Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.


melting points coupled with high corjosion resistance so that they and their
alloys join the ranks of the expensive 'superalloys'.
18.81 Beryllium was in fact discovered as long ago as 1797 by Vauque-
lin. The famous chemist Wohler first produced the metal in quantity in
1828 but commercial development did not begin until 1916 in the USA.
Beryllium bronze (16.71) first made its appearance commercially in the
early 'thirties' but it is only in recent years that interest has been focused
on the possibilities of using beryllium in the pure form or as an alloy base.
For a 'light' metal beryllium has a very high melting point (1285°C) so
that it can be used over a wide range of temperatures. It also has good
strength and reasonable corrosion resistance. Since beryllium is less dense
than aluminium but potentially stronger, the aerospace industries would
be much more interested in the metal than they are, were it not for diffi-
culties in forming but above all its extreme scarcity. The only significant
source of beryllium is the mineral beryl, a semi-precious stone, mined in
a number of countries but notably Brazil, Argentina and Central Africa.
It is also present in the precious stone emerald.
In France beryllium is known as 'glucinium', a reference to the sweet
taste of some of its compounds. The metal is, however, extremely toxic
giving rise to a chronic form of poisoning called 'berylliosis'. This resembles
septic pneumonia and constitutes a further hazard in fabricating the metal.
Moreover, although malleable the metal lacks ductility, a fact which
restricts the methods which can be used to shape it. Beryllium crystallises
in the CPH form and metals with this type of structure are generally
more brittle than those which crystallise in one of the cubic forms (4.12).
Nevertheless beryllium is successfully extruded to produce bar, tube and
various sections. Hot-working the metal in a mild steel sheath is a common
method of shaping beryllium.
One property which interests the nuclear engineers is the low neutron
absorption of beryllium. That is, it does not significantly impede the pass-
age of neutrons. For this reason beryllium was initially popular as a canning
material for nuclear fuel but partly because of its scarcity it has been largely
replaced by zirconium alloys. Since it is transparent to X-rays beryllium is
used for X-ray windows. Its low inertia has led to use in some expensive
camera shutters whilst its high melting point has made it useful in neon-sign
electrodes and targets for cyclotrons.
Beryllium has found limited use in aerospace structures, jet-engine tur-
bine parts and also as a neutron source using a-particles from plutonium.
Commercial quality beryllium (92Be; 4.5BeO; 0.2 max.Al; 0.5 max.C; 0.3
max.Fe) has a yield strength of approximately 560 N/mm
2
and a tensile
strength of 890 N/mm
2
.
18.82 Zirconium In 1789 Klaproth identified a new metal in the min-
eral zircon, but it was Berzelius who, in 1824, succeeded in isolating zir-
conium. It was not until 1914 that the metal was produced in sufficient
quantity to show that it was ductile. Then, during the First World War,
rumours circulated that Germany was developing zirconium steels. Inten-
sive research by the Allies, however, failed to produce an alloy of this type
with useful properties. Eventually zirconium became known chiefly as an

alloying addition to magnesium-base alloys (18.12), whilst its oxide zircona
was developed as a high-temperature refractory. In more recent years it
has been used in some of the titanium-base alloys (18.62).
In 1944 research was instituted with the object of producing high-purity
zirconium and to-day it is probably the most useful nuclear-engineering
material for use where low neutron absorption is necessary. In other
respects too zirconium is an attractive proposition in nuclear engineering.
As compared with beryllium it is relatively plentiful, and is also superior
to beryllium in corrosion resistance. Moreover, zirconium and its alloys
can be fabricated with comparative ease provided that the metal is not
heated in contact with either oxygen, nitrogen or hydrogen. Each of these
gases will form interstitial solid solutions in zirconium leading to its em-
brittlement.
Unalloyed zirconium tends to be mechanically weak at high tempera-
tures.
In addition, under such conditions it is rapidly corroded by water
vapour and carbon dioxide. These difficulties have been largely overcome
by alloying and most of the zirconium now used in nuclear engineering is
supplied as alloys such as 'Zircaloy IF (1.5Sn; 0.12Fe; 0.05Ni; 0.1Cr;
BaI.Zr) or 'Zircaloy IV (1.5Sn; 0.2Fe; 0.1Cr). These alloys are used for
fuel sheathing and structural components in pressurised-water reactors
because of their combination of low neutron absorption, high strength and
good corrosion resistance.
Since zirconium is chemically reactive in the powdered form it is com-
pacted with lead to make lighter 'flints'. Before the days of 'electronic
flash' it constituted the wire in many consumable photographic flash bulbs.
In the form of ferro-zirconium or ferro-silico-zirconium it can be used as
a 'scavenger' in steel making since it removes traces of both oxygen and
sulphur. Any residue refines the grain of steel whilst the strength of some
alloy steels is increased. Zirconium occurs chiefly as 'zircon sands' and is
mined in Brazil, India, the USA and around the Australian coast. It is
extracted from its minerals in a similar way to titanium.
18.83 Hafnium One of the main difficulties in the use of zirconium as
a nuclear canning material is the presence of small amounts of hafnium
which materially increase neutron absorption. Unfortunately hafnium is
always closely associated with zirconium in the mineral deposits of the
latter, and the reduction of the hafnium content to the few parts per
million which is all that can be tolerated in reactor-quality zirconium, is an
expensive operation. Both zirconium and hafnium belong to the same
'transition group' in the Periodic Classification. Consequently their chemi-
cal properties are so alike that their separation is difficult and, hence,
costly. In respect of neutron absorption, however, they are at opposite
ends of the scale for, whilst zirconium has a very low neutron absorption,
that of hafnium is extremely high.
For some time hafnium was used as control rods in nuclear reactors
because of its high neutron absorption and because the 'daughter' atoms
formed by such neutron bombardment were hafnium isotopes. This led to
little dimensional change in the rods. Of recent years hafnium has been
replaced by less expensive materials so that it now finds little industrial use.

The existence of hafnium was first suspected towards the end of the
nineteenth century and it was discovered in rare-earth residues in 1911,
though its properties were not verified until 1923. It appears to be named
after Hafnia, the ancient name for Kobenhavn (Copenhagen).
18.84 Tantalum is important because it combines high ductility and
exceptional corrosion resistance with a very high melting point. In the pure
state tantalum is corrosion resistant to most acids and alkalis. Since, when
pure,
it is also very ductile, it is used in acid-proof equipment in chemical
plant. Being impervious to acid attack, sections as thin as 0.3 mm can be
used in heat-transfer equipment. It is used in the form of plugs to repair
damage to vitreous lined steel tanks in distilleries and chemical plant.
Not only is pure tantalum unreactive towards animal fluids but it also
provides a surface upon which tissue will grow. Consequently, it is used in
bone surgery, plates to replace skull tissue and for other 'implants' in the
human body. Woven tantalum gauze is used as a reinforcement to the
abdominal wall in some hernia operations.
Tantalum can be anodically treated (21.91) and the film so formed is very
stable and self-sealing. Moreover, this oxide film has excellent dielectric
properties and, being impervious to electrolytes used, has made tantalum
a very useful metal for the manufacture of very small sized electrolytic
capacitors. Foil of the order of 0.012 mm thick can be produced resulting
in electrolytic capacitors about one-tenth of the volume of ordinary alu-
minium-foil capacitors of equivalent capacitance. These small tantalum-foil
capacitors are widely used in modern electronic circuitry.
In modern engineering tantalum alloys (Table 18.9) are used for steam-
turbine blades, valves, nozzles, stills, agitators, containers and pipes in
chemical industries; and for the tips of fountain-pen nibs. Its minerals are
mined principally in Central and Southern Africa, Australia and Portugal.
Though generally assumed to be a 'new' metal the presence of tantalum
was first detected early in the nineteenth century. Because of the great
difficulty in isolating it from its minerals the metal was named after Tanta-
lus,
son of Zeus, of Greek mythology. It was Tantalus who, for his alleged
crimes, was stood in water up to his chin but as he attempted to drink the
water always receded—hence to 'tantalize'. Although it was detected so
early, a hundred years passed before the pure metal was isolated in 1905.
18.85 Niobium was first discovered near Connecticut in 1801 by a
British chemist named Hatchett. In recognition of its source he called it
'columbium'. At about the same time Ekeburg in Sweden discovered a
'new' metal in close association with tantalum, and when later, in 1844, it
was isolated from tantalum by Rose he appropriately named it 'niobium'
after the sad Niobe, daughter of Tantalus. Soon afterwards 'columbium'
and 'niobium' were identified as being the same metal, but the two names
continued to be used on their respective sides of the Atlantic until some
years ago, by international agreement, the name 'niobium' was officially
adopted. Niobium was first produced in quantity in 1929 and has for a
considerable time been used as a 1.0% addition to 18-8 stainless steels in
which it induces resistance to 'weld decay' (20.93).
Like zirconium, niobium has a low neutron absorption. Moreover it has
a very high melting point (2468°C) suggesting that it would be useful for
nuclear-fuel sheathing where higher reactor temperatures are involved.
Unfortunately niobium also reacts with the pile gases, as well as with other
substances with which it is likely to come into contact in the reactor, at
temperatures in excess of 500
0
C. However some of the alloys of niobium
have very good resistance to oxidation and a number (Table 18.9) are used
for high-temperature applications.
18.86 Cobalt is second only to iron in terms of its strong ferromagnetic
properties and finds use in permanent magnet alloys (14.30). It is also
important as a constituent of super high-speed steels and as the binding
matrix in some cemented carbide tool materials (14.20).
Although the corrosion-resistance of pure cobalt is poor that of some of
its alloys is excellent, particularly at high temperatures. Most of these
alloys are difficult to work and are therefore shaped by investment casting
(5.40—Pt.2). They have good high-temperature strength. 'FSX.414' (52Co;
29.5Cr; 10.5Ni; 7W; 0.25C; 0.01B) is used for gas-turbine vanes; whilst
'MAR-M322' (60Co; 21.5Cr; 9W; 4.5Ta; 2.25Zr; IC; 0.75Ti) is an invest-
ment casting alloy for jet-engine blades. 'NAS Co-W-Re' (67Co; 25W;
3Cr; 2Re; IZr; ITi; 0.4C) has a rupture strength of 45N/mm
2
at 1300
0
C
for 1000 hours and is used for high-temperature space applications.
Whilst the current tendency is towards the development of plastics
materials and ceramics for surgical purposes because of their greater chemi-
cal inactivity, some cobalt-base alloys are used in orthopaedic implants. A
popular cast alloy contains 65% cobalt, 28% chromium, 6% molybdenum
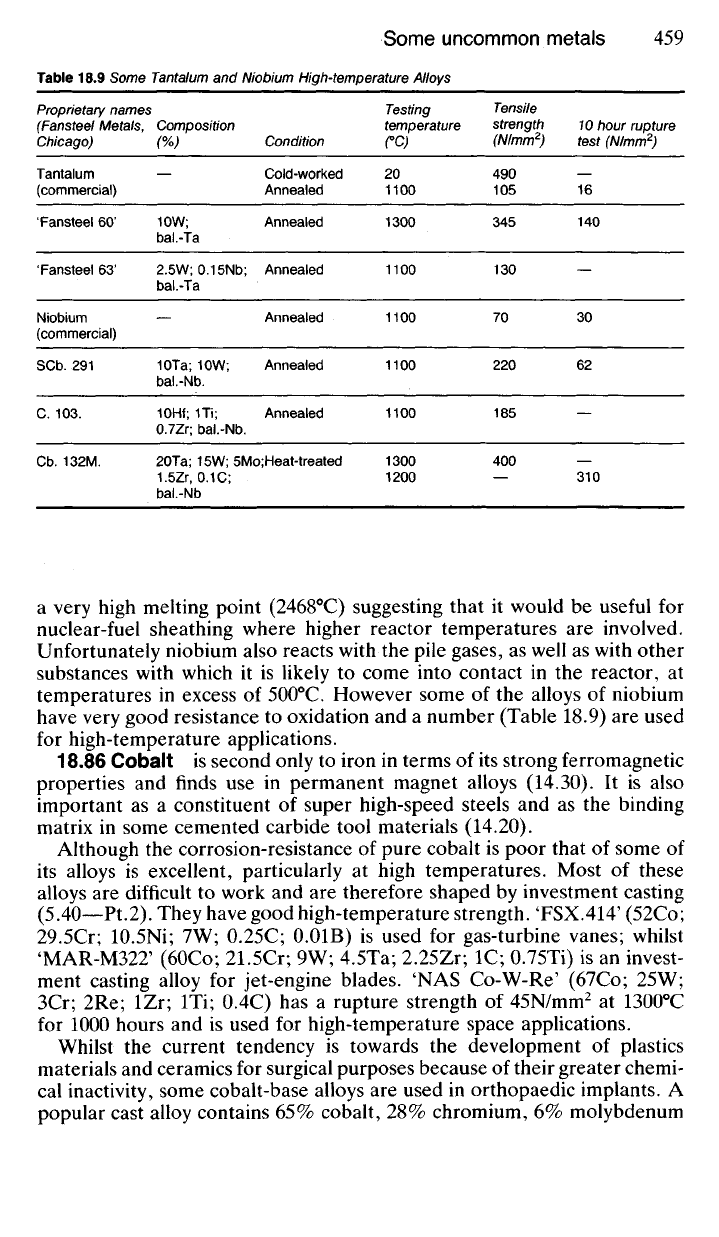
Table 18.9 Some Tantalum and Niobium High-temperature Alloys
Proprietary names
(Fansteel Metals,
Chicago)
Tantalum
(commercial)
'Fansteel 60'
'Fansteel 63'
Niobium
(commercial)
SCb.
291
C. 103.
Cb.
132M.
Composition
(%)
10W;
bal.-Ta
2.5W;0.15Nb;
bal.-Ta
10Ta;
10W;
bal.-Nb.
10Hf; 1Ti;
0.7Zr;
bal.-Nb.
Condition
Cold-worked
Annealed
Annealed
Annealed
Annealed
Annealed
Annealed
20Ta;
15W; 5Mo;Heat-treated
1.5Zr,
0.1 C;
bal.-Nb
Testing
temperature
CC)
20
1100
1300
1100
1100
1100
1100
1300
1200
Tensile
strength
(N/mm
2
)
490
105
345
130
70
220
185
400
10 hour rupture
test (N/mm
2
)
16
140
30
62
310

and 1% nickel whilst a suitable wrought alloy contains 20% chromium,
10%
nickel, 0.1% carbon and the balance cobalt. However even with alloys
of the highest corrosion resistance some interaction may occur with the
surrounding tissue, releasing potentially toxic substances.
18.90 As more becomes known of the rarer and more elusive of the metallic
elements so are their properties exploited. Thus metals like yttrium (Table
18.5),
samarium and strontium (Table 14.3) now find use in engineering
alloys, whilst another 'rare earth' neodymium is used in a new generation
of permanent magnets (14.30) as well as in the colouring of glass and the
manufacture of some capacitors. Yet another 'rare earth', praseodymium,
along with cerium, is used in the decolourising of some optical glasses,
whilst lanthanum is used in high refractive index glasses. As long ago as
the 1960s europium and yttrium compounds were developed for the coating
of TV screens, whilst during the same period the nuclear energy pro-
gramme was interested in europium, samarium, gadolinium and dys-
prosium as high neutron cross-section materials. The high thermal stability
of cerium and yttrium hydrides led to their use as neutron moderators in
atomic piles. In the field of medicine even the man-made element tech-
netium (with a half-life of only a few hours) is used as a radioactive isotope.
Exercises
1.
By reference to Fig. 18.1 estimate the maximum and minimum solution-
treatment temperatures (prior to precipitation-hardening) for magnesium-base
alloys which contain singly: (i) 10% aluminium; (ii) 5% zinc; (iii) 3% thorium.
2.
By reference to Fig. 18.4 estimate the proportion of primary aluminium to
eutectic in a slowly cooled bearing metal in the cast condition containing 80
Al-20 Sn.
3.
Which of the following alloy compositions could be hardened by some form of
heat-treatment: (i) 90Mg-IOAl; (ii) 90Al-IOMg; (iii) 90Cu-IOZn; (iv) 90Cu-
10Al; (v) 90Cu-IONi? Give reasons for your answers.
4.
What are the principal difficulties encountered during the manufacture of mag-
nesium-base alloys?
Where do most of these alloys find application? (18.10)
5.
Write a short essay on light-weight non-ferrous alloys, their properties and
uses (Ch. 17; 18.10)
6. Outline a process by which zinc-base alloys are generally shaped. What are (i)
the attractive properties; (ii) possible difficulties, involved in the use of these
materials? (6.20; 18.20)
7.
Discuss the factors which influence the choice of nickel-base alloys for high-
temperature engineering. Outline the mechanisms used to strengthen these
materials. (18.30)
8. The development of materials to operate at elevated temperatures has been a
most important aspect of metallurgical progress. Give a comprehensive account
of the ferrous and non-ferrous alloys at present available for operation in the
600-1000
0
C range, discussing such factors as resistance to oxidation, resistance
to corrosion and retention of strength and showing how these are obtained in
particular alloys. (13.70; 18.30)
9. List, and briefly describe, the essential requirements of a bearing metal. Show,

by discussing the constitution and structure of materials, how the requirements
are realised in: (i) certain copper-base alloys and (ii) white metals.
Compare the applications of the two groups and give typical
compositions. (18.40)
10.
What particular properties of titanium and its alloys are important in consider-
ing them for aero-space design?
Outline the principles by which high strength is developed in these materials.
(18.60)
11.
Outline the roles played by (i) uranium; (ii) beryllium; (iii) zirconium; and (iv)
hafnium, in 'nuclear engineering'. (18.70; 18.80)
Bibliography
Betteridge, W., Cobalt and its Alloys, Ellis Horwood, 1982
Betteridge, W., Nickel and its Alloys, Ellis Horwood, 1984
Betteridge, W. and Heslop, J., The Nimonic Alloys and Other Nickel-base High-
temperature Alloys, Edward Arnold, 1974.
Hausner, H. H., Beryllium, its Metallurgy and
Properties,
University of California
Press,
1966.
Kleefisch, E. W., Industrial Applications of Titanium and Zirconium, American
Society for Testing and Materials, 1981.
Morgan, S. W. E., Zinc and its Alloys, Macdonald and Evans, 1977.
Polmear, I. J., Light Alloys
(Metallurgy
of the Light Alloys), Edward Arnold, 1989.
Sims,
C. T. and Hagel, W. C, The Superalloys, John Wiley, 1973.
BS 2970: 1972 Magnesium Alloy Ingots and Castings.
BS 3370: 1970 Wrought Magnesium Alloys for General
Engineering
Purposes:
Plate,
Sheet and Strip.
BS 3372: 1970 Wrought Magnesium Alloys for General
Engineering
Purposes:
Forg-
ings and Cast Forging
Stock.
BS
3373:
1970 Wrought Magnesium Alloys for General
Engineering
Purposes:
Bars,
Sections and Tubes, Including Extruded Forging
Stock.
BS 1004: 1985 Zinc Alloys for Die Casting and Zinc Alloy Die Castings.
BS 3072: 1983
Specifications
for Nickel and Nickel Alloys: Sheet and Plate.
BS
3073:
1989
Specifications
for Nickel and Nickel Alloys: Strip.
BS 3074: 1983
Specifications
for Nickel and Nickel Alloys: Seamless Tube.
BS 3075: 1989
Specifications
for Nickel and Nickel Alloys: Wire.
BS 3076: 1983
Specifications
for Nickel and Nickel Alloys: Bar.
BS 115: 1987 Metallic Resistance
Materials
for Electrical Purposes.
BS TA 1 to TA 59: 1973/1986
Specifications
for Titanium and Titanium Alloys (The
TA series covers 'Aerospace' uses.)
BS 2TA 100: 1973
Procedure
for Inspection and Testing of Wrought Titanium and
Titanium Alloys.

The Surface Hardening
of Steels
19.10 The service conditions of many steel components such as cams,
gears and shafts make it necessary for them to possess both hard, wear-
resistant surfaces and at the same time, tough, shock-resistant cores. In
plain carbon steels these two different sets of properties exist only in alloys
of different carbon content. A low-carbon steel, containing approximately
0.1%
carbon, will be tough but soft, whilst a high-carbon steel of 0.8% or
more carbon will be hard when suitably heat-treated but will also be rela-
tively brittle.
19.11 The situation can best be met by employing a low-carbon steel
with suitable core properties and then causing either carbon or nitrogen to
penetrate to a regulated depth to produce a potentially hard surface skin;
as in the principal surface-hardening processes of carburising and nitriding.
Alternatively, a medium-carbon steel can be used, heat-treated first to
produce desirable core properties and local hardness at the surface then
introduced by one of the flame-hardening or induction-hardening pro-
cesses. In the first case the hardenable material is localised, whilst in the
second case it is the heat-treatment itself which is localised.
Rapid penetration of the surface of steel can only be effective if the
solute element dissolves interstitially. This is the case with the elements
used, viz. carbon, nitrogen and, to a small extent, boron. Once dissolved,
the elements increase the hardness of the surface by forming interstitial
compounds—carbides, nitrides or borides.
Case-hardening
19.20 The principles of case-hardening were used centuries ago in the
conversion of wrought iron to steel by the 'cementation' process. Both this
ancient process and case-hardening make use of the fact that carbon will
19

diffuse into iron provided that the latter is in the FCC (y) form which exists
above 910
0
C. Thus carburising consists in surrounding the component with
suitable carbonaceous material and heating it to above its upper critical
temperature for long enough to produce a carbon-enriched layer of suf-
ficient depth.
Solid, liquid and gaseous carburising media are used. The nature and
scope of the work involved will govern which medium it is best to employ.
The function of the carburising medium is to release atoms of carbon at
the surface of the work piece so that, at the carburising temperature, they
will be absorbed interstitially into the steel.
The rate at which the released carbon atoms diffuse beneath the surface
of the work piece is governed by Fick's Law (8.25):
J =
SV-JT
dx
where / = the amount of carbon which passes in unit time across area S
in a plane normal to the 'JC' axis;
— = the variation of carbon content with depth below the surface
"
x
(the concentration gradient of the carbon);
D = the diffusion coefficient of carbon in y-iron.
D is very dependent upon temperature but in this instance is of the order
of 10~
7
cm
2
/s at the carburising temperature (900
0
C).
From the Diffusion Equation (8.25):
dt dx
2
the depth of carbon penetration could be derived using one of the standard
methods for solving differential equations. However, a simple formula
(13.84—Pt.2), derived originally by Einstein from mathematical studies of
diffusion, can be applied:
x = y/{2Di)
where D = the diffusion coefficient (cm
2
/s);
and t = time of diffusion (s).
This can be written:
Case depth = kyjt
where k = V5D and gives a rough estimate of the time required to produce
a case of given depth.
A very rough estimate of the depth of case may be made from a fractured
section of a test piece carburised along with the work but a more accurate
measurement may be made metallographically. A cross section of the test
piece is polished and etched and its image projected on to the screen of a
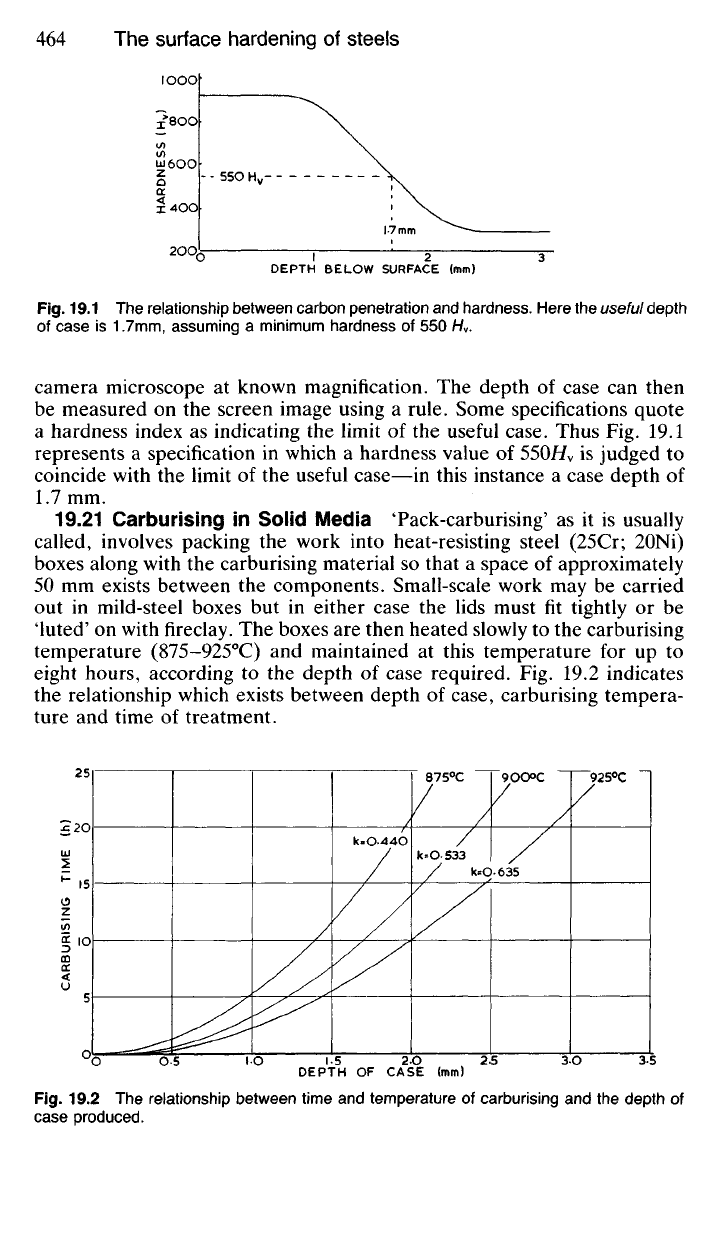
Fig.
19.1 The relationship between carbon penetration and hardness. Here the useful depth
of case is 1.7mm, assuming a minimum hardness of 550 H
v
.
camera microscope at known magnification. The depth of case can then
be measured on the screen image using a rule. Some specifications quote
a hardness index as indicating the limit of the useful case. Thus Fig. 19.1
represents a specification in which a hardness value of
55OH
V
is judged to
coincide with the limit of the useful case—in this instance a case depth of
1.7 mm.
19.21 Carburising in Solid Media Tack-carburising' as it is usually
called, involves packing the work into heat-resisting steel (25Cr; 20Ni)
boxes along with the carburising material so that a space of approximately
50 mm exists between the components. Small-scale work may be carried
out in mild-steel boxes but in either case the lids must fit tightly or be
luted' on with fireclay. The boxes are then heated slowly to the carburising
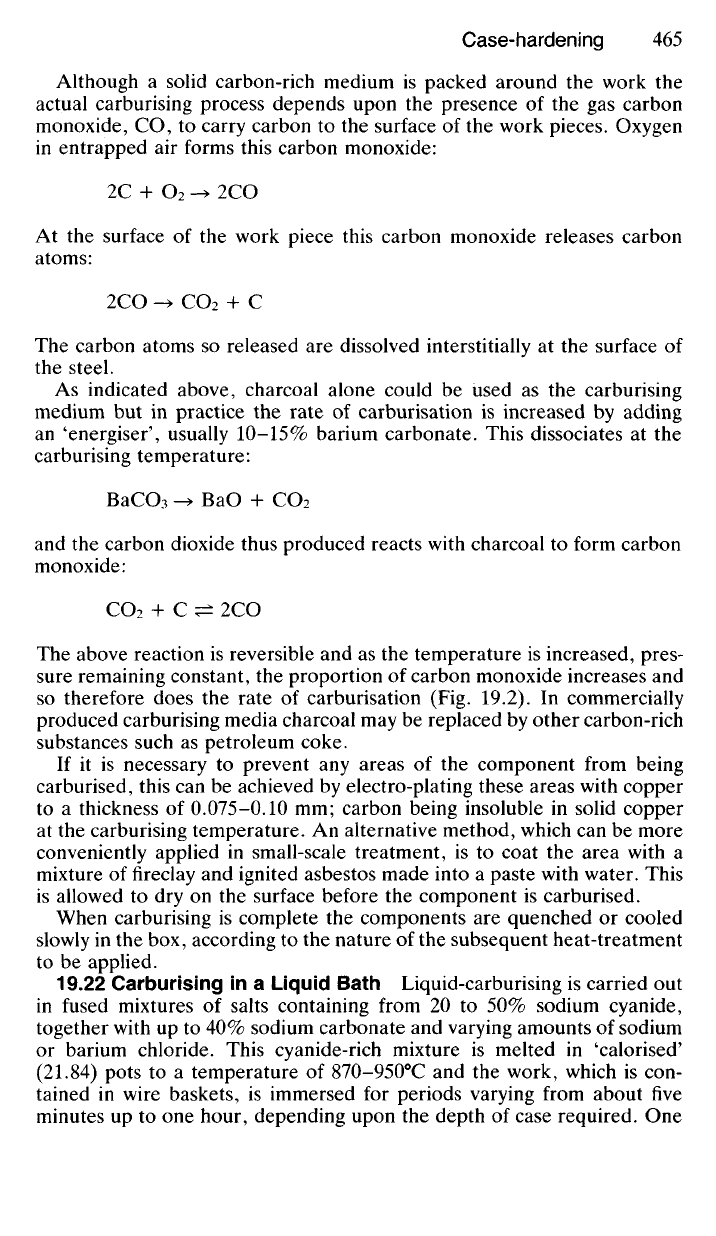
temperature (875-925°C) and maintained at this temperature for up to
eight hours, according to the depth of case required. Fig. 19.2 indicates
the relationship which exists between depth of case, carburising tempera-
ture and time of treatment.
HARDNESS
(H
v
)
CARBURISING
TIME
(h)
DEPTH BELOW SURFACE (mm)
DEPTH OF CASE (mm)
Fig.
19.2 The relationship between time and temperature of carburising and the depth of
case produced.
Although a solid carbon-rich medium is packed around the work the
actual carburising process depends upon the presence of the gas carbon
monoxide, CO, to carry carbon to the surface of the work pieces. Oxygen
in entrapped air forms this carbon monoxide:
2C + O
2
-> 2CO
At the surface of the work piece this carbon monoxide releases carbon
atoms:
2CO -> CO
2
+ C
The carbon atoms so released are dissolved interstitially at the surface of
the steel.
As indicated above, charcoal alone could be used as the carburising
medium but in practice the rate of carburisation is increased by adding
an 'energiser', usually 10-15% barium carbonate. This dissociates at the
carburising temperature:
BaCO
3
-> BaO + CO
2
and the carbon dioxide thus produced reacts with charcoal to form carbon
monoxide:
CO
2
+ C ^± 2CO
The above reaction is reversible and as the temperature is increased, pres-
sure remaining constant, the proportion of carbon monoxide increases and
so therefore does the rate of carburisation (Fig. 19.2). In commercially
produced carburising media charcoal may be replaced by other carbon-rich
substances such as petroleum coke.
If it is necessary to prevent any areas of the component from being
carburised, this can be achieved by electro-plating these areas with copper
to a thickness of 0.075-0.10 mm; carbon being insoluble in solid copper
at the carburising temperature. An alternative method, which can be more
conveniently applied in small-scale treatment, is to coat the area with a
mixture of fireclay and ignited asbestos made into a paste with water. This
is allowed to dry on the surface before the component is carburised.
When carburising is complete the components are quenched or cooled
slowly in the box, according to the nature of the subsequent heat-treatment
to be applied.
19.22 Carburising in a Liquid Bath Liquid-carburising is carried out
in fused mixtures of salts containing from 20 to 50% sodium cyanide,
together with up to 40% sodium carbonate and varying amounts of sodium
or barium chloride. This cyanide-rich mixture is melted in 'calorised'
(21.84) pots to a temperature of 870-950
0
C and the work, which is con-
tained in wire baskets, is immersed for periods varying from about five
minutes up to one hour, depending upon the depth of case required. One
