ensure
its austenitisation and the water jets, following closely behind the flame,
effect quenching.
Symmetrical components such as gears and shafts can be spun between
centres within a ring burner. The work piece rotates quite slowly at about
one revolution per second and as soon as the surface has reached its austen-
itic state it is quenched either by complete immersion in a bath or by water
jets built into the burner ring. For the progressive surface hardening of
long shafts a gas ring burner combined with a water-spraying system can
be used.
19.52 Induction Hardening is similar in principle to flame hardening
in that only the surface is austenitised prior to being quenched. Heating
of the surface is achieved by surrounding the component with an inductor
coil carrying a high-frequency alternating current (in the range 2-500 kHz).
The smaller the work pieces the higher the frequency used since this results
in a shallower depth of penetration of the heated zone.
When an electric current passes through a coil a magnetic field surrounds
the coil (Fig. 19.6 (i)) and a steel bar introduced into the field will carry a
magnetic flux. Since the magnetic flux in this case is created by a high-
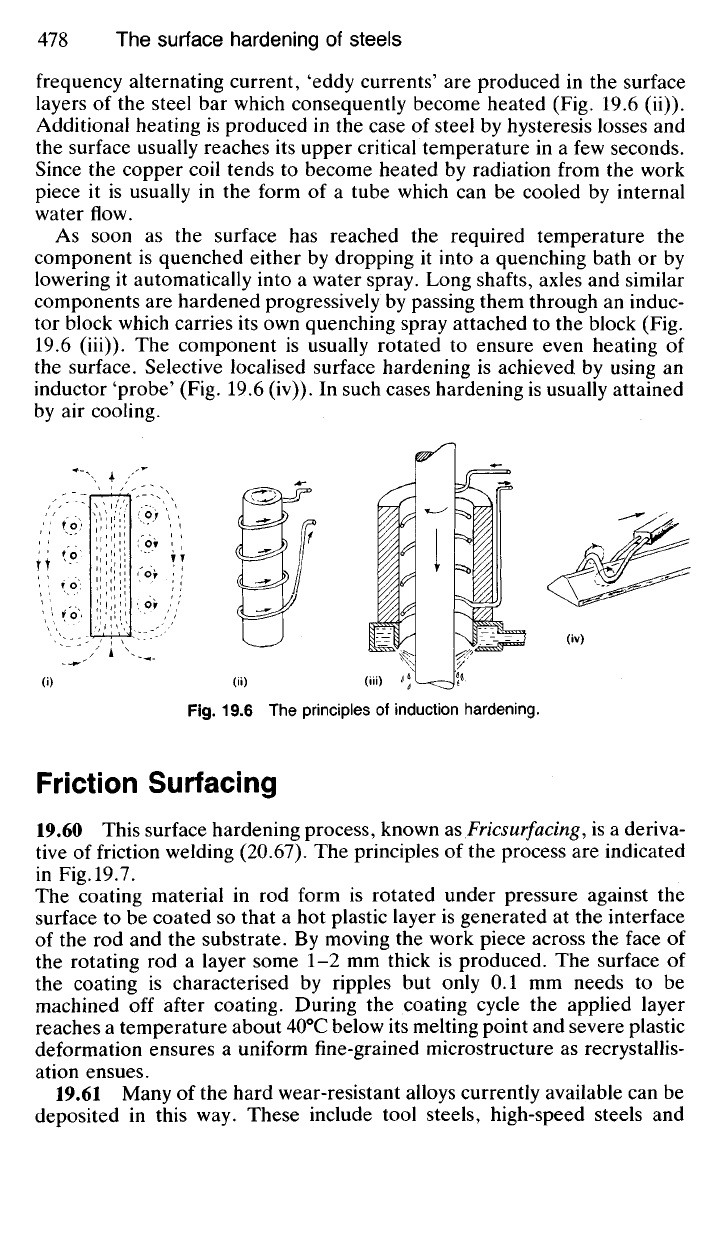
frequency alternating current, 'eddy currents' are produced in the surface
layers of the steel bar which consequently become heated (Fig. 19.6 (ii)).
Additional heating is produced in the case of steel by hysteresis losses and
the surface usually reaches its upper critical temperature in a few seconds.
Since the copper coil tends to become heated by radiation from the work
piece it is usually in the form of a tube which can be cooled by internal
water flow.
As soon as the surface has reached the required temperature the
component is quenched either by dropping it into a quenching bath or by
lowering it automatically into a water spray. Long shafts, axles and similar
components are hardened progressively by passing them through an induc-
tor block which carries its own quenching spray attached to the block (Fig.
19.6 (Hi)). The component is usually rotated to ensure even heating of
the surface. Selective localised surface hardening is achieved by using an
inductor 'probe' (Fig. 19.6 (iv)). In such cases hardening is usually attained
by air cooling.
Fig.
19.6 The principles of induction hardening.
Friction Surfacing
19.60 This surface hardening process, known as
Friesurfacing,
is a deriva-
tive of friction welding (20.67). The principles of the process are indicated
inFig.19.7.
The coating material in rod form is rotated under pressure against the
surface to be coated so that a hot plastic layer is generated at the interface
of the rod and the substrate. By moving the work piece across the face of
the rotating rod a layer some 1-2 mm thick is produced. The surface of
the coating is characterised by ripples but only 0.1 mm needs to be
machined off after coating. During the coating cycle the applied layer
reaches a temperature about 40
0
C below its melting point and severe plastic
deformation ensures a uniform fine-grained microstructure as recrystallis-
ation ensues.
19.61 Many of the hard wear-resistant alloys currently available can be
deposited in this way. These include tool steels, high-speed steels and
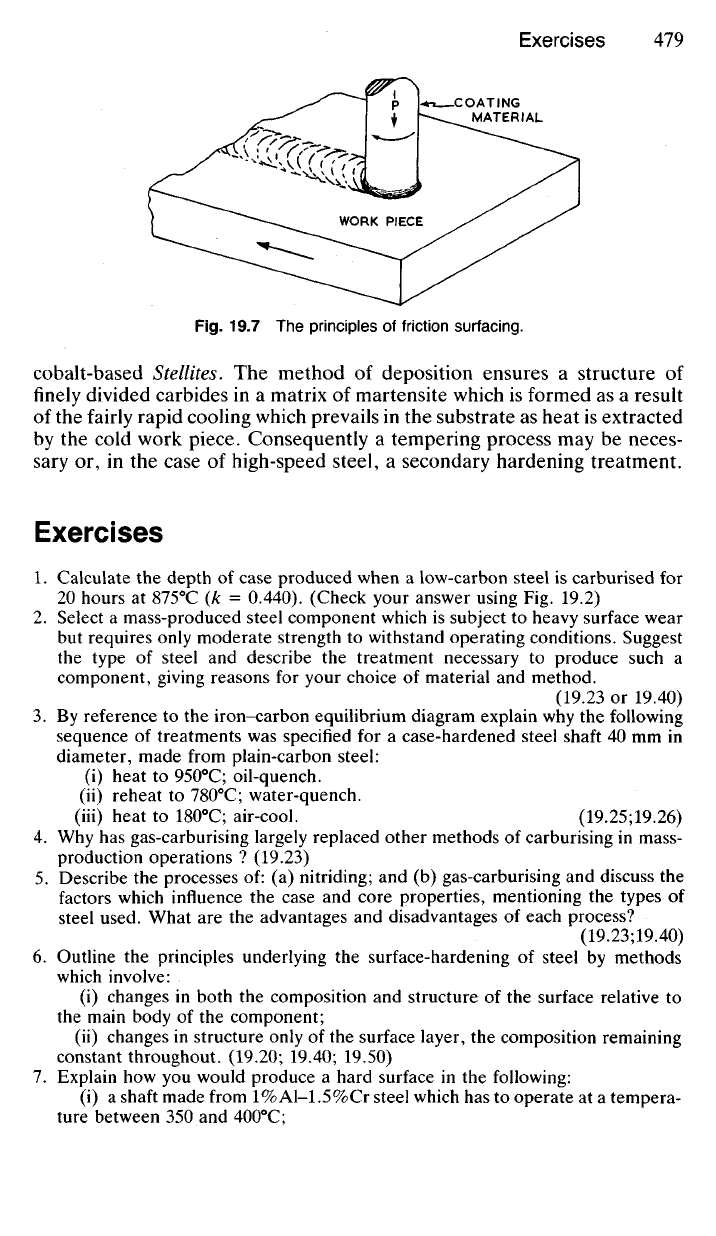
Fig.
19.7 The
principles
of
friction surfacing.
cobalt-based Stellites. The method of deposition ensures a structure of
finely divided carbides in a matrix of martensite which is formed as a result
of the fairly rapid cooling which prevails in the substrate as heat is extracted
by the cold work piece. Consequently a tempering process may be neces-
sary or, in the case of high-speed steel, a secondary hardening treatment.
Exercises
1.
Calculate
the
depth
of
case produced when
a
low-carbon steel
is
carburised
for
20 hours
at
875°C
(A: =
0.440). (Check your answer using
Fig. 19.2)
2.
Select
a
mass-produced steel component which
is
subject
to
heavy surface wear
but requires only moderate strength
to
withstand operating conditions. Suggest
the type
of
steel
and
describe
the
treatment necessary
to
produce such
a
component, giving reasons
for
your choice
of
material
and
method.
(19.23
or
19.40)
3.
By
reference
to the
iron-carbon equilibrium diagram explain
why the
following
sequence
of
treatments
was
specified
for a
case-hardened steel shaft
40 mm in
diameter, made from plain-carbon steel:
(i) heat
to
950
0
C; oil-quench,
(ii) reheat
to
780
0
C; water-quench,
(in) heat
to
180
0
C; air-cool. (19.25;19.26)
4.
Why has
gas-carburising largely replaced other methods
of
carburising
in
mass-
production operations
?
(19.23)
5.
Describe
the
processes
of: (a)
nitriding;
and (b)
gas-carburising
and
discuss
the
factors which influence
the
case
and
core properties, mentioning
the
types
of
steel used. What
are the
advantages
and
disadvantages
of
each process?
(19.23;19.40)
6. Outline
the
principles underlying
the
surface-hardening
of
steel
by
methods
which involve:
(i) changes
in
both
the
composition
and
structure
of the
surface relative
to
the main body
of the
component;
(ii) changes
in
structure only
of the
surface layer,
the
composition remaining
constant throughout. (19.20; 19.40; 19.50)
7.
Explain
how you
would produce
a
hard surface
in the
following:
(i)
a
shaft made from l%Al-1.5%Cr steel which
has to
operate
at a
tempera-
ture between
350 and
400
0
C;
WORK PIECE
.COATING
MATERIAL

(ii) a gear wheel made from a 0.5%C steel which requires a hard surface to
improve its wear-resistant properties.
Give reasons for the choice you make and a brief description of the process
chosen. (19.40; 19.50)
8. Outline the methods available for the surface-hardening of gears. Explain the
principles underlying these processes. (19.20; 19.50)
9. Outline the process known as 'ionitriding' and show how it differs from the
orthodox nitriding process.
(19.41;
19.43)
Bibliography
Child, H. C, Surface Hardening of Steel (Engineering Design Guide), Oxford
University Press, 1980.
Thelning, K-E., Steel and its Heat Treatment: Bofors Handbook, Butterworths,
1984.
BS 970: 1988 Wrought Steels in the Form of Blooms, Billets, Bars and Forgings.
(Part 2 includes steels capable of surface hardening by nitriding.)

Metallurgical Principles
of the Joining of Metals
20.10 Apart from purely mechanical methods such
as
riveting
the
chief
methods available
for
joining metals
are
soldering, brazing, welding
or the
use
of
resin-based adhesives. Industrial applications
of
these processes
are
many
and
varied
and
range from
the
soldering
of
sardine cans
to the
fabrication
by
welding
of
mass-produced ships
by
Henry
J.
Kaiser during
the Second World
War.
Indeed
the
enormous progress
in
welding tech-
niques since then
has led to the
replacement
of
riveted joints
in
steel
structures
by
welded ones
for
almost every application. Modern welding
produces
a
joint which saves
up to 15% of the
mass
of the
structure
as
compared with riveting. Moreover
the
joint
is
free from gaps
and
crevices
and
is
easier
to
maintain
by
surface coating.
The
development
of
welding
has made possible
the
replacement
of
many
of the
larger iron castings
by
weldments, resulting
in
tougher, lighter
and
sounder structures.
At the
same time
it
must
be
admitted that
the
relatively high labour costs, associ-
ated with welding
and its
allied processes, have
led in
recent years
to the
development
of a
host
of
metal fastening devices such
as
self-piercing
rivets,
self-clinching captive fasteners, thread-forming screws, toggle
latches
and
pop-rivets which
in
many cases will provide
an
inexpensive
alternative
to a
continuous metallic joint.
In soldering, brazing
or
welding complete
or
incipient fusion takes place
at
the
surfaces
of the two
pieces
of
metal being joined
so
that
a
more
or
less continuous crystal structure exists
as we
pass across
the
region
of the
joint. Soldering
and
brazing
are
fundamentally similar processes
in
that
the joining material always melts
at a
temperature which
is
lower than
that
of the
work pieces,
but the
distinction between
the two
processes
is
imprecise. However,
it is
generally agreed that soldering—or soft soldering
as
it is
often called—can
be
described
as a
process
in
which temperatures
below 450
0
C
are
involved, whereas brazing temperatures
are
generally
between 600
0
C
and
900
0
C. Included
in the
brazing processes
are
hard
soldering
or
silver soldering, terms tending
to
proliferate confusion.
20

20.11 In the most ancient welding operation—that used by the black-
smith—the two pieces of metal are hammered together whilst at a high
temperature, so that crystal growth occurs across the surfaces in contact,
thus knitting the halves firmly together. A welding process of this type
was probably used by Tubal Cain. By the time the Parthenon was being
constructed in Ancient Greece, on the initiative of Pericles, between 447
and 438
BC,
welding played an important part in civil engineering. In the
Parthenon the marble blocks used were not joined by mortar but by a
system of iron dowels and double T-shaped clamps. Metallographic exam-
ination of surviving specimens has shown that the latter were made by
welding the feet of two T-shaped pieces of iron together.
Soldering
20.20 A solder must be capable of 'wetting', that is, alloying with, the
metals to be joined and at the same time have a freezing range appreciably
lower so that the work itself is in no danger of fusion or deterioration of
mechanical properties. The mechanical strength of the solder must also be
adequate though it is often necessary to overlap workpieces considerably
so that a joint of sufficient strength is obtained.
20.21 Alloys based on tin or lead fulfil most of these requirements for
a wide range of metallurgical materials which need to be joined, since tin
will alloy readily with iron and steel, copper and its alloys, nickel and
its alloys and lead. At the same time tin-lead alloys possess mechanical
toughness, and melt at temperatures between 183°C and 250
0
C, which is
comfortably below the point at which deterioration in properties of the
metals to be joined will take place. Those metals which coat themselves
with a tenacious oxide skin are very difficult to solder. This is true of
aluminium though some success is possible if a suitable flux and a tin-zinc
solder are used. Zinc-base die-casting alloys containing aluminium are
difficult to solder for the same reason, whilst the extremely dense oxide
films on titanium and tantalum make them impossible to solder.
Solders are of two main types depending upon the freezing range
required. Thus in the electronics industries and for the manufacture of tin
cans,
motor-car radiators and the like, a 'tinman's solder' is required which
will solidify quickly over a narrow temperature range. Therefore an alloy
is used which is as near to the eutectic mixture (62Sn-38Pb) as economic
circumstances will allow, bearing in mind that the cost of tin is many times
that of lead. 'Best' tinman's solder solidifies over the range 183-185°C
whilst a cheaper compromise, 'coarse' tinman's solder (50Sn-50Pb) solidi-
fies over the range 183-220
0
C. An increased awareness of the poisonous
nature of lead has resulted in its prohibition for plumbing purposes. Tin-
lead solders too are outlawed for domestic plumbing, even for the joining
of copper pipes and fittings. Tin-silver solders or tin-copper alloys
(Table 20.1) are now employed instead. Although these alloys are used by
'plumbers'—perhaps they should now be called 'coppersmiths'—they are
not required to have the long freezing range of the original plumber's
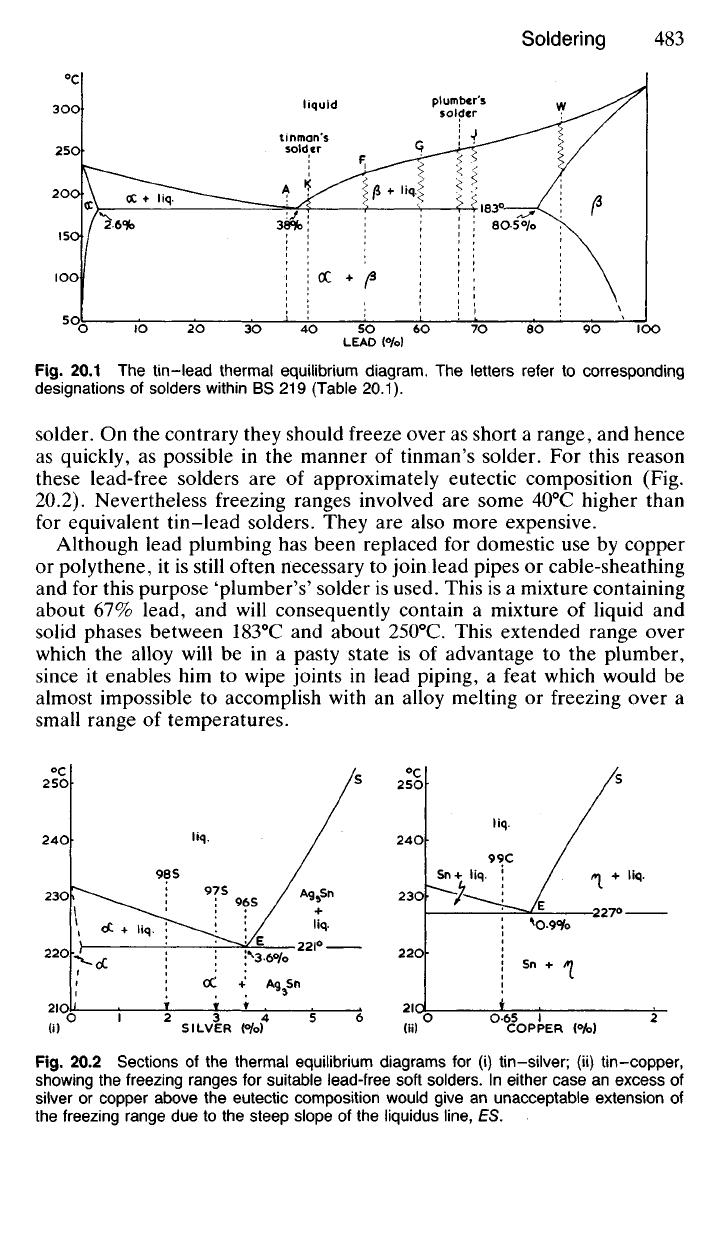
LEAD {%>)
Fig.
20.1 The tin-lead thermal equilibrium diagram. The letters refer to corresponding
designations of solders within BS 219 (Table 20.1).
solder. On the contrary they should freeze over as short a range, and hence
as quickly, as possible in the manner of tinman's solder. For this reason
these lead-free solders are of approximately eutectic composition (Fig.
20.2).
Nevertheless freezing ranges involved are some 40
0
C higher than
for equivalent tin-lead solders. They are also more expensive.
Although lead plumbing has been replaced for domestic use by copper
or polythene, it is still often necessary to join lead pipes or cable-sheathing
and for this purpose 'plumber's' solder is used. This is a mixture containing
about 67% lead, and will consequently contain a mixture of liquid and
solid phases between 183°C and about 250
0
C. This extended range over
which the alloy will be in a pasty state is of advantage to the plumber,
since it enables him to wipe joints in lead piping, a feat which would be
almost impossible to accomplish with an alloy melting or freezing over a
small range of temperatures.
°c
liquid
tinman's
solder
plumber's
solder
Fig.
20.2 Sections of the thermal equilibrium diagrams for (i) tin-silver; (ii) tin-copper,
showing the freezing ranges for suitable lead-free soft solders. In either case an excess of
silver or copper above the eutectic composition would give an unacceptable extension of
the freezing range due to the steep slope of the liquidus line, ES.
SILVER (°/o) COPPER (°/o)
°c
°c
liq.
liq.
Ag
5
Sn
+
liq.
/^ + liq.
Sn + liq.
cC + liq.
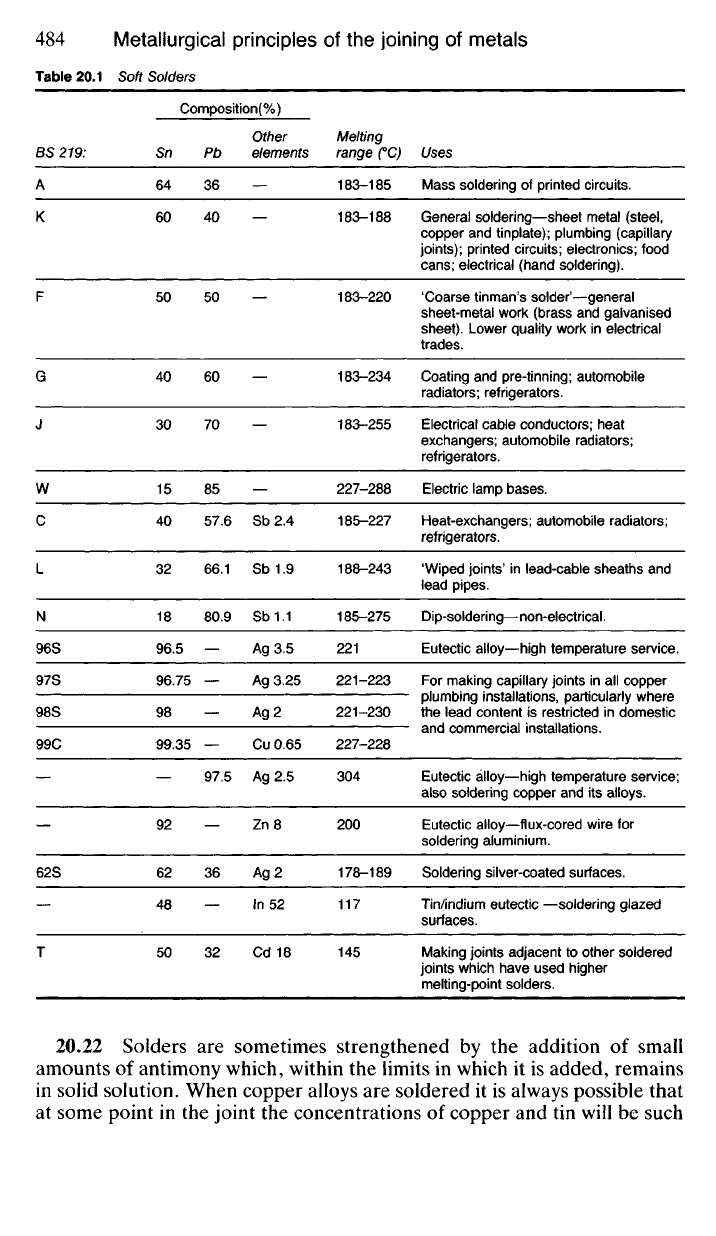
Table 20.1 Soft Solders
Composition(%)
BS 219:
A
K
F
G
J
W
C
L
N
96S
97S
98S
99C
62S
T
Sn
64
60
50
40
30
15
40
32
18
96.5
96.75
98
99.35
92
62
48
50
Pb
36
40
50
60
70
85
57.6
66.1
80.9
97.5
36
32
Other
elements
Sb 2.4
Sb 1.9
Sb 1.1
Ag 3.5
Ag 3.25
Ag 2
Cu 0.65
Ag 2.5
Zn 8
Ag 2
In 52
Cd 18
Melting
range CC)
183-185
183-188
183-220
183-234
183-255
227-288
185-227
188-243
185-275
221
221-223
221-230
227-228
304
200
178-189
117
145
Uses
Mass soldering of printed circuits.
General soldering—sheet metal (steel,
copper and tinplate); plumbing (capillary
joints); printed circuits; electronics; food
cans;
electrical (hand soldering).
'Coarse tinman's solder'—general
sheet-metal work (brass and galvanised
sheet). Lower quality work in electrical
trades.
Coating and pre-tinning; automobile
radiators; refrigerators.
Electrical cable conductors; heat
exchangers; automobile radiators;
refrigerators.
Electric lamp bases.
Heat-exchangers; automobile radiators;
refrigerators.
'Wiped joints' in lead-cable sheaths and
lead pipes.
Dip-soldering—non-electrical.
Eutectic alloy—high temperature service.
For making capillary joints in all copper
plumbing installations, particularly where
the lead content is restricted in domestic
and commercial installations.
Eutectic alloy—high temperature service;
also soldering copper and its alloys.
Eutectic alloy—flux-cored wire for
soldering aluminium.
Soldering silver-coated surfaces.
Tin/indium eutectic —soldering glazed
surfaces.
Making joints adjacent to other soldered
joints which have used higher
melting-point solders.
20,22 Solders are sometimes strengthened by the addition of small
amounts of antimony which, within the limits in which it is added, remains
in solid solution. When copper alloys are soldered it is always possible that
at some point in the joint the concentrations of copper and tin will be such

that one of the hard, brittle copper-tin intermetallic compounds, such as
CU31S118 or CusSn6 will be formed in sufficient quantity to cause brittleness
of the joint. This will be accentuated if the joint is subjected during service
to high temperatures, which, though below the melting point of the solder,
promote diffusion of copper and tin within the joint leading to an increase
in the amount of compound formed. Such a joint will tear through at the
interface containing these compounds revealing their characteristic bluish
colour. This difficulty can be overcome by soldering copper with an alloy
consisting of 97.5Pb-2.5Ag, for, whilst lead and copper are insoluble in
each other, silver alloys with each, and thus acts as a metallic bond between
the two without forming any brittle intermetallic compounds. A list of
representative solders is given in Table 20.1.
20.23 In order that the solder shall 'wet', or alloy with, the surfaces of
the work pieces, the latter must be clean and free from oxide film. Initial
cleaning may involve pickling or some form of mechanical abrasion of the
surface, but the thin layer of oxide which immediately forms on the cleaned
surface of most metals must be dissolved by a suitable flux during the
actual soldering process. The traditional soldering flux for iron and steel
was 'killed spirits of salts'—hydrochloric acid to which had been added
excess granulated zinc. Modern fluxes of this type contain zinc, ammonium,
sodium or tin chlorides either separately or together. Orthophosphoric
acid is a component of fluxes used for soldering stainless steel.
Whilst the above fluxes are very effective in action they leave behind a
corrosive deposit. It is often inconvenient or impossible to wash this off,
particularly in the case of electrical assemblies and some organic type of
flux is then preferable. These are generally organic acids such as lactic,
oleic or glutamic acids or their halogen compounds. Many of these volatil-
ise during soldering or are easily water-soluble. They can be used for
soldering steel, copper, brass and many electro-plated surfaces. For electri-
cal work—including modern electronics—rosin-based fluxes are used. The
least corrosive soldering flux is a solution of pure white rosin dissolved in
propanol-2.
20.24 The reader will be familiar with simple hand-soldering using a
'soldering iron' and flux-cored solder. Whilst this method is still useful,
mass production demands more sophisticated automatic methods. Thus,
the solder may be 'pre-placed' in the form of washers, rings, discs, pellets
or powder (generally a suspension in paste or liquid flux). The assembly,
along with the pre-placed solder and flux is then heated on a hot-plate, or
in some form of oven, or by high-frequency induction. Dip- or mass-
soldering is also used where the work pieces are brought into contact with
molten solder which also acts as the heat source. Printed-circuit soldering
generally involves a modified form of dip-soldering, known as 'wave-
soldering' in which the circuit board is passed over a wave-peak generated
in the molten solder bath (Fig. 20.3).
20.25 Diffusion Soldering The growth of the electronics industry has
generated a need for soldering processes in which thermal damage to the
workpiece is minimised. Such a process is diffusion soldering the elements
of which are shown in Fig. 20.4. Here the workpieces to be joined are
